A Coronary Artery Disease Monitoring Model Built from Clinical Data and Alpha-1-Antichymotrypsin
Abstract
1. Introduction
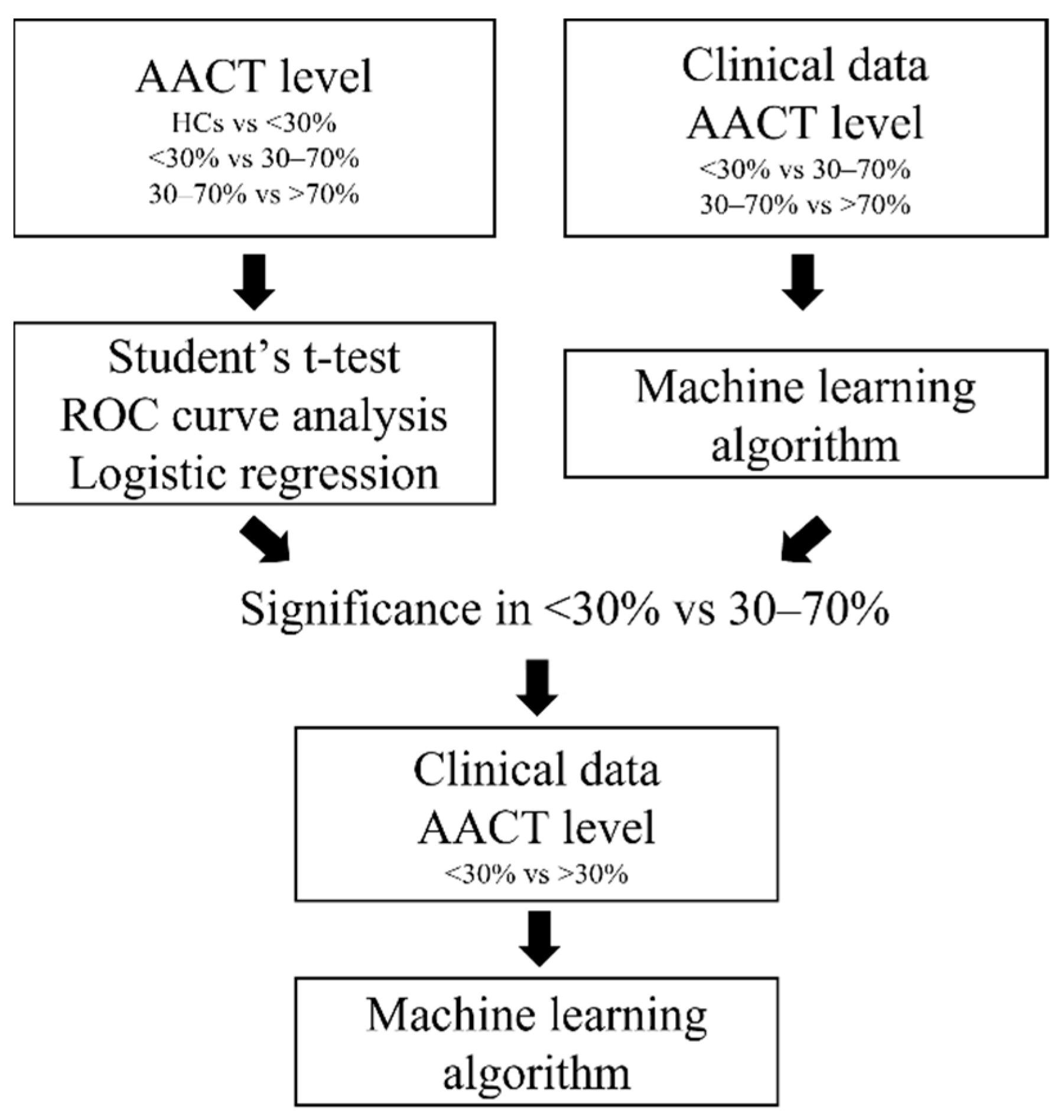
2. Materials and Methods
2.1. Patient Cohort
2.2. Determination of AACT
2.3. Statistical Analysis
3. Results
3.1. Clinical Demographics from Patients with CAD
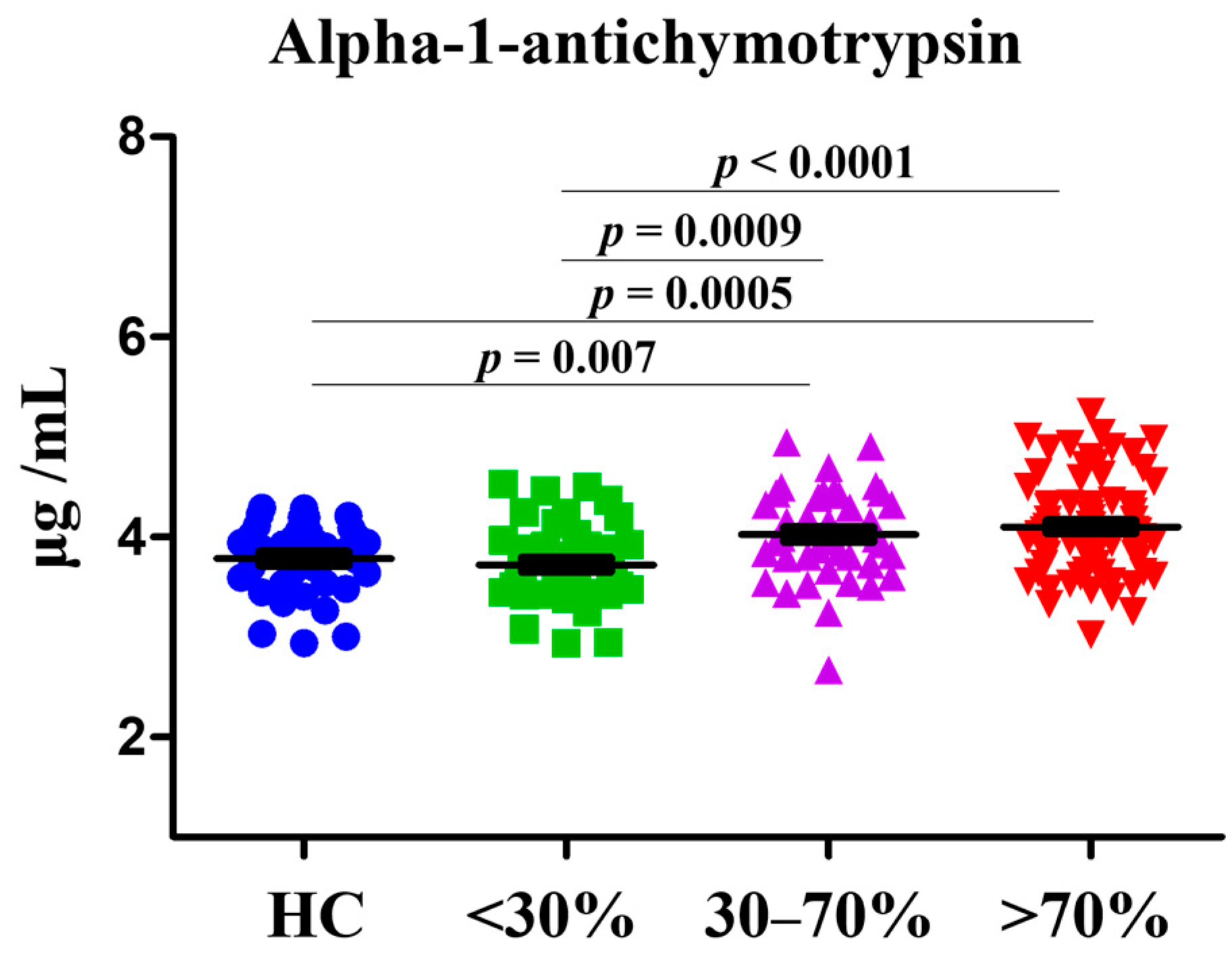
3.2. Determination of AACT Level in HCs and Patients with CAD
3.3. Associations of AACT with CAD in Different Stenosis Rate
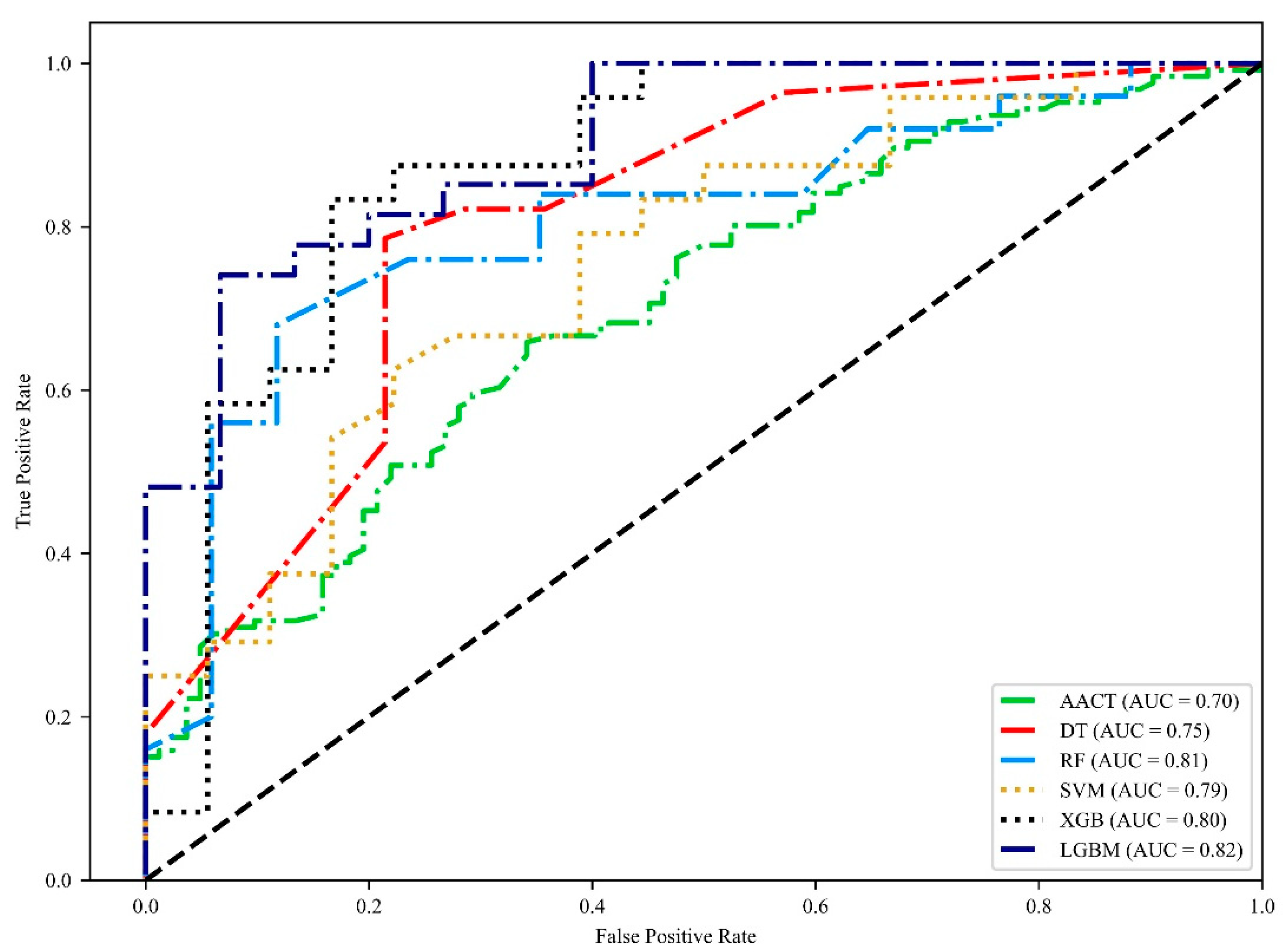
3.4. Incorporating AACT to Identify the Severity of Patients with CAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- NHS. Cardiovascular Disease. Available online: https://www.nhs.uk/conditions/cardiovascular-disease/ (accessed on 20 April 2022).
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 2 May 2022).
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef] [PubMed]
- Flora, D.G.; Nayak, K.M. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N. Cardiovascular Disease Progression: A Target for Therapy? Am. J. Med. 2018, 131, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, M.M.; Zanetti, D.; Carreras-Torres, R.; Moral, P.; Athanasiadis, G. A survey of sub-Saharan gene flow into the Mediterranean at risk loci for coronary artery disease. Eur. J. Hum. Genet. 2017, 25, 472–476. [Google Scholar] [CrossRef]
- Gupta, R.; Deedwania, P.C.; Sharma, K.; Gupta, A.; Guptha, S.; Achari, V.; Asirvatham, A.J.; Bhansali, A.; Gupta, B.; Gupta, S.; et al. Association of educational, occupational and socioeconomic status with cardiovascular risk factors in Asian Indians: A cross-sectional study. PLoS ONE 2012, 7, e44098. [Google Scholar] [CrossRef]
- Saunders-Hastings, P.; Heong, S.W.; Srichaikul, J.; Wong, H.L.; Shoaibi, A.; Chada, K.; Burrell, T.A.; Dores, G.M. Acute myocardial infarction: Development and application of an ICD-10-CM-based algorithm to a large U.S. healthcare claims-based database. PLoS ONE 2021, 16, e0253580. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Metra, M.; Morais, J.; Atar, D. The myth of ‘stable’ coronary artery disease. Nat. Rev. Cardiol. 2020, 17, 9–21. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK402282/ (accessed on 22 April 2022).
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- McCarthy, C.P.; McEvoy, J.W.; Januzzi, J.L., Jr. Biomarkers in stable coronary artery disease. Am. Heart J. 2018, 196, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, K.; Zhao, R.; Hu, J.; Hao, Z.; Wang, F.; Lu, Y.; Liu, F.; Zhang, Y. Inflammatory biomarkers of coronary heart disease. Front. Biosci. (Sch. Ed.) 2018, 10, 185–196. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Koźma, M.; Mariusz, K.; Piotr, S. High-sensitivity troponin T is a prognostic marker of hemodynamic instability in patients undergoing valve surgery. Biomark. Med. 2018, 12, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Pais de Lacerda, A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev. Port. Cardiol. (Engl. Ed.) 2012, 31, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Masri, A.A.A. Relationship of high sensitivity C-reactive protein with presence and severity of coronary artery disease. Pak. J. Med. Sci. 2013, 29, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Doust, J.; Lehman, R.; Glasziou, P. The role of BNP testing in heart failure. Am. Fam. Phys. 2006, 74, 1893–1898. [Google Scholar]
- Zimmerli, L.U.; Schiffer, E.; Zürbig, P.; Good, D.M.; Kellmann, M.; Mouls, L.; Pitt, A.R.; Coon, J.J.; Schmieder, R.E.; Peter, K.H.; et al. Urinary proteomic biomarkers in coronary artery disease. Mol. Cell Proteom. 2008, 7, 290–298. [Google Scholar] [CrossRef]
- Law, R.H.P.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Zheng, M.; Guo, Z.; Li, K.; Liu, Y.; Chen, M.; Yang, X. Circulating Serpina3 levels predict the major adverse cardiac events in patients with myocardial infarction. Int. J. Cardiol. 2020, 300, 34–38. [Google Scholar] [CrossRef]
- Kalsheker, N.A. α1-antichymotrypsin. Int. J. Biochem. Cell Biol. 1996, 28, 961–964. [Google Scholar] [CrossRef]
- Ganz, P.; Heidecker, B.; Hveem, K.; Jonasson, C.; Kato, S.; Segal, M.R.; Sterling, D.G.; Williams, S.A. Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients With Stable Coronary Heart Disease. JAMA 2016, 315, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.I.; Lok, D.J.; van der Weide, P.; Winkens, B.; Bruggink-André de la Porte, P.W.; Doevendans, P.A.; de Weger, R.A.; van der Meer, P.; de Jonge, N. Plasma levels of alpha-1-antichymotrypsin are elevated in patients with chronic heart failure, but are of limited prognostic value. Neth. Heart J. 2014, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Setia, M.S. Methodology Series Module 1: Cohort Studies. Indian J. Derm. 2016, 61, 21–25. [Google Scholar] [CrossRef]
- Goldbohm, R.A.; van den Brandt, P.A.; Brants, H.A.; van’t Veer, P.; Al, M.; Sturmans, F.; Hermus, R.J. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr. 1994, 48, 253–265. [Google Scholar]
- Dekker, L.H.; Snijder, M.B.; Beukers, M.H.; de Vries, J.H.M.; Brants, H.A.M.; de Boer, E.J.; van Dam, R.M.; Stronks, K.; Nicolaou, M. A prospective cohort study of dietary patterns of non-western migrants in the Netherlands in relation to risk factors for cardiovascular diseases: HELIUS-Dietary Patterns. BMC Public Health 2011, 11, 441. [Google Scholar] [CrossRef]
- Khatun, T.; Maqbool, D.; Ara, F.; Sarker, M.R.; Anwar, K.S.; Hoque, A. Dietary habits of patients with coronary artery disease in a tertiary-care hospital of Bangladesh: A case-controlled study. J. Health Popul. Nutr. 2021, 40, 3. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; González-Soria, I.; Caldiño-Bohn, R.; Bobadilla, N.A. An integrative view of serpins in health and disease: The contribution of SerpinA3. Am. J. Physiol. Cell Physiol. 2020, 320, C106–C118. [Google Scholar] [CrossRef]
- Li, B.; Lei, Z.; Wu, Y.; Li, B.; Zhai, M.; Zhong, Y.; Ju, P.; Kou, W.; Shi, Y.; Zhang, X.; et al. The Association and Pathogenesis of SERPINA3 in Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 756889. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Z.; Wang, P.; Zheng, M.; Yang, X.; Liu, Y.; Ma, Z.; Chen, M.; Yang, X. Proteomics of epicardial adipose tissue in patients with heart failure. J. Cell Mol. Med. 2020, 24, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Fukumoto, Y.; Sugimura, K.; Miura, Y.; Aoki, T.; Nochioka, K.; Tatebe, S.; Miyamichi-Yamamoto, S.; Shimizu, T.; Osaki, S.; et al. Plasma Cyclophilin A Is a Novel Biomarker for Coronary Artery Disease. Circ. J. 2013, 77, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.-L.; Zhang, Y.-Y.; Ke, J.; Wang, Z.-W.; Zhang, B.-Y.; Ma, Y.; Yang, L.-Y.; Zhao, D. AK098656: A new biomarker of coronary stenosis severity in hypertensive and coronary heart disease patients. Diabetol. Metab. Syndr. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; Januzzi, J.L.; Magaret, C.A.; Gaggin, H.K.; Rhyne, R.F.; Gandhi, P.U.; Kelly, N.; Simon, M.L.; Motiwala, S.R.; Belcher, A.M.; et al. A Clinical and Biomarker Scoring System to Predict the Presence of Obstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Rehman, T.B.; Gangadhar, C.; Anand, R.; Sindhwani, N.; Babu, M.V.S. Accuracy detection of coronary artery disease using machine learning algorithms. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
| HC | Stenosis Rate of Patients | |||
|---|---|---|---|---|
| <30% | 30–70% | >70% | ||
| n = 36 | n = 45 | n = 49 | n = 68 | |
| Age | 38.8 ± 10.13 | 62.89 ± 10.26 ** | 63.08 ± 9.88 ** | 62.63 ± 9.20 ** |
| Sex | 22 (61.1%) | 30 (66.6%) | 31 (63.2%) | 61 (89.7%) * |
| Hypertension | 1 (2.7%) | 24 (53.3%) ** | 37 (75.5%) ** | 50 (73.5%) ** |
| Hyperglycemia | 0 | 22 (48.8%) ** | 30 (61.2%) ** | 51 (75%) ** |
| Diabetes | 1 (2.7%) | 8 (17.7%) * | 13 (26.5%) ** | 33 (48.5%) ** |
| ESRD | 0 | 1 (2.2%) | 2 (4.08%) | 13 (19.1%) ** |
| Smoking | 10 (27.7%) | 20 (44.4%) * | 20 (40.8%) * | 40 (58.8%) * |
| Drinking | 9 (25%) | 9 (20%) | 12 (24.4%) | 20 (29.4%) |
| Use of lipid-lowering agents | - | 16 (35.5%) | 20 (40.8%) | 45 (66.1%) |
| Total Cholesterol (mg/dL) | 186.03 ± 36.67 | 154.9 ± 31.84 ** | 154.08 ± 27.68 ** | 154.13 ± 30.93 ** |
| HDL-C (mg/dL) | 54.5 ± 13.95 | 53.67 ± 19.41 | 49.22 ± 17.05 * | 43.8 ± 15.8 ** |
| LDL-C (mg/dL) | 131.08 ± 37.05 | 95.09 ± 26.94 ** | 95.43 ± 28.8 ** | 96.39 ± 31.3 ** |
| TG (mg/dL) | 154.5 ± 47.26 | 146.64 ± 123.52 | 144.95 ± 103.08 | 174.12 ± 152.09 * |
| Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | p-Value | |
|---|---|---|---|---|---|
| HC vs. 30% | 3.743 | 58.7 (43.2–73) | 63.89 (46.2–79.2) | 0.598 (0.483–0.704) | 0.1303 |
| 30% vs. 30–70% | 3.810 | 73.47 (58.9–85.1) | 63.04 (47.5–76.8) | 0.726 (0.625–0.813) | <0.0001 |
| 30–70% vs. 70% | 4.027 | 50.65 (39.0–62.2) | 53.06 (38.3–67.5) | 0.526 (0.436–0.616) | 0.6144 |
| <30% vs. >30% | 3.927 | 65.87 (56.9–74.1) | 65.85 (54.6–76) | 0.702 (0.635–0.763) | <0.0001 |
| Groups | Multivariate Logistic Regression Model | |||
|---|---|---|---|---|
| Cut off | HC (n = 36) | <30% (n = 46) | ORs (95% CI) | p-value |
| 3.481 | 10 | 22 | 3.172 (0.625–16.093) | 0.1637 |
| 26 | 24 | |||
| <30% (n = 46) | 30–70% (n = 49) | ORs (95% CI) | p-value | |
| 3.546 | 22 | 8 | 4.845 (1.850–12.691) | 0.0013 |
| 24 | 41 | |||
| 30–70% (n = 49) | >70% (n = 77) | ORs (95% CI) | p-value | |
| 3.800 | 8 | 12 | 1.071 (0.397–2.892) | 0.8921 |
| 41 | 65 | |||
| HC and <30% (n = 82) | >30% (n = 126) | ORs (95% CI) | p-value | |
| 3.623 | 32 | 20 | 4.235 (2.035–8.816) | 0.0001 |
| 50 | 106 | |||
| Training and Validating without AACT | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Models | Accuracy (95% CI) | Precision (95% CI) | f1 Score (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
| <30% vs. 30–70% | decision tree | 48.9% (40.3–57.6%) | 49.1% (32.3–66%) | 43.7% (32.7–54.8%) | 46% (23.8–68.2%) | 55.7% (35.7–75.6%) | 0.519 (0.412–0.626) |
| random forest | 44.7% (35.4–54.1%) | 37.5% (27.9–47.1%) | 36.6% (24.4–48.8%) | 38.8% (21.4–56.2%) | 51.9% (42.7–61.2%) | 0.46 (0.402–0.518) | |
| SVM | 45.3% (37–53.6%) | 39.5% (26.6–52.4%) | 38.5% (24.6–52.5%) | 46.3% (16.2–76.4%) | 48.8% (25.6–72%) | 0.52 (0.386–0.653) | |
| XGBoost | 42.6% (34.6–50.7%) | 47.6% (34.7–60.5%) | 41.8% (29.2–54.4%) | 41.7% (23.1–60.2%) | 48.7% (31.6–65.7%) | 0.496 (0.411–0.582) | |
| lightGBM | 44.2% (24.6–63.8%) | 41.7% (22.4–61%) | 43.2% (23.4–62.9%) | 48.5% (23.8–73.2%) | 42.3% (16.3–68.3%) | 0.442 (0.262–0.622) | |
| 30–70% vs. >70% | decision tree | 51.5% (42.6–60.5%) | 61.4% (51.9–70.9%) | 59.1% (47.6–70.6%) | 59.9% (41.5–78.3%) | 40.4% (26.9–53.9%) | 0.531 (0.45–0.611) |
| random forest | 55.4% (45.9–64.8%) | 65.5% (54.6–76.5%) | 63.7% (54.7–72.8%) | 63.2% (52.2–74.2%) | 43.2% (32.2–54.2%) | 0.571 (0.507–0.634) | |
| SVM | 56.2% (46.7–65.6%) | 62.8% (56.9–68.8%) | 68.8% (59.9–77.7%) | 76.9% (62.8–91%) | 18.1% (9.8–26.3%) | 0.466 (0.337–0.596) | |
| XGBoost | 57.3% (50.2–64.4%) | 66% (55–76.9%) | 68% (62–73.9%) | 71.8% (64.5–79.1%) | 33% (17.7–48.2%) | 0.51 (0.425–0.595) | |
| lightGBM | 50.8% (41.2–60.3%) | 58% (48.5–67.4%) | 61.9% (52.9–70.9%) | 68.6% (53.4–83.7%) | 26.1% (11.8–40.3%) | 0.483 (0.375–0.592) | |
| Training and Validating with AACT | |||||||
| Groups | Models | Accuracy (95% CI) | Precision (95% CI) | f1 Score (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
| <30% vs. 30–70% | decision tree | * 58.4% (47.8–69.1%) | 63.8% (46.7–80.9%) | 55.5% (42.5–68.5%) | 50.9% (36–65.9%) | 68.7% (54.8–82.6%) | * 0.631 (0.536–0.725) |
| random forest | * 59.5% (50.2–68.8%) | 57.5% (44–71.1%) | * 52.9% (43.9–61.9%) | 51.8% (38–65.6%) | * 66.8% (50.6–83%) | * 0.604 (0.484–0.724) | |
| SVM | 44.2% (34.2–54.2%) | 39.4% (22.2–56.5%) | 36.8% (23.7–49.9%) | 43% (18.4–67.6%) | 49.8% (22.8–76.7%) | 0.486 (0.399–0.572) | |
| XGBoost | 58.4% (47.2–69.6%) | * 49.2% (36.6–61.8%) | 52% (38.8–65.2%) | 58.5% (39.7–77.4%) | * 58.1% (44.1–72.1%) | 0.656 (0.547–0.765) | |
| lightGBM | * 65.3% (53.1–77.5%) | * 63.8% (45.1–82.5%) | 60.9% (47.8–74.1%) | 62.5% (45.7–79.2%) | 68.4% (49–87.8%) | 0.669 (0.59–0.748) | |
| 30–70% vs. >70% | decision tree | 54.2% (46.7–61.8%) | 60.4% (50.7–70.1%) | 63.6% (55.6–71.6%) | 69.3% (56.1–82.5%) | 34.5% (21.2–47.7%) | 0.516 (0.436–0.597) |
| random forest | 54.2% (45.5–63%) | 61% (52.1–70%) | 65.2% (58.5–72%) | 73.9% (56.7–91.1%) | 29.2% (13–45.4%) | 0.546 (0.401–0.691) | |
| SVM | 55.8% (43.6–68%) | 59.2% (48.5–69.8%) | 69.3% (57.3–81.3%) | 85% (68.3–101.7%) | 9.3% (1.5–17.1%) | 0.39 (0.272–0.507) | |
| XGBoost | 51.2% (42.8–59.5%) | 59.6% (48.7–70.5%) | 61.1% (53.9–68.2%) | 64.4% (54.6–74.2%) | 33.6% (15.6–51.5%) | 0.505 (0.387–0.622) | |
| lightGBM | 51.9% (42.6–61.2%) | 63.9% (52.9–74.9%) | 62.4% (51.2–73.6%) | 62.1% (48.5–75.7%) | 30.2% (17.7–42.6%) | 0.466 (0.395–0.537) | |
| Groups | Models | Accuracy (95% CI) | Precision (95% CI) | f1 Score (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| <30% vs. >30% | decision tree | 70.7% (67–74.4%) | 71.8% (62.6–80.9%) | 75.8% (71.8–79.8%) | 81.9% (74.5–89.3%) | 57.2% (45.1–69.3%) | 0.751 (0.692–0.809) |
| random forest | 75.2% (68.4–82.1%) | 77.9% (68.7–87.1%) | 81.4% (75.2–87.6%) | 86.3% (77.2–95.4%) | 56% (41.7–70.3%) | 0.816 (0.75–0.882) | |
| SVM | 75% (69.5–80.5%) | 76% (71.6–80.4%) | 81.5% (77.1–85.8%) | 88.6% (79.1–98.1%) | 52.5% (40.9–64.1%) | 0.793 (0.72–0.865) | |
| XGBoost | 75% (69.1–80.9%) | 77.1% (71.3–82.8%) | 79.3% (73.8–84.9%) | 81.8% (75.9–87.6%) | 65.1% (58.7–71.6%) | 0.805 (0.741–0.87) | |
| lightGBM | 76.2% (71–81.3%) | 79.1% (73.2–85.1%) | 80.1% (74.4–85.7%) | 81.4% (73.1–89.7%) | 67.3% (57.7–77%) | 0.822 (0.77–0.874) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Tsai, I.-J.; Shen, W.-C.; Chen, H.-Y.; Hsu, P.-W.; Lin, C.-Y. A Coronary Artery Disease Monitoring Model Built from Clinical Data and Alpha-1-Antichymotrypsin. Diagnostics 2022, 12, 1415. https://doi.org/10.3390/diagnostics12061415
Chang C-C, Tsai I-J, Shen W-C, Chen H-Y, Hsu P-W, Lin C-Y. A Coronary Artery Disease Monitoring Model Built from Clinical Data and Alpha-1-Antichymotrypsin. Diagnostics. 2022; 12(6):1415. https://doi.org/10.3390/diagnostics12061415
Chicago/Turabian StyleChang, Chen-Chi, I-Jung Tsai, Wen-Chi Shen, Hung-Yi Chen, Po-Wen Hsu, and Ching-Yu Lin. 2022. "A Coronary Artery Disease Monitoring Model Built from Clinical Data and Alpha-1-Antichymotrypsin" Diagnostics 12, no. 6: 1415. https://doi.org/10.3390/diagnostics12061415
APA StyleChang, C.-C., Tsai, I.-J., Shen, W.-C., Chen, H.-Y., Hsu, P.-W., & Lin, C.-Y. (2022). A Coronary Artery Disease Monitoring Model Built from Clinical Data and Alpha-1-Antichymotrypsin. Diagnostics, 12(6), 1415. https://doi.org/10.3390/diagnostics12061415






