Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Testing
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Kozłowska, D.; Anyszek, T.; Dorycka, M.; Martin, E.; Piotrowska-Mietelska, A. Atypical pneumonia diagnosed as coronavirus disease 2019 by a serologic test (patient -1 in Poland). Pol. Arch. Intern. Med. 2020, 130, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.M.; Eisinger, R.W.; Lowy, D.R.; Petersen, L.R.; Humes, R.; Hepburn, M.; Cassetti, M.C. The COVID-19 Serology Studies Workshop: Recommendations and Challenges. Immunity 2020, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. [Google Scholar] [CrossRef]
- Chukwu, C.A.; Mahmood, K.; Elmakki, S.; Gorton, J.; Kalra, P.A.; Poulikakos, D.; Middleton, R. Evaluating the antibody response to SARS-COV-2 vaccination amongst kidney transplant recipients at a single nephrology centre. PLoS ONE 2022, 17, e0265130. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Lancet, J.E.; Pilon-Thomas, S.; Dong, N.; Jain, A.G.; Tan, E.; Ball, S.; Tworoger, S.S.; Siegel, E.M.; Whiting, J.; et al. Evaluation of Antibody Response to SARS-CoV-2 mRNA-1273 Vaccination in Patients With Cancer in Florida. JAMA Oncol. 2022, 8, 748–754. [Google Scholar] [CrossRef]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Martin, E. A comparison of 7 commercial anti-SARS-CoV-2 antibody immunoassays. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Kozlowska, D.; Panek, A.; Martin, E. The real life performance of 7 automated anti-SARS-CoV-2 IgG and IgM/IgA immunoassays. Pract. Lab. Med. 2021, 25, e00212. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 478–1488. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Anyszek, T.; Panek, A.; Martin, E. Anti-Spike SARS-CoV-2 IgG Assessment with a Commercial Assay during a 4-Month Course after COVID-19 Vaccination. Vaccines 2021, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Marklund, E.; Leach, S.; Nyström, K.; Lundgren, A.; Liljeqvist, J.; Nilsson, S.; Yilmaz, A.; Andersson, L.-M.; Bemark, M.; Gisslén, M. Longitudinal Follow Up of Immune Responses to SARS-CoV-2 in Health Care Workers in Sweden With Several Different Commercial IgG-Assays, Measurement of Neutralizing Antibodies and CD4+ T-Cell Responses. Front. Immunol. 2021, 12, 750448. [Google Scholar] [CrossRef]
- First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human). NIBSC Code: 20/136. Instructions for Use 480 (Version 2.0, Dated 17 December 2020). Available online: https://www.nibsc.org/documents/ifu/20-136.pdf (accessed on 4 March 2022).
- Soleimani, R.; Khourssaji, M.; Gruson, D.; Rodriguez-Villalobos, H.; Berghmans, M.; Belkhir, L.; Yombi, J.; Kabamba-Mukadi, B. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID-19 patients. J. Med. Virol. 2020, 93, 1465–1477. [Google Scholar] [CrossRef]
- Higgins, V.; Fabros, A.; Kulasingam, V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation. J. Clin. Microbiol. 2021, 59, e03149-20. [Google Scholar] [CrossRef]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance evaluation of three automated quantitative immunoassays and their correlation with a surrogate virus neutralization test in coronavirus disease 19 patients and pre-pandemic controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef]
- Tang, M.S.; Case, J.B.; E Franks, C.; E Chen, R.; Anderson, N.W.; Henderson, J.P.; Diamond, M.S.; Gronowski, A.M.; Farnsworth, C.W. Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays. Clin. Chem. 2020, 66, 1538–1547. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available online: https://www.R-project.org/ (accessed on 31 January 2022).
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency: EMA/707383/2020 Assessment Report. June 2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 4 March 2022).
- European Medicines Agency: EMA/15689/2021 Assessment Report. June 2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 4 March 2022).
- European Medicines Agency: EMA/94907/2021 Assessment Report. June 2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-public-assessment-report_en.pdf (accessed on 4 March 2022).
- European Medicines Agency: EMA/158424/2021 Assessment Report. June 2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf (accessed on 4 March 2022).
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Sasso, B.L.; Giglio, R.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.; Ciaccio, A.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Clementi, N.; Criscuolo, E.; Ambrosi, A.; Corea, F.; Di Resta, C.; Tomaiuolo, R.; Mancini, N.; Locatelli, M.; Plebani, M.; et al. Antibody Titer Kinetics and SARS-CoV-2 Infections Six Months after Administration with the BNT162b2 Vaccine. Vaccines 2021, 9, 1357. [Google Scholar] [CrossRef]
- Lukaszuk, K.; Kiewisz, J.; Rozanska, K.; Dabrowska, M.; Podolak, A.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, A.; Rabalski, L. Usefulness of IVD Kits for the Assessment of SARS-CoV-2 Antibodies to Evaluate the Humoral Response to Vaccination. Vaccines 2021, 9, 840. [Google Scholar] [CrossRef]
- Hibino, M.; Watanabe, S.; Kamada, R.; Tobe, S.; Maeda, K.; Horiuchi, S.; Kondo, T. Antibody Responses to the BNT162b2 mRNA Vaccine in Healthcare Workers in a General Hospital in Japan: A Comparison of Two Assays for Anti-spike Protein Immunoglobulin G. Intern. Med. 2022, 61, 811–819. [Google Scholar] [CrossRef]
- Giavarina, D.; Carta, M. Improvements and limits of anti SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization. Diagnosis 2021, 9, 274–279. [Google Scholar] [CrossRef]
- Carta, M.; Marinello, I.; Cappelletti, A.; Rodolfi, A.; Cerrito, E.; Bernasconi, C.; Gottardo, M.; Lago, F.D.; Rizzetto, D.; Barzon, E.; et al. Comparison of Anti–SARS-CoV-2 S1 Receptor-Binding Domain Antibody Immunoassays in Health Care Workers Before and After the BNT162b2 mRNA Vaccine. Am. J. Clin. Pathol. 2022, 157, 212–218. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef]
- Cox, R.J.; Brokstad, K.A. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat. Rev. Immunol. 2020, 20, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.S.; Farnsworth, C.W. Associating SARS-CoV-2 Serological Assays with Protection: Where the Field Stands. Clin. Chem. 2021, 67, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.J.; Kuivanen, S.; Kekäläinen, E.; Ahava, M.J.; Loginov, R.; Kallio-Kokko, H.; Vapalahti, O.; Jarva, H.; Kurkela, S.; Lappalainen, M. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J. Clin. Virol. 2020, 129, 104512. [Google Scholar] [CrossRef]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. eBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef]
- Bal, A.; Pozzetto, B.; Trabaud, M.-A.; Escuret, V.; Rabilloud, M.; Langlois-Jacques, C.; Paul, A.; Guibert, N.; D’Aubarède-Frieh, C.; Massardier-Pilonchery, A.; et al. Evaluation of High-Throughput SARS-CoV-2 Serological Assays in a Longitudinal Cohort of Patients with Mild COVID-19: Clinical Sensitivity, Specificity, and Association with Virus Neutralization Test. Clin. Chem. 2021, 67, 742–752. [Google Scholar] [CrossRef]
- Padoan, A.; Cosma, C.; Bonfante, F.; della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. SARS-CoV-2 neutralizing antibodies after one or two doses of Comirnaty (BNT162b2, BioNTech/Pfizer): Kinetics and comparison with chemiluminescent assays. Clin. Chim. Acta 2021, 523, 446–453. [Google Scholar] [CrossRef]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021, 12, 6379. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
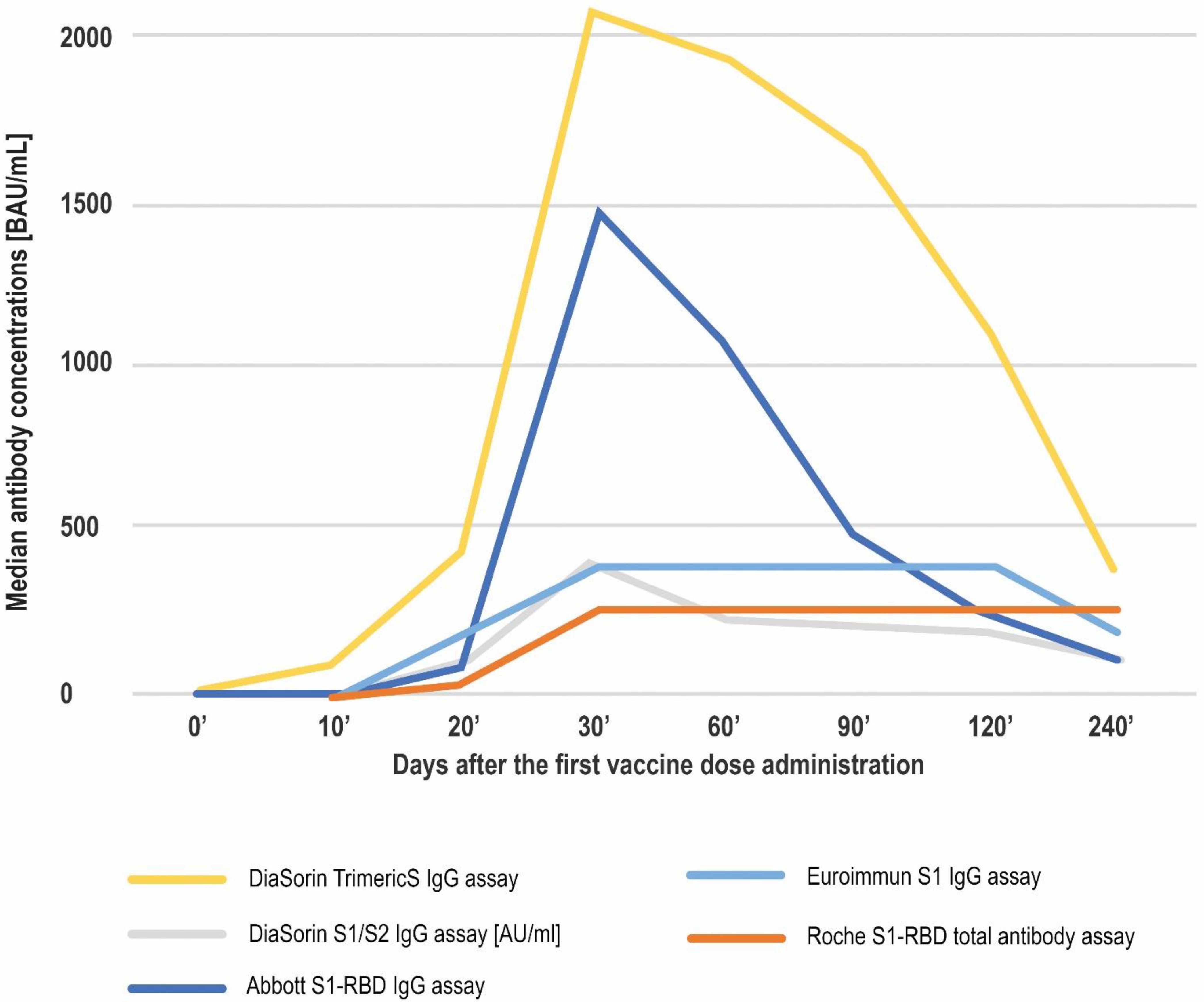


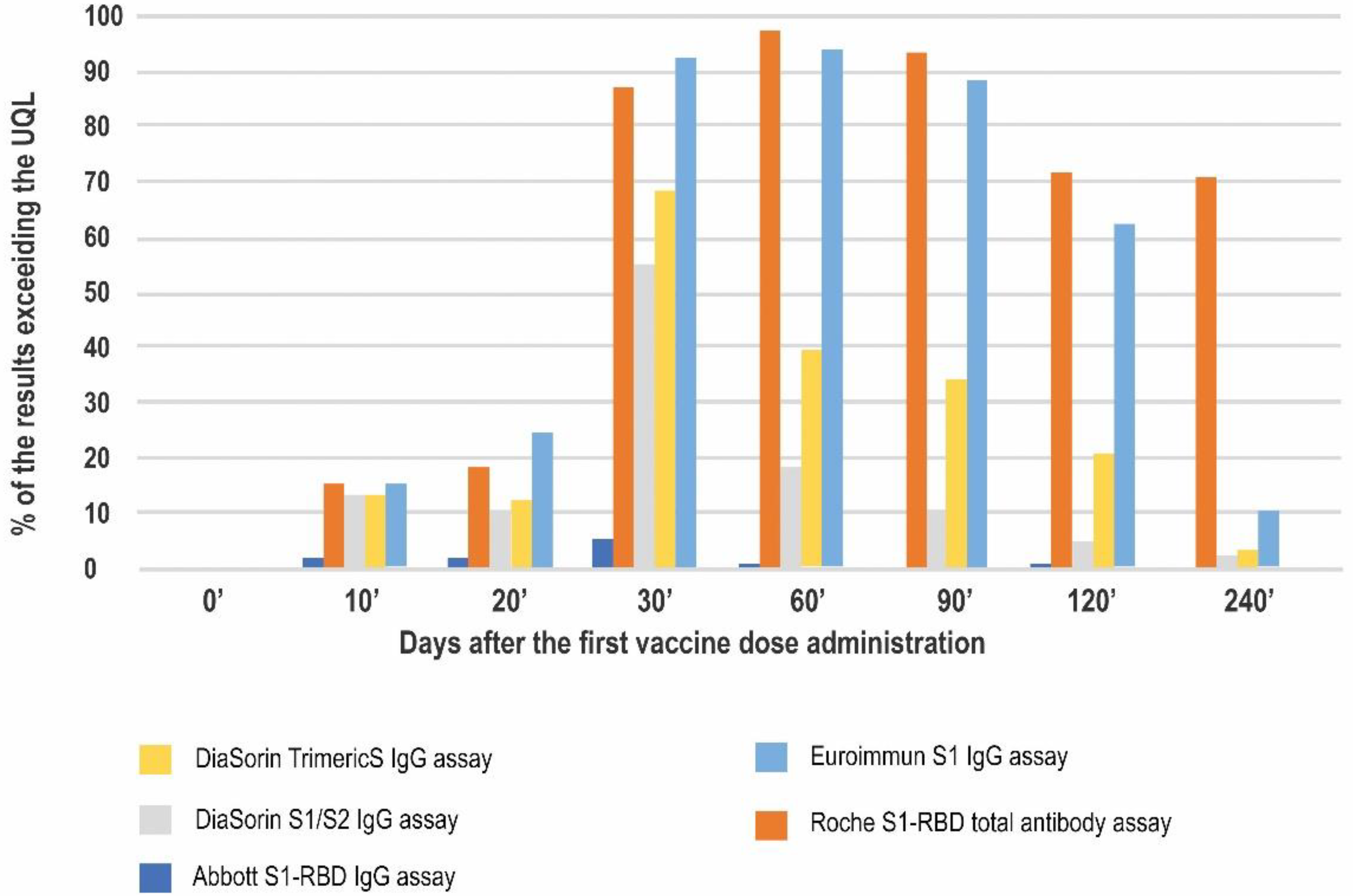
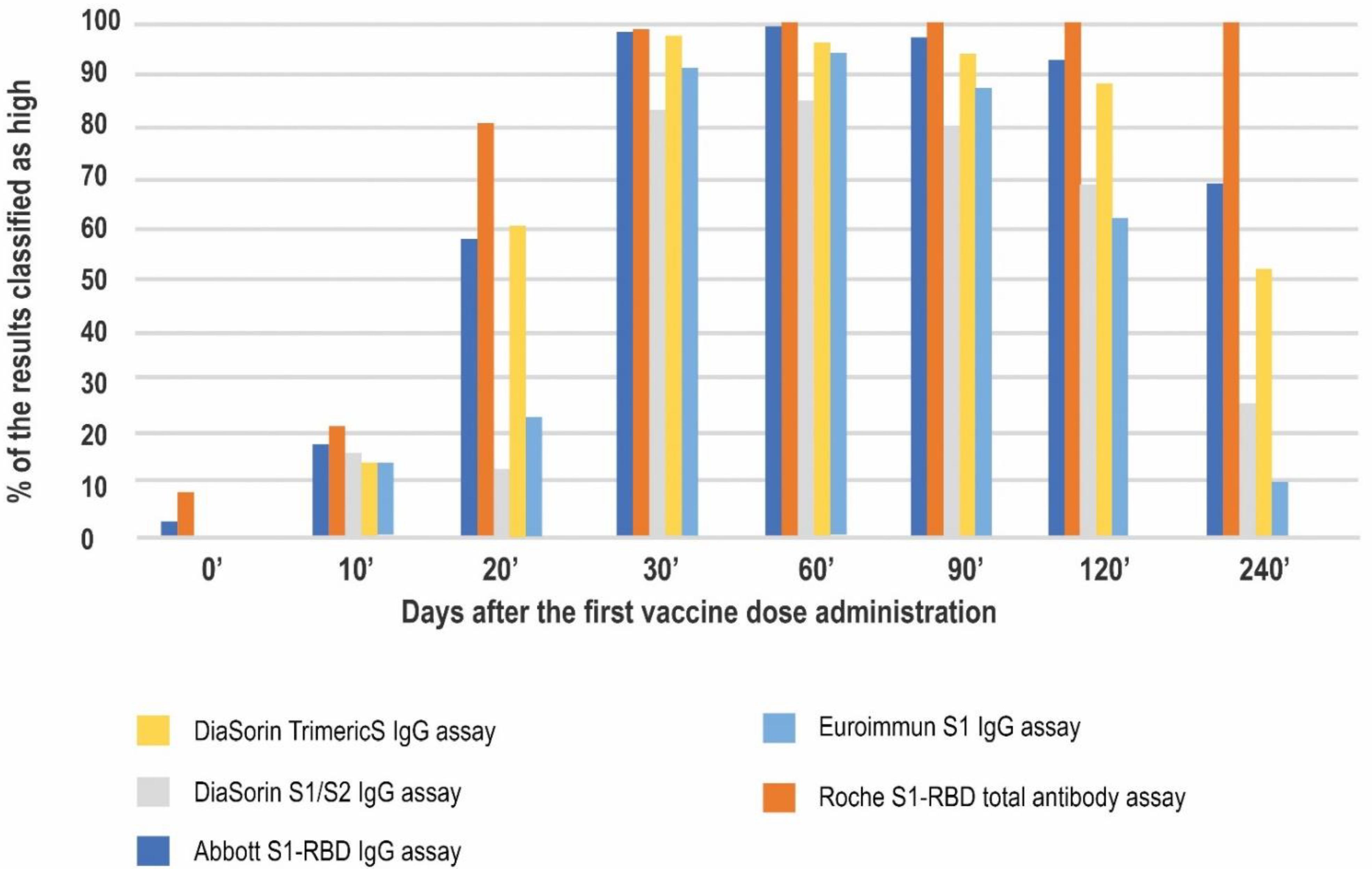
| Antibody Class | Antigen | Conversion to BAU/mL | LQL [BAU/mL] | Positive Result Cut-off [BAU/mL] | UQL | UQL/Positive Cut-off | High Result Adjusted Cut-off (10.9× Positive Cut-off) | |
|---|---|---|---|---|---|---|---|---|
| DiaSorin TrimericS IgG assay | IgG | Trimeric S | AU/mL × 2.6 | 4.81 | 33.8 | 2080 | 61.54 | 368 |
| Abbott S1-RBD IgG assay | IgG | S1-RBD | AU/mL × 0.142 | 2.98 | 7.1 | 5680 | 800 | 77 |
| Roche S1-RBD total antibody assay | Total Ab | S1-RBD | U/mL: 0.972 | 0.41 | 0.82 | 257.2 | 313.6 | 9 |
| Euroimmun S1 IgG assay | IgG | S1 | RU/mL × 3.2 | 3.2 | 35.2 | 384 | 10.9 | 384 |
| DiaSorin S1/S2 IgG assay | IgG | S1/S2 | n/a | 3.8 AU/mL | 15 AU/mL | 400 AU/mL | 26.67 | 163 |
| Median Antibody Concentration [BAU/mL] (Min–Max) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0′ | 10′ | 20′ | 30′ | 60′ | 90′ | 120′ | 240′ | |
| DiaSorin TrimericS IgG assay | 4.81 (4.81–256) | 93.21 (4.81–2080) | 435.5 (18.4–2080) | 2080 (100.62–2080) | 1931.8 (186.94–2080) | 1669.2 (116.47–2080) | 1090 (46–2080) | 379 (26.4–2080) |
| Euroimmun S1 IgG assay | 3.2 (3.2–132) | 6.6 (3.2–384) | 202.8 (5.9–384) | 384 (133.3–384) | 384 (116.4–384) | 384 (149.2–384 | 384 (29.2–384) | 178.05 (19.2–384) |
| DiaSorin S1/S2 IgG assay (results expressed in AU/mL) | 3.8 (3.8–72.7) | 7.0 (3.8–400) | 74.05 (3.8–400) | 400.0 (17.2–400) | 247 (60–400) | 223 (67.4–400) | 194 (30.2–400) | 117 (18.2–400) |
| Roche S1-RBD total antibody assay | 0.4 (0.41–161.5) | 0.4 (0.41–257.2) | 31.74 (0.41–257.2) | 257.2 (2.12–257.2) | 257.2 (13.7–257.2) | 257.2 (75–257.2) | 257.2 (18.11–257.2) | 257.2 (35.19–257.2) |
| Abbott S1-RBD IgG assay | 2.98 (2.98–93.79) | 4.07 (2.98–5680) | 83.95 (2.98–5680) | 1471.8 (11.94–5680) | 1075.99 (16.06–5680) | 490.26 (56.25–3034.85) | 236.71 (9.81–5680) | 110.115 (12.68–2898.75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swadźba, J.; Anyszek, T.; Panek, A.; Chojęta, A.; Wyrzykowska, K.; Martin, E. Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course. Diagnostics 2022, 12, 1426. https://doi.org/10.3390/diagnostics12061426
Swadźba J, Anyszek T, Panek A, Chojęta A, Wyrzykowska K, Martin E. Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course. Diagnostics. 2022; 12(6):1426. https://doi.org/10.3390/diagnostics12061426
Chicago/Turabian StyleSwadźba, Jakub, Tomasz Anyszek, Andrzej Panek, Agnieszka Chojęta, Kinga Wyrzykowska, and Emilia Martin. 2022. "Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course" Diagnostics 12, no. 6: 1426. https://doi.org/10.3390/diagnostics12061426
APA StyleSwadźba, J., Anyszek, T., Panek, A., Chojęta, A., Wyrzykowska, K., & Martin, E. (2022). Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course. Diagnostics, 12(6), 1426. https://doi.org/10.3390/diagnostics12061426






