Study of the Anatomical Association between Morton’s Neuroma and the Space Inferior to the Deep Transverse Metatarsal Ligament Using Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedure and Intruments
2.3. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Quinn, T.J.; Jacobson, J.A.; Craig, J.G.; van Holsbeeck, M.T. Sonography of Morton’s neuromas. Am. J. Roentgenol. 2000, 174, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, N.; Lampridis, V.; Sakellariou, A. Morton’s interdigital neuroma: Instructional review. EFORT Open Rev. 2019, 4, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, T.J.; Choi, G.W.; Kim, H.J. Prediction of Clinical Prognosis according to Intermetatarsal Distance and Neuroma Size on Ultrasonography in Morton Neuroma: A Prospective Observational Study. J. Ultrasound Med. 2019, 38, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, J.H.; Park, J.; Wang, J.; Lee, I. An anatomical study of Morton’s interdigital neuroma: The relationship between the occurring site and the Deep Transverse Metatarsal Ligament (DTML). Foot Ankle Int. 2007, 28, 1007–1010. [Google Scholar] [CrossRef]
- Hulstaert, T.; Shahabpour, M.; Provyn, S.; Lenchik, L.; Simons, P.; Vanheste, R.; De Maeseneer, M. Forefoot Pain in the Lesser Toes: Anatomical Considerations and Magnetic Resonance Imaging Findings. Can. Assoc. Radiol. J. 2019, 70, 408–415. [Google Scholar] [CrossRef]
- Ruiz-Herrera, M.D.M.; Marcos-Tejedor, F.; Aldana-Caballero, A.; Calvo-Lobo, C.; Rodriguez-Sanz, D.; Moroni, S.; Konschake, M.; Mohedano-Moriano, A.; Aceituno-Gómez, J.; Criado-Álvarez, J.J. Novel Ultrasound Anatomical Measurement of the Deep Transverse Metatarsal Ligament: An Intra-Rater Reliability and Inter-Rater Concordance Study. J. Clin. Med. 2022, 11, 2553. [Google Scholar] [CrossRef]
- Matthews, B.G.; Hurn, S.; Harding, M.P.; Henry, R.A.; Ware, R.S. The effectiveness of non-surgical interventions for common plantar digital compressive neuropathy (Morton’s neuroma): A systematic review and meta-analysis. J. Foot Ankle Res. 2019, 12, 12. [Google Scholar] [CrossRef]
- Symeonidis, P.D.; Iselin, L.D.; Simmons, N.; Fowler, S.; Dracopoulos, G.; Stavrou, P. Prevalence of interdigital nerve enlargements in an asymptomatic population. Foot Ankle Int. 2012, 33, 543–547. [Google Scholar] [CrossRef]
- Díaz, J.F.J.; Rey, G.A.; Matas, R.B.; De La Rosa, F.J.B.; Padilla, E.L.; Vicente, J.G.V. New technologies applied to ultrasound diagnosis of sports injuries. Adv. Ther. 2008, 25, 1315–1330. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, I.Y.; Ryu, C.H.; Lee, J.W.; Kang, S.W. Ultrasound-Guided Hyaluronic Acid Injection for the Management of Morton’s Neuroma. Foot Ankle Int. 2018, 39, 201–204. [Google Scholar] [CrossRef]
- Casais, M.C.A.; Crujeiras, C.V.; Ariza, M.V.T.; Gutierrez, B. Objetivo docente. Seram 2012, S-1113. [Google Scholar] [CrossRef]
- De La Peña, C.H. Neuroma de Morton: Diagnóstico por Imagen. Rev. Int. Ciencias Podol. 2010, 4, 37–43. [Google Scholar] [CrossRef]
- Stecco, C.; Fantoni, I.; Macchi, V.; Del Borrello, M.; Porzionato, A.; Biz, C.; De Caro, R. The role of fasciae in Civinini-Morton’s syndrome. J. Anat. 2015, 227, 654–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadas, D.; Pérez, M.; Sosa, H.; Ticse, R. Variabilidad intra e inter examinador de la medición del índice tobillo braquial palpatorio utilizando un método estandarizado. Rev. Med. Hered. 2013, 24, 199. [Google Scholar] [CrossRef]
- Petscavage-Thomas, J. Clinical applications of dynamic functional musculoskeletal ultrasound. Rep. Med. Imaging 2014, 7, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Casasola, G.; Sánchez, F.J.G.; Luordo, D.; Zapata, D.F.; Frías, M.C.; Garrido, V.V.; Martínez, J.V.; de la Sotilla, A.F.; Rojo, J.M.C.; Macho, J.T. Basic abdominal point-of-care ultrasound training in the undergraduate: Students as mentors. J. Ultrasound Med. 2016, 35, 2483–2489. [Google Scholar] [CrossRef]
- Prieto, L.; Lamarca, R.; Casado, A. La evaluación de la fiabilidad en las observaciones clínicas: El coeficiente de correlación intraclase. Med. Clin. 1998, 110, 142–145. Available online: http://ci.nii.ac.jp/naid/10019307727/en/ (accessed on 17 November 2020).
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef]
- Alshami, A.M.; Cairns, C.W.; Wylie, B.K.; Souvlis, T.; Coppieters, M.W. Reliability and Size of the Measurement Error when Determining the Cross-Sectional Area of the Tibial Nerve at the Tarsal Tunnel with Ultrasonography. Ultrasound Med. Biol. 2009, 35, 1098–1102. [Google Scholar] [CrossRef]
- Catani, O.; Corrado, G.; Sergio, F.; Zappia, M.; D’Apice, A. Notre expérience chirurgicale sur le névrome de Morton avec la technique mini-invasive. Med. Chir. du Pied. 2015, 31, 23–31. [Google Scholar] [CrossRef]
- Obón-Azuara, B.; Gasch-Gallén, Á.; Gutiérrez-Cía, I.; Tomás-Aznar, C. Health policies, gender perspective and affective- sexual diversity: An unresolved matter? Aten. Primaria 2020, 52, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Campillo Ibáñez, M.; Zabala Ferrer, S. Las metatarsalgias. Rev Española Reum. Órgano La Soc Española Reumatol. 2003, 30, 478–488. [Google Scholar]
- Yalawar, R.; Bhuyan, D.; Desai, R.; Goswami, G. A rare case of symptomatic os supranaviculare in a sportsman. IOSR J. Appl. Phys. 2014, 6, 15–17. [Google Scholar] [CrossRef]
- Uden, H.; Jones, S.; Grimmer, K. Foot Pain and Cycling: A survey of frequency, type, location, associations and amelioration of foot pain. J. Sci. Cycl. 2012, 1, 28–34. [Google Scholar]
- Dufour, A.B.; Losina, E.; Menz, H.B.; LaValley, M.P.; Hannan, M.T. Obesity, foot pain and foot disorders in older men and women. Obes. Res. Clin. Pract. 2017, 11, 445–453. [Google Scholar] [CrossRef]
- Naraghi, R.; Bremner, A.; Slack-Smith, L.; Bryant, A. The relationship between foot posture index, ankle equinus, body mass index and intermetatarsal neuroma. J. Foot Ankle Res. 2016, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Orejana Garcia, A.M.; Monzó Pérez, F. Modelo de estrés de tejidos. Aplicaciones clínicas en la patología del pie. Rev. Española Podol. 2018, 29, 101–112. [Google Scholar] [CrossRef]
- Barouk, L.S. The effect of gastrocnemius tightness on the pathogenesis of juvenile hallux valgus: A preliminary study. Foot Ankle Clin. 2014, 19, 807–822. [Google Scholar] [CrossRef]
- Bignotti, B.; Signori, A.; Sormani, M.P.; Molfetta, L.; Martinoli, C.; Tagliafico, A. Ultrasound versus magnetic resonance imaging for Morton neuroma: Systematic review and meta-analysis. Eur. Radiol. 2015, 25, 2254–2262. [Google Scholar] [CrossRef]
- Downey, M.S. Tratamiento mediante descompresión del neuroma de Morton: Visión actual y recomendaciones. Rev. Española Podol. 2021, 32, 63–69. [Google Scholar] [CrossRef]
- Pinter, Z.; Odom, C.; McGee, A.; Paul, K.; Huntley, S.; Johnson, J.L.; Shah, A. Morton’s Neuroma Excision: What Are We Really Doing? Which Retractor Is Superior? Foot Ankle Spec. 2019, 12, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A. Treatment of Morton’s neuroma as a nerve compression. The role for neurolysis. J. Am. Podiatr. Med. Assoc. 1992, 82, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Elghazy, M.A.; Whitelaw, K.C.; Waryasz, G.R.; Guss, D.; Johnson, A.H.; DiGiovanni, C.W. Isolated Intermetatarsal Ligament Release as Primary Operative Management for Morton’s Neuroma: Short-term Results. Foot Ankle Spec. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lui, T.H. Endoscopic Intermetatarsal Ligament Decompression. Arthrosc. Tech. 2015, 4, e807–e810. [Google Scholar] [CrossRef] [Green Version]
- Chu, I.; Jang, H.; Park, H.-W. Corrective Osteotomy of Metatarsal Bone for Surgical Treatment of Morton’s Neuroma. J. Korean Foot Ankle Soc. 2015, 19, 58. [Google Scholar] [CrossRef] [Green Version]
- Rungprai, C.; Cychosz, C.C.; Phruetthiphat, O.; Femino, J.E.; Amendola, A.; Phisitkul, P. Simple Neurectomy Versus Neurectomy with Intramuscular Implantation for Interdigital Neuroma. Foot Ankle Int. 2015, 36, 1412–1424. [Google Scholar] [CrossRef]
- Mohr, L.; Vogt, L.; Wilke, J. Use of reflective tape to detect ultrasound transducer movement: A validation study. Life 2021, 11, 104. [Google Scholar] [CrossRef]
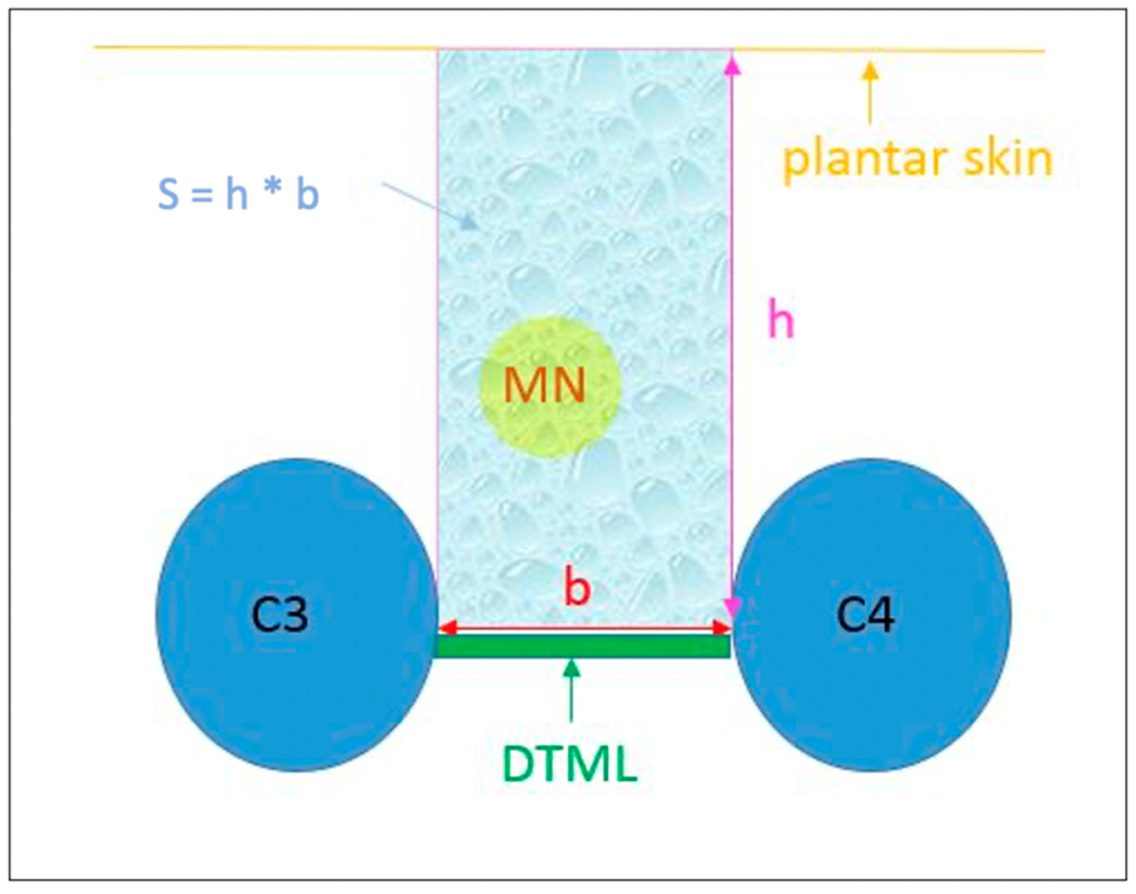
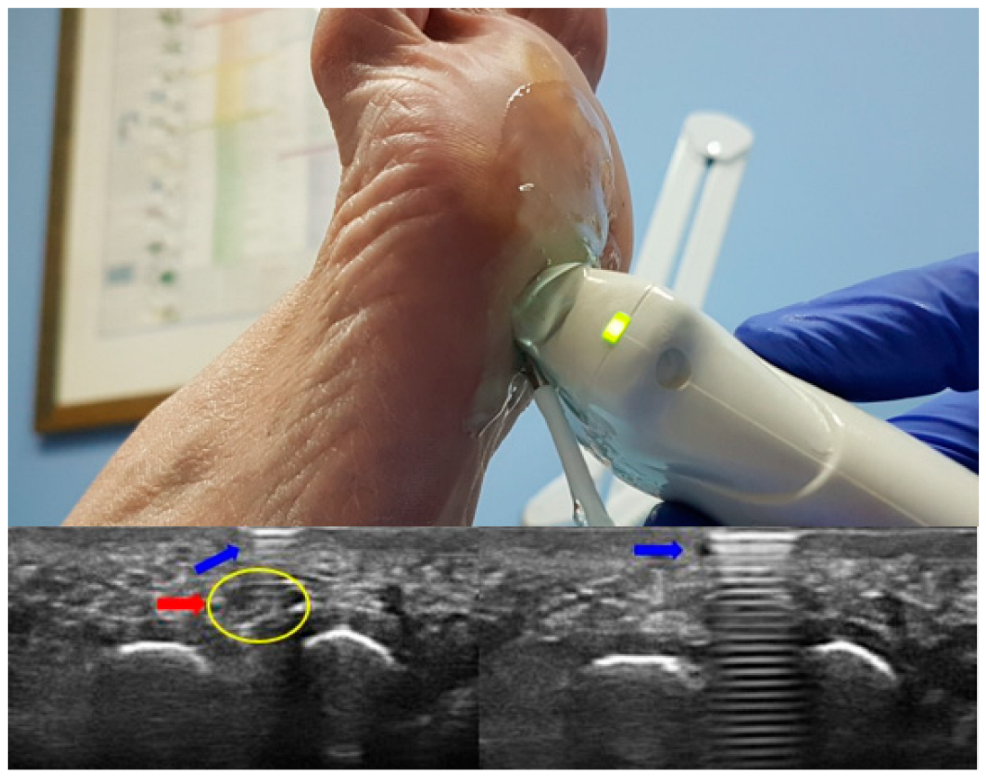
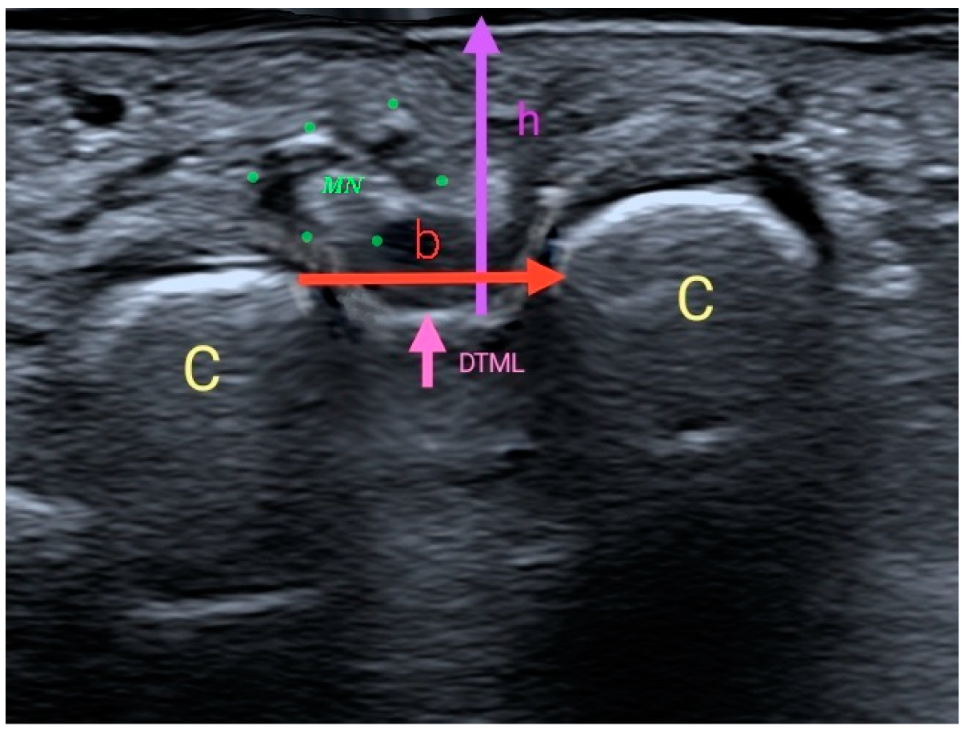
| Sexo | ||||
|---|---|---|---|---|
| Men | Women | Total | ||
| Group | Control | 16 (25.8%) | 46 (74.2%) | 62 |
| Case | 17 (44.7%) | 21 (55.3%) | 38 | |
| Total | 33 (33.0%) | 67 (67.0%) | 100 | |
| Sex | Variable | Group | Statistical Significance | |
|---|---|---|---|---|
| Control | Case | |||
| Men | right height (mm) | 16.4 (SD: 2.28) | 12.6 (SD: 1.77) | p < 0.05 * |
| right base (mm) | 6.4 (SD: 1.41) | 3.6 (SD: 0.76) | p < 0.05 * | |
| right surface (mm2) | 108.1 (SD: 34.84) | 45.3 (SD: 9.27) | p < 0.05 * | |
| left height (mm) | 16.5 (SD: 2.12) | 12.5 (SD: 1.77) | p < 0.05 * | |
| left base (mm) | 6.5 (SD: 1.37) | 3.6 (SD: 0.60) | p < 0.05 * | |
| left surface (mm2) | 109.5 (SD: 34.55) | 44.7 (SD: 6.64) | p < 0.05 * | |
| Women | right height (mm) | 11.8 (SD: 1.14) | 10.4 (SD: 1.38) | p < 0.05 * |
| right base (mm) | 4.6 (SD: 0.45) | 3.4 (SD: 1.00) | p < 0.05 * | |
| right surface (mm2) | 54.6 (SD: 10.40) | 36.8 (SD: 15.51) | p < 0.05 * | |
| left height (mm) | 11.8 (SD: 1.13) | 9.6 (SD: 0.99) | p < 0.05 * | |
| left base (mm) | 4.5 (SD: 0.45) | 3.3 (SD: 0.66) | p < 0.05 * | |
| left surface (mm2) | 54.2 (SD: 10.54) | 33.0 (SD: 9.91) | p < 0.05 * | |
| Sex | Variable | Group | Statistical Significance | |
|---|---|---|---|---|
| Control | Case | |||
| Men | Sitting (hours) | 25.4 (SD: 16.86) | 51.3 (SD: 18.34) | p < 0.05 * |
| Bicycle (hours) | 2.8 (SD: 2.70) | 4.0 (SD: 4.28) | p > 0.05 | |
| Exercise (hours) | 4.4 (SD: 4.36) | 3.4 (SD: 3.41) | p > 0.05 | |
| Woman | Sitting (hours) | 25.2 (SD: 11.12) | 55.2 (SD: 16.97) | p < 0.05 * |
| Bicycle (hours) | 0.9 (SD: 1.50) | 3.6 (SD: 5.64) | p < 0.05 * | |
| Exercise (hours) | 1.5 (SD: 2.32) | 1.4 (SD: 1.88) | p > 0.05 | |
| Variables | B | Statistical Significance | Exp(B) | 95%CI for Exp(B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Weight | 0.07 | 0.018 | 1.073 | 1.012 | 1.137 |
| Left Base | −2.916 | 0.000 | 0.054 | 0.013 | 0.225 |
| Right Base | −1.011 | 0.021 | 0.364 | 0.154 | 0.857 |
| Constant | 10.206 | 0.004 | 27,061.890 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Mar Ruiz-Herrera, M.; Criado-Álvarez, J.J.; Suarez-Ortiz, M.; Konschake, M.; Moroni, S.; Marcos-Tejedor, F. Study of the Anatomical Association between Morton’s Neuroma and the Space Inferior to the Deep Transverse Metatarsal Ligament Using Ultrasound. Diagnostics 2022, 12, 1367. https://doi.org/10.3390/diagnostics12061367
del Mar Ruiz-Herrera M, Criado-Álvarez JJ, Suarez-Ortiz M, Konschake M, Moroni S, Marcos-Tejedor F. Study of the Anatomical Association between Morton’s Neuroma and the Space Inferior to the Deep Transverse Metatarsal Ligament Using Ultrasound. Diagnostics. 2022; 12(6):1367. https://doi.org/10.3390/diagnostics12061367
Chicago/Turabian Styledel Mar Ruiz-Herrera, María, Juan José Criado-Álvarez, Mario Suarez-Ortiz, Marko Konschake, Simone Moroni, and Félix Marcos-Tejedor. 2022. "Study of the Anatomical Association between Morton’s Neuroma and the Space Inferior to the Deep Transverse Metatarsal Ligament Using Ultrasound" Diagnostics 12, no. 6: 1367. https://doi.org/10.3390/diagnostics12061367
APA Styledel Mar Ruiz-Herrera, M., Criado-Álvarez, J. J., Suarez-Ortiz, M., Konschake, M., Moroni, S., & Marcos-Tejedor, F. (2022). Study of the Anatomical Association between Morton’s Neuroma and the Space Inferior to the Deep Transverse Metatarsal Ligament Using Ultrasound. Diagnostics, 12(6), 1367. https://doi.org/10.3390/diagnostics12061367









