Abstract
Cardiovascular malformations (CVM) represent the most common structural anomalies, occurring in 0.7% of live births. The CVM prenatal suspicion should prompt an accurate investigation with fetal echocardiography and the assessment through genetic counseling and testing. In particular, chromosomal microarray analysis (CMA) allows the identification of copy number variations. We performed a systematic review and meta-analysis of the literature, studying the incremental diagnostic yield of CMA in fetal isolated CVM, scoring yields for each category of heart disease, with the aim of guiding genetic counseling and prenatal management. At the same time, we report 59 fetuses with isolated CVM with normal karyotype who underwent CMA. The incremental CMA diagnostic yield in fetuses with isolated CVM was 5.79% (CI 5.54–6.04), with conotruncal malformations showing the higher detection rate (15.93%). The yields for ventricular septal defects and aberrant right subclavian artery were the lowest (2.64% and 0.66%). Other CVM ranged from 4.42% to 6.67%. In the retrospective cohort, the diagnostic yield was consistent with literature data, with an overall CMA diagnostic yield of 3.38%. CMA in the prenatal setting was confirmed as a valuable tool for investigating the causes of fetal cardiovascular malformations.
1. Introduction
Cardiovascular anomalies occur in about 0.7% of live births [1,2,3], excluding small muscular ventricular septal defects (VSDs) that are detected in 5–6% of neonates and 95% of these close spontaneously in the first year of life [4]. The prevalence is higher in the prenatal setting because some VSDs identified in utero can close spontaneously before birth. Cardiovascular malformations include a heterogeneous group of anomalies that can present either as an isolated finding or as part of a conspicuous number of chromosomal, genomic or monogenic conditions. Associated structural malformations are not always identified prenatally, with potential repercussions on the clinical outcome. Many of the children diagnosed with congenital heart disease undergo cardiac surgery with favourable results [5]. However, knowing if cardiac malformation is part of a syndromic picture is important for planning the timing of the intervention and for avoiding the most frequent complications in the specific subgroup of patients. According to the literature, in some cases the prognosis depends on the presence of further structural anomalies or associated genetic conditions [6] and knowing the underlying disease can help to predict outcome and complications.
Prenatal suspicion of heart disease during an obstetric ultrasound scan should prompt the investigation of intracardiac and vascular morphology with fetal echocardiography. A careful medical history of the pregnant woman and the whole family can rule out possible underlying causes and guide diagnostic investigations after genetic counseling. Fetal echocardiography is a detailed ultrasound examination focused on the morphology of the heart and cardiovascular system, which can be performed from the late first trimester to term [7], due to fetal factors, maternal or familial diseases or environmental exposure [8].
Genetic counseling allows an overall assessment of the case, based on imaging investigations and on the information acquired by the consultants. Cytogenetics and molecular testing after sample collection can be requested. Conventional karyotyping in some countries is still considered the first-tier test when structural anomalies are detected. It is able to diagnose chromosomal numerical and structural anomalies starting from 5–10 megabases (Mb). Karyotype anomalies are identified in up to 30–40% of fetuses with cardiovascular malformations, and the higher prevalence of chromosomopathies in this population, if compared to postnatal cases, is attributable to the high intrauterine mortality related to these conditions [7]. Chromosomal microarray analysis (CMA) is a tool capable of detecting copy number variations (CNVs) with a 50 Kb resolution. It can be performed in fetuses with structural anomalies after or alongside standard karyotype or as a first-tier test. Fetuses with heart diseases, as well as most structural anomalies, may benefit from this assay, whose incremental diagnostic rate over standard karyotyping ranges from 3.18% (CI 3.11–3.25) to 6.47% (CI 6.23–6.71) in this subgroup of isolated malformations [9].
In the literature, most of the series concerning the association between heart malformations and CNVs were postnatally performed. In the prenatal setting, no distinction is usually made between the different diagnostic yields of laboratory techniques with respect to the specific cardiac malformation.
We performed a systematic review and meta-analysis of the literature, studying the incremental yield of CMA in fetal-isolated cardiovascular anomalies and calculating the specific yield based on the category of heart disease, with the aim of guiding genetic counseling after assessment of each subgroup of cardiovascular malformation. At the same time, we compared a series of fetuses with isolated cardiovascular malformation, who showed normal karyotype and underwent CMA, with the literature.
2. Materials and Methods
2.1. Systematic Review of the Literature
The research was conducted following PRISMA guidelines [10]. We searched the Pubmed database (https://pubmed.ncbi.nlm.nih.gov/), accessed on 25 April 2022 for (“fetus” OR “fetuses” OR “foetus” OR “foetuses” OR “fetal” OR “foetal” OR “prenatal” OR “pre-natal”) AND (“congenital heart disease” OR “congenital heart diseases” OR “congenital heart defect” OR “congenital heart defects” OR “congenital cardiac malformation” OR “congenital cardiac malformations” OR “heart disease” OR “heart diseases” OR “heart defect” OR “heart defects” OR “cardiac malformation” OR “cardiac malformations” OR “Crisscross heart” OR “Dextrocardia” OR “Ebstein Anomaly” OR “Ventricular Septal Defect” OR “Atrial Septal Defect” OR “Aortopulmonary Window” OR “Persistent Truncus Arteriosus” OR “Heterotaxy Syndrome” OR “Heterotaxia” OR “Right Isomerism” OR “Left Isomerism” OR “Atrioventricular Canal” OR “Atrioventricular Septal Defect” OR “Hypoplastic Left Heart Syndrome” OR “Tetralogy of Fallot” OR “Transposition of Great Vessels” OR “Transposition of Great Arteries” OR “Double Outlet Right Ventricle” OR “Double Outlet Left Ventricle” OR “Double Inlet Left Ventricle” OR “Double Inlet Right Ventricle” OR “Tricuspid Atresia” OR “Mitral Atresia” OR “Aortic Stenosis” OR “Aortic Atresia” OR “Pulmonary Stenosis” OR “Pulmonary Atresia”) AND (“molecular cytogenetic” OR “molecular cytogenetics” OR “CMA” OR “chromosomal microarray analysis” OR “comparative genomic hybridization” OR “comparative genomic hybridization” OR “hybridization” OR “hybridization” OR “microarray” OR “microarrays” OR “array” OR “arrays” OR “CGH” OR “array-CGH” OR “array CGH” OR “single nucleotide polymorphism” OR “SNP” OR “SNP-array” OR “microdeletion” OR “microduplication” OR “CNV” OR “CNVs” OR “copy number variant” OR “copy number variants” OR “copy number variation” OR “copy number variations”).
All titles and abstracts were examined. Only papers with full text available in the English language were retained. Papers not describing the prenatal diagnostic application of CMA on invasive samples in fetuses detected with cardiac anomalies were excluded. Case reports were excluded. Cases with postnatal diagnosis, fetuses enrolled for known chromosomal/molecular variants and familiarity/recurrence for genetic disorders, cases enrolled after fetal demise and twin pregnancies were excluded. Papers reporting only cases with associated structural anomalies were excluded. Papers in which these categories were included but could not be separated from eligible cases were secondarily excluded.
Cases from eligible papers were classified into different subgroups based on the type of heart disease. We excluded those conditions suspected in the prenatal period but requiring a postnatal confirmatory diagnosis or dynamic entities that did not meet the definition of fetal structural anomalies, such as aortic coarctation or suspicion of atrial septal defects and imbalanced ventricles [11].
Cases included in this meta-analysis were divided according to the type of heart disease. The incremental yield of CMA over standard karyotyping was assessed for each class of anomaly as “pooled number of cases with a Pathogenic or Likely Pathogenic (P/LP) CNV”/“pooled number of recruited cases”. We excluded cases for which the original paper reported the specific data as statistically non-relevant. We excluded from quantitative analysis and data pooling heart conditions for which less than three papers were available or in which less than thirty fetuses were analyzed.
Standard deviations and 95% confidence intervals were scored with the = STDEV.S and = CONFIDENCE functions on Microsoft Excel (Office 365).
2.2. Retrospective Cohort
We retrospectively collected fetuses detected with isolated cardiac malformations between March 2018 and April 2022. Invasive sampling (chorionic villus of amniotic fluid) was offered in each case. After informed consent, karyotyping and CMA were performed.
We excluded fetuses detected with additional malformations in organs not included in the cardiovascular system and cases in which a monogenic condition was highly suspected based on family history and/or known familial variants. We excluded twin pregnancies because they present additional risks for heart diseases [12]. We noted soft markers and the mode of conception.
Echocardiographic scans were performed by two operators using 3d convex volumetric probe with frequency range of 4–8 MHz of a Samsung Echocardiography Accuvix A30, HS60 and 3d convex volumetric probe with frequency range of 1–8 MHz of a Samsung Medison HS70A Prime, in accordance with the American Society of Echocardiography guidelines for fetal echocardiography [13].
Genomic screening for CNVs was performed on fetal and parental DNA using the Cytoscan HD (Thermo Fisher Scientific, Waltham, MA, USA) or the 180K oligonucleotide-array (Agilent Technologies, Waldbronn, Germany) microarray platform, following the manufacturer’s instructions and using the ChAS (Thermo Fisher Scientific) or Cytogenomics (Agilent Technologies) analysis software, respectively. Both microarray platforms had 75 Kb effective resolution. Rearrangements were confirmed by real-time quantitative PCR. In accordance with the guidelines of the American College of Medical Genetics (ACMG), the detected CNVs were classified as pathogenic (P), probably pathogenic (LP), variants of uncertain significance (VUS), probably benign (LB) or benign (B) [14].
The diagnostic yield was calculated both on the total number of cardiopathic fetuses analyzed and by excluding the pregnancies arising as a result of assisted reproductive technologies, due to the increased risk of cardiac malformations [15].
3. Results
3.1. Systematic Review of the Literature
The literature search led to the identification of 742 papers. A total of 648 were excluded from the review for not being in line with the objectives of the study. A total of 92 papers were retained and analyzed. A further 74 articles were secondarily excluded from the quantitative analysis. Of these, 38 did not provide quantitative data [6,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], in 22 articles it was not possible to infer a quantitative analysis of incremental yield of CMA over standard karyotyping [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74], 3 papers concerned only prenatal cardiac soft-markers and not cardiovascular malformations [75,76,77], 7 articles did not present the full-text in English [78,79,80,81,82,83,84] and the full-text of 4 articles was not accessible [85,86,87,88]. A total of 18 articles were eligible for quantitative analysis [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] (Figure 1, Table 1).
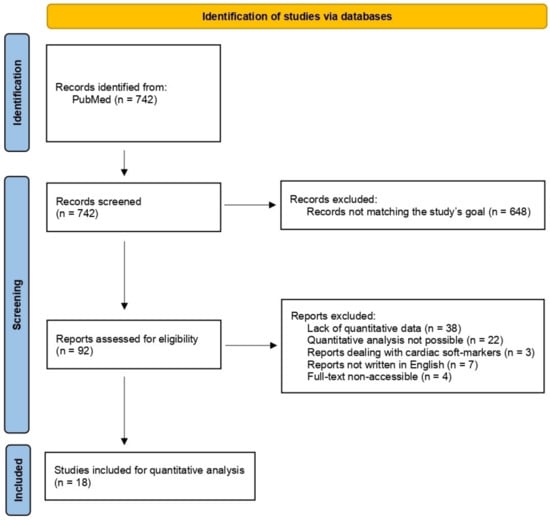
Figure 1.
PRISMA flowchart of the systematic review.

Table 1.
Systematic review of incremental diagnostic yield and VUS rate detected by CMA for each subgroup of CVM.
Table 1.
Systematic review of incremental diagnostic yield and VUS rate detected by CMA for each subgroup of CVM.
| Article (Area) | Test | US Anomaly | Diagnostic Yield | VUSs | Negative Results | |||
|---|---|---|---|---|---|---|---|---|
| Fu, 2017 [89] (China) | SNP | VSD | 4/73 | (5.48%) | 3/73 | (4.11%) | 66/73 | (90.41%) |
| Gindes, 2021 [90] (Israel) | CMA | PAVSD + MAPCAs | 0/3 | (0%) | - | - | 3/3 | (100%) |
| Hureaux, 2019 [91] (France) | CGH or SNP | isolated CVM | 17/160 | (10.63%) | 4/160 | (2.5%) | 139/160 | (86.88%) |
| Septal Defects | 3/38 | (7.89%) | 1/38 | (2.63%) | 34/38 | (89.47%) | ||
| RVOTD | 2/13 | (15.38%) | 1/13 | (7.69%) | 10/13 | (76.92%) | ||
| LVOTD | 2/49 | (4.08%) | 2/49 | (4.08%) | 45/49 | (91.84%) | ||
| CTD | 10/60 | (16.67%) | 0/60 | (0%) | 50/60 | (83.33%) | ||
| Lazier, 2016 [92] (Canada) | CGH | isolated CVM | 2/8 | (25%) | 0/8 | (0%) | 6/8 | (75%) |
| HLHS | 0/2 | (0%) | 0/2 | (0%) | 2/2 | (100%) | ||
| IAA | 0/2 | (0%) | 0/2 | (0%) | 2/2 | (100%) | ||
| TOF | 1/3 | (33.33%) | 0/3 | (0%) | 2/3 | (66.67%) | ||
| Hypoplastic RV + PA | 1/1 | (100%) | 0/1 | (0%) | 0/1 | (0%) | ||
| Lee, 2020 [93] (Korea) | SNP | D-TGA | 2/23 | (8.7%) | 0/23 | (0%) | 21/23 | (91.30%) |
| Lu, 2022 [94] (China) | SNP | isolated CVM | 18/116 | (15.52%) | - | - | 98/116 | (84.48%) |
| Maya, 2017 [95] (Israel) | CGH | ARSA | 0/36 | (0%) | - | - | 36/36 | (100%) |
| Maya, 2020 [96] (Israel) | CGH, SNP or hybrid | VSD | 6/566 | (1.06%) | - | - | 560/566 | (98.94%) |
| Mustafa, 2020 [97] (USA) | SNP | isolated CVM | 8/118 | (6.78%) | - | - | 110/118 | (93.22%) |
| Qiao, 2021 [98] (China) | SNP | isolated CVM | 18/247 | (7.29%) | - | - | 229/247 | (92.71%) |
| VSD | 7/92 | (7.61%) | - | - | 85/92 | (92.39%) | ||
| AVSD | 1/13 | (7.69%) | - | - | 12/13 | (92.31%) | ||
| DORV | 1/17 | (5.88%) | - | - | 16/17 | (94.12%) | ||
| D-TGA | 0/13 | (0%) | - | - | 13/13 | (100%) | ||
| IAA B | 0/2 | (0%) | - | - | 2/2 | (100%) | ||
| TOF | 6/34 | (17.65%) | - | - | 28/34 | (82.35%) | ||
| TA | 0/3 | (0%) | - | - | 3/3 | (100%) | ||
| AS | 0/4 | (0%) | - | - | 4/4 | (100%) | ||
| HLHS | 1/16 | (6.25%) | - | - | 15/16 | (93.75%) | ||
| Ebstein Anomaly | ½ | (50%) | - | - | 1/2 | (50%) | ||
| Pulmonary Stenosis | 1/16 | (6.25%) | - | - | 15/16 | (93.75%) | ||
| Tricuspid Atresia | 0/6 | (0%) | - | - | 6/6 | (100%) | ||
| heterotaxy | 0/17 | (0%) | - | - | 17/17 | (100%) | ||
| RAA | 0/11 | (0%) | - | - | 11/11 | (100%) | ||
| Cor Triatriatum | 0/1 | (0%) | - | - | 1/1 | (100%) | ||
| Sagi-Dain, 2021 [99] (Israel) | SNP or hybrid | isolated CVM | 27/1365 | (1.98%) | - | - | 1338/1365 | (98.02%) |
| VSD | 8/623 | (1.28%) | - | - | 615/623 | (98.72%) | ||
| ARSA | 3/381 | (0.79%) | - | - | 378/381 | (99.21%) | ||
| RAA | 5/136 | (3.68%) | - | - | 131/136 | (96.32%) | ||
| TOF | 6/67 | (8.96%) | - | - | 61/67 | (91.04%) | ||
| AVC | 1/42 | (2.38%) | - | - | 41/42 | (97.62%) | ||
| TGA | 3/30 | (10%) | - | - | 27/30 | (90%) | ||
| Vessel Anomaly | 1/27 | (3.7%) | - | - | 26/27 | (96.3%) | ||
| HLHS | 0/16 | (0%) | - | - | 16/16 | (100%) | ||
| SIT | 0/15 | (0%) | - | - | 15/15 | (100%) | ||
| Interrupted inferior vena cava | 0/7 | (0%) | - | - | 7/7 | (100%) | ||
| PLSVC | 0/7 | (0%) | - | - | 7/7 | (100%) | ||
| Aortic diameter anomaly | 0/6 | (0%) | - | - | 6/6 | (100%) | ||
| DORV | 0/5 | (0%) | - | - | 5/5 | (100%) | ||
| TA | 0/3 | (0%) | - | - | 3/3 | (100%) | ||
| Shaffer, 2012 [100] (USA) | CGH | isolated CVM | 4/119 | (3.36%) | - | - | 115/119 | (96.64%) |
| HLHS | 4/42 | (9.52%) | - | - | 38/42 | (90.48%) | ||
| TOF | 0/18 | (0%) | - | - | 18/18 | (100%) | ||
| VSD | 0/38 | (0%) | - | - | 38/38 | (100%) | ||
| Dextrocardia/SIT | 0/21 | (0%) | - | - | 21/21 | (100%) | ||
| Song, 2019 [101] (China) | CMA | isolated CVM | 17/138 | (12.32%) | 10/138 | (7.25%) | 111/138 | (80.43%) |
| VSD | 8/82 | (9.76%) | 5/82 | (6.1%) | 69/82 | (84.15%) | ||
| VSD + Aortic Abnormality | 3/8 | (37.5%) | 1/8 | (12.5%) | 4/8 | (50%) | ||
| VSD + Pulmonary Abnormality | 1/5 | (20%) | 0/5 | (0%) | 4/5 | (80%) | ||
| DORV | 1/1 | (100%) | 0/1 | (0%) | 0/1 | (0%) | ||
| VSD, VR | 0/1 | (0%) | 1/1 | (100%) | 0/1 | (0%) | ||
| TOF | 1/10 | (10%) | 0/10 | (0%) | 9/10 | (90%) | ||
| Single Ventricle | 2/5 | (40%) | 0/5 | (0%) | 3/5 | (60%) | ||
| VR | 1/21 | (4.76%) | 1/21 | (4.76%) | 19/21 | (90.48%) | ||
| AS, IAA | 0/5 | (0%) | 2/5 | (40%) | 3/5 | (60%) | ||
| Svirsky, 2019 [102] (Israel) | SNP | muscular VSD | 0/29 | (0%) | - | - | 29/29 | (100%) |
| Turan, 2018 [103] (USA) | SNP | isolated CVM | 16/86 | (18.6%) | - | - | 70/86 | (81.4%) |
| LVOTD | 3/22 | (13.64%) | - | - | 19/22 | (86.36%) | ||
| CTD | 5/34 | (14.71%) | - | - | 29/34 | (85.29%) | ||
| AVSD | 0/2 | (0%) | - | - | 2/2 | (100%) | ||
| VSD | 2/7 | (28.57%) | - | - | 5/7 | (71.43%) | ||
| RSD | 3/13 | (23.08%) | - | - | 10/13 | (76.92%) | ||
| RAA | 2/6 | (33.33%) | - | - | 4/6 | (66.67%) | ||
| ASD + PLSVC | ½ | (50%) | - | - | 1/2 | (50%) | ||
| Wang, 2018 [104] (China) | SNP | isolated CVM | 27/359 | (7.52%) | - | - | 332/359 | (92.48%) |
| VSD | 6/169 | (3.55%) | - | - | 163/169 | (96.45%) | ||
| TA | 3/6 | (50%) | - | - | 3/6 | (50%) | ||
| IAA B | 3/11 | (27.27%) | - | - | 8/11 | (72.72%) | ||
| D-TGA | 0/11 | (0%) | - | - | 11/11 | (100%) | ||
| DORV | 2/11 | (18.18%) | - | - | 9/11 | (81.81%) | ||
| TOF | 8/63 | (12.7%) | - | - | 55/63 | (87.30%) | ||
| HLHS | 1/16 | (6.25%) | - | - | 15/16 | (93.75%) | ||
| AS | 1/3 | (33.33%) | - | - | 2/3 | (66.67%) | ||
| AS + Pulmonary Stenosis | 0/2 | (0%) | - | - | 2/2 | (100%) | ||
| Pulmonary Stenosis | 1/16 | (6.25%) | - | - | 15/16 | (93.75%) | ||
| Pulmonary Atresia | 0/9 | (0%) | - | - | 9/9 | (100%) | ||
| Tricuspid Atresia | 0/3 | (0%) | - | - | 3/3 | (100%) | ||
| AVSD | 0/4 | (0%) | - | - | 4/4 | (100%) | ||
| Heterotaxy | 2/19 | (10.53%) | - | - | 17/19 | (89.47%) | ||
| Single Ventricle | 0/1 | (0%) | - | - | 1/1 | (100%) | ||
| L-TGA | 0/1 | (0%) | - | - | 1/1 | (100%) | ||
| RAA | 0/9 | (0%) | - | - | 9/9 | (100%) | ||
| PLSVC | 0/2 | (0%) | - | - | 2/2 | (100%) | ||
| DAA | 0/2 | (0%) | - | - | 2/2 | (100%) | ||
| Aortopulmonary window | 0/1 | (0%) | - | - | 1/1 | (100%) | ||
| Wu, 2020 [105] (China) | CMA | ARSA | 0/35 | (0%) | 0/35 | (0%) | 35/35 | (100%) |
| RAA | 1/19 | (5.26%) | 0/19 | (0%) | 18/19 | (94.74%) | ||
| Zhu, 2016 [106] (China) | CMA | isolated CVM | 6/58 | (10.34%) | 2/58 | (3.45%) | 50/58 | (86.21%) |
| CTD | 3/19 | (15.79%) | 1/19 | (5.26%) | 15/19 | (78.95%) | ||
| AVSD | 0/3 | (0%) | 0/3 | (0%) | 3/3 | (100%) | ||
| LVOTD | 0/4 | (0%) | 0/4 | (0%) | 4/4 | (100%) | ||
| RVOTD | ¼ | (25%) | 1/4 | (25%) | 2/4 | (50%) | ||
| VSD | 2/28 | (7.14%) | 0/28 | (0%) | 26/28 | (92.86%) | ||
ARSA: aberrant right subclavian artery; AS: aortic stenosis; AVC: atrioventricular canal; AVSD: atrioventricular septal defect; CGH: comparative genomic hybridization; CMA: chromosomal microarray analysis; CTD: conotruncal defect; CVM: cardiovascular malformation; DAA: double aortic arch; D-TGA: dextro-transposition of the great arteries; DORV: Double outlet right ventricle; HLHS: hypoplastic left heart syndrome; IAA: interrupted aortic arch; LVOTD: left ventricular outflow tract defect; L-TGA: levo-transposition of the great arteries; MAPCAs: major aortopulmonary collateral arteries; PA: pulmonary atresia; PAVSD: pulmonary atresia with ventricular septal defect; PLSVC: persistent left superior vena cava; RAA: right aortic arch; RV: right ventricle; RVOTD: right ventricular outflow tract defect; RSD: right sided defect; SIT: situs inversus; SNP: single-nucleotide polymorphism; TA: tricuspid atresia; TOF: tetralogy of Fallot; TGA: transposition of the great arteries; US: ultrasound; VR: vascular ring; VSD: ventricular septal defect; VUS: variants of uncertain significance.
We scored the incremental diagnostic yield of CMA over standard karyotyping for pooled isolated cardiovascular malformations (from papers enrolling all fetuses presenting any cardiovascular malformation) and for eight different individual cardiovascular malformations. Data concerning VUS rates did not meet the criteria for a meta-analysis. The incremental diagnostic yield in fetuses with isolated cardiovascular malformations was 5.79% (5.54–6.04), with a 95% confidence interval (Table 2).

Table 2.
Meta-analysis of each subgroup of CVM.
Table 2.
Meta-analysis of each subgroup of CVM.
| CVM Type | References | Diagnostic Yield (95% CI) |
|---|---|---|
| Any CVM | [91,92,94,97,98,99,100,101,103,104,106] | 5.79% (5.54–6.04) |
| CTD | [91,103,106] | 15.93% (15.75–16.11) |
| TOF | [92,98,99,100,101,104] | 11.28% (9.7–12.86) |
| LVOTD | [91,103,106] | 6.67% (5.51–7.83) |
| HLHS | [92,98,99,100,104] | 6.52% (5.64–7.4) |
| D-TGA | [93,98,99,104] | 6.49% (5.26–7.72) |
| RAA | [98,99,103,104,105] | 4.42% (2.36–6.48) |
| VSD | [89,91,96,98,99,100,101,102,103,104,106] | 2.64% (2.26–3.02) |
| ARSA | [95,99,105] | 0.66% (0.62–0.7) |
ARSA: aberrant right subclavian artery; CTD: conotruncal defect; CVM: cardiovascular malformation; D-TGA: dextro-transposition of the great arteries; HLHS: hypoplastic left heart syndrome; LVOTD: left ventricular outflow tract defect; RAA: right aortic arch; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
3.2. Retrospective Cohort
We selected 59 fetuses (33 males and 26 females), detected with isolated cardiovascular malformations and normal karyotype, who underwent CMA (Table 3). Five fetuses were conceived through assisted reproductive technologies, in particular, three of the couples underwent fertilization in vitro and embryo transfer (FIVET) and two women underwent intracytoplasmic sperm injection (ICSI). Seven fetuses were detected with soft markers (four with choroid plexus cysts, one with hyperechogenic bowel, one with increased nuchal translucency, and one with short femur and borderline ventriculomegaly) and polyhydramnios was also detected in one of these.

Table 3.
Retrospective cohort of fetuses with isolated CVM.
Forty-eight fetuses presented with intracardiac malformations, yielding the following CMA results: eighteen ventricular septal defects (VSD; CMA diagnostic yield 0/18; VUS 3/18), six tetralogy of Fallot (TOF; CMA diagnostic yield 1/6; VUS 0/6), two tricuspid anomalies (CMA diagnostic yield 0/2; VUS 0/2), two truncus arteriosus (TA; CMA diagnostic yield 0/2; VUS 0/2), two hypoplastic left heart syndrome (HLHS; CMA diagnostic yield 0/2; VUS 0/2), two pulmonary atresia with intact ventricular septum (PA-IVS; CMA diagnostic yield 0/2; VUS 0/2), two pulmonary atresia with ventricular septal defect (PA-VSD; CMA diagnostic yield 1/2; VUS 0/2), two aortic valve anomalies (CMA diagnostic yield 0/2; VUS 0/2), one pulmonary stenosis (PS; CMA diagnostic yield 0/1; VUS 0/1), one dysplastic mitral valve (CMA diagnostic yield 0/1; VUS 0/1), one L-looped transposition of great arteries (L-TGA; CMA diagnostic yield 0/1; VUS 0/1), one D-looped transposition of great arteries (D-TGA; CMA diagnostic yield 0/1; VUS 0/1), one atrioventricular canal (AVC; CMA diagnostic yield 0/1; VUS 0/1), one partial atrioventricular canal (pAVC; CMA diagnostic yield 0/1; VUS 0/1), one univentricular heart (CMA diagnostic yield 0/1; VUS 0/1), one left interatrial membrane (CMA diagnostic yield 0/1; VUS 1/1), one TOF with absent pulmonary valve (D-TGA; CMA diagnostic yield 0/1; VUS 0/1) [107], one endocardial fibroelastosis and aortic stenosis (CMA diagnostic yield 0/1; VUS 0/1), one AVC, TGA, PS (CMA diagnostic yield 0/1; VUS 0/1), one D-TGA and tricuspid atresia (CMA diagnostic yield 0/1; VUS 0/1) and one L-TGA, VSD, PS (CMA diagnostic yield 0/1; VUS 0/1).
Twenty-two fetuses were detected with extracardiac cardiovascular malformations, yielding a VUS in one case of right aortic arch (RAA). The cohort encompassed six cases of RAA, four hypoplastic aortic arch, four RAA with aberrant left subclavian artery (ALSA), one RAA with discontinuous pulmonary arteries, one double aortic arch (DAA), one aberrant right subclavian artery (ARSA), one aortic dextroposition, one hypoplastic left pulmonary artery (LPA) with persistent left superior vena cava (PLSVC), one ARSA with PLSVC, one hypoplastic aortic arch with PLSVC and one narrowing of aortic isthmus with PLSVC.
Overall the incremental diagnostic yield for CMA was 2/59 (3.38%), with 6/59 (10.17%) VUSs and 51 (86.44%) negative results. Excluding fetuses conceived with assisted reproductive technologies, which can increase the rate of cardiac malformations, the diagnostic yield was 2/54 (3.70%), with 6/54 (11.11%) VUSs and 46 (85.19%) negative results.
In particular, one likely pathogenic and one pathogenic result were reported. One male fetus, presenting with PA-VSD and whose medical records report increased nuchal translucency in the first trimester examination, was diagnosed with 22q11.2 deletion syndrome. One female fetus with TOF was detected with a 10.7 Mb 21q21.1q21.3 deletion.
4. Discussion
The association between cardiac malformations and genetic conditions is widely known. Cytogenetics and molecular investigations are therefore fundamental to guaranteeing the couple an informed choice about the ongoing pregnancy, to formulate the recurrence risks and to guide the most appropriate obstetric management and genetic counseling.
Every genetic investigation must be preceded and followed by non-directive genetic counseling with the aim of educating consultants about the possible conditions related to the malformation and the available testing, providing the basis for thoughtful decision making, according to psychological, socio-economic, cultural and religious backgrounds [9].
CMA allows the detection of small rearrangements that can underlie several structural anomalies [108,109,110,111,112]. This technique has largely replaced conventional karyotyping, becoming the first-tier genetic investigation after the detection of fetal structural anomalies in several countries, even if the most used approach (array-comparative genomic hybridization: a-CGH) is not able to identify balanced chromosomal aberrations, triploidies and mosaicisms below 30%. Some authors suggest replacing standard karyotype with CMA, but also adding a rapid method for detection of aneuploidies and triploidies (e.g., quantitative fluorescent polymerase chain reaction) [113]. In our opinion, it is important to include karyotype examination in any case, due to the possibility of low-rate mosaicism and the importance of chromosomal structure analysis for recurrence risks. However, the abnormal result of a rapid method for detection of aneuploidies and triploidies can avoid the use of CMA in fetuses with these conditions, reducing both turnaround time and costs.
CMA presents a higher resolution and a faster turnaround time, not requiring cell culturing [114], and it is based on different platforms: bacterial artificial chromosome, oligonucleotide and single nucleotide polymorphism-array (SNP-array) [115,116]. Using the first two array platforms, DNA of patient and control samples are marked through different fluorochromes and hybridized to complementary probes on a chip. The colours’ intensity are compared, revealing the presence of CNVs [22]. a-CGH and SNP-array analysis show similar sensitivities in detection of CNVs [117], but SNP-array, using probes with alternative alleles (A and B) of given polymorphic loci, allows the detection of triploidies, lower-level mosaics compared to a-CGH and regions of homozygosity, which can suggest consanguinity or uniparental disomy. CMA is a genome-wide quantitative analysis. This means that it can result in the detection of incidental findings, such as variants in a susceptibility locus for neurodevelopmental disorder, regions encompassing genes associated with diseases with incomplete penetrance and predisposition to late-onset diseases or to variants of uncertain significance (VUSs), such as rearrangements involving candidate genes for disease association [22]. During pretest genetic counseling, the couple should be informed about the possibility of obtaining these results. It is known that unexpected findings can cause a negative psychological impact (anxiety, improper pregnancy management) and clarity on the possible results can encourage the couple to adopt a more rational attitude [118].
4.1. CMA in the Literature and in the Present Cohort
In a recent meta-analysis, we calculated the P/LP CMA rate in pregnancies without indications for chromosomal analysis and in advanced maternal age cohorts, which amount, respectively, to 0.79% and 0.84% [9].
In the same work we extrapolated the CMA diagnostic rate of fetuses with cardiovascular malformations from cohorts of fetuses enrolled for any structural anomaly and from papers enrolling fetuses specifically for cardiac defects, yielding different percentages: 3.18% and 6.47%. In particular, in the second group cardiovascular anomalies had the highest detection rate, 6.47% if isolated, compared to the other malformations. This difference prompted us to investigate the detection rates of the subgroups of heart disease. Concerning the detection of VUSs, they represented 2.49% in the first group and 4.74% in the second group.
In the present systematic review and meta-analysis of the literature, the overall detection rate of CMA for pooled isolated cardiovascular malformation was 5.79%, representing a high yield, which differs significantly from the rate obtained from papers enrolling all fetuses for any isolated structural anomaly (3.66–5.64%) [9], while it approaches the percentage obtained from fetuses specifically enrolled in heart disease cohorts from the previous meta-analysis. The reason for this discrepancy can be identified in the different inclusion criteria and in the different approaches that the clinician may have.
Among the subgroups of heart diseases, the one that showed the highest detection rate was TOF (11.28%). It is one of the most common heart diseases in 22q11.2 deletion syndrome, along with other cardiovascular (especially conotruncal) malformations, such as PA-VSD, TA and interrupted aortic arch [119]. The strong association with this genetic condition could explain the higher diagnostic yield of CMA in this heart disease. Since the diagnostic rate of TOF is so high, it is not surprising that it also appears high in overall conotruncal heart disease, amounting to 15.93%.
The diagnostic yield of VSDs was, as expected, lower than most of the other heart diseases analyzed.
The yield of ARSA was 0.66%, resulting even lower than the sample of fetuses without structural anomalies. It is a frequent vascular anomaly in the population, often misdiagnosed and identified as an incidental finding following investigations carried out for another cause. Although the diagnostic yield is so low, it is important to remember that in the prenatal setting it is not always possible to identify all the morphological anomalies. Due to these limitations that it is still advisable to undergo diagnostic investigations since an anomaly of this type is identified.
The other subgroups of heart disease (RAA, D-TGA, HLHS and left ventricle outflow tract defect) present a detection rate ranging from 4.42% to 6.67%, consistent with the average yield for cardiopathies.
In the retrospective cohort we enrolled, the diagnostic yield was consistent with the data extrapolated from the literature. Overall, the CMA diagnostic yield amounts to 3.38%, with 10.17% VUSs. Excluding fetuses conceived with assisted reproductive technologies, which can increase the rate of cardiac malformations, the diagnostic yield was 3.70%, with 11.11% VUSs.
4.2. CMA Compared to Other Techniques
We recently performed a review of the literature scoring the incremental detection rate of prenatal exome sequencing (ES) over CMA [120] and a meta-analysis focusing on the comparison of the available molecular techniques in the prenatal setting [9]. In the first review [120] we analyzed the cohorts of fetuses enrolled for ultrasound anomalies (regardless of the affected organ). The incremental diagnostic yield of prenatal exome sequencing of the included papers ranges from 9% to 47% (average 28%), with a higher rate for fetuses showing multiple malformations. We also analyzed the subgroups of fetuses selected for specific anomalies. In this case, the diagnostic yield ranged from 6% to 92% (average 32%). In particular, the detection rate of heart malformations was 11%, and it seemed to represent the most solid prediction because it was consistent among vast cohorts [98,121,122,123,124]. In the second review and meta-analysis [9] we analyzed cohorts of fetuses enrolled isolated anomalies. In this case, the pooled diagnostic rate for ES was 14.77% (13.23–16.32%) in the group of any cardiovascular anomaly. The rate for isolated or non-isolated cardiovascular anomalies in papers focusing on this subtype of malformation was 11.02% (10.65–11.39%). The high incremental rate of ES is promising, but the possible clinical impact of these diagnoses is still unclear, and the management of molecular VUS, which occur at a high rate, still poses limits to its widespread application.
4.3. Proposed Diagnostic Workflow in Fetal Cardiovascular Malformations
In order to avoid inaccurate or fragmentary information, the achievement of a well-defined morphological analysis plays a pivotal role in pretest genetic counseling.
Ideally the first genetic counseling should be performed immediately after the suspect of the structural anomaly. If such tempestivity is not possible, the highest priority is a fetal echocardiography, in order to validate or disconfirm the suspicion. Furthermore, with the proper definition of the anatomy of the fetal cardiovascular system, genetic counseling can be targeted on the specific heart anomaly and on the possibly related syndromic conditions. The detection of a fetal cardiovascular anomaly should prompt the proposal of further examination, either of the fetus or the parents. These investigations can include morphological evaluation or genetic testing (Figure 2).
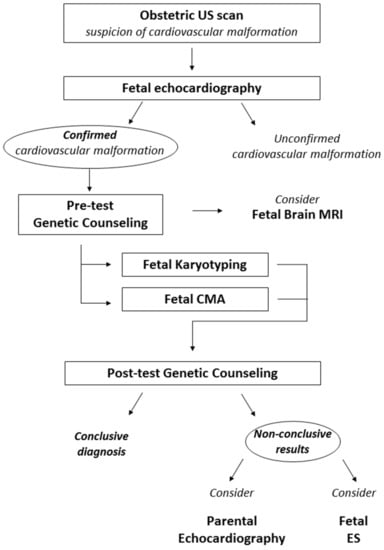
Figure 2.
Proposed diagnostic workflow in fetal CVM. The suspicion of a cardiovascular anomaly should always be confirmed with fetal echocardiography. Fetal Karyotyping and CMA should be proposed as first-tier genetic investigation. We also suggest considering further investigations which lack the bulk of evidence for systematic application but can prove to be useful in selected cases. Imaging assessments to be considered include a fetal brain MRI and the study of parental heart morphology with US examination. Fetal ES should be considered in cases with high suspicion of an underlying monogenic cause after non-conclusive karyotyping and CMA. US: ultrasound; MRI: magnetic resonance imaging; CMA: chromosomal microarray analysis; ES: exome sequencing.
During the pretest genetic counseling, familial history of the couple is gathered and represented graphically as a pedigree. All exams already performed are collected. After the detection of fetal cardiovascular anomalies, standard and molecular karyotyping (CMA) should be requested. These approaches provide complementary information and should not be mutually exclusive [9].
Brain magnetic resonance imaging (MRI) of the fetus should be considered. In fact, central nervous system anomalies often coexist with cardiac malformations, occurring in several syndromes. This can be useful to better define the clinical picture that the fetus will present after birth, if abnormalities of karyotype or CMA are found, or it can guide the analysis of specific monogenic disorders. Moreover, recent studies have confirmed that brain and cerebellar involvement secondary to heart disease can occur, but the clinical consequences of such findings are still under investigation [125,126,127,128].
If both karyotype and CMA yield inconclusive results, the echocardiography of parents and siblings can be taken into consideration. In our experience, it is not uncommon to find slight morphological anomalies or anatomical variants of the main structures of the cardiovascular system, which may be ascribed to the same spectrum of the fetal heart disease, guiding further molecular investigations. Data concerning the cost-effectiveness of this approach and the potential benefit of a systematic application are still lacking.
Post-test genetic counseling should be performed after the results of cytogenetic testing. During this counseling, all investigations carried out should be summarized and discussed.
Although Mendelian inheritance patterns are usually not observed, in the last decade an increasing number of families with cardiac malformations due to monogenic conditions has been described [16], and fetal exome sequencing shows promising diagnostic yields, despite a high VUS burden. For this reason, if there was a suspicion of a monogenic condition, it would be advisable to perform fetal exome sequencing, whose incremental detection rate from CMA amounts 11–14% in fetuses with cardiovascular anomalies [9,120].
5. Conclusions
CMA in the prenatal setting represents a valuable tool for investigating the causes of fetal cardiovascular malformations. The overall diagnostic incremental yield of CMA in pooled cardiovascular anomalies accounts for 5.79% and is therefore higher than the average for structural anomalies, confirming the importance of this tool. Most of the heart diseases analyzed (RAA, D-TGA, HLHS and left ventricle outflow tract defect) presented a detection rate ranging from 4.42% to 6.67%, not deviating excessively from the overall rate for cardiopathies. The heart disease that showed the highest detection rate was TOF (11.28%), probably due to the association with 22q11.2 deletion syndrome. Since the diagnostic rate of TOF is so high, it is not surprising that it also appears high in overall conotruncal heart disease, amounting to 15.93%. Despite the yield of ARSA amounts of 0.66%, it is important to perform diagnostic investigations, because not always it is possible to identify all the morphological anomalies in the prenatal setting. In the retrospective cohort, diagnostic yield was consistent with the data extrapolated from the literature. The prenatal management of CVMs is challenging for all professionals involved. Providing couples with knowledge of the specific risks for each malformation can be extremely valuable in a tailored genetic counseling.
Author Contributions
Study conceptualization, G.M. and D.G.; methodology, G.M.; formal analysis, G.M., N.K.H. and D.G.; data curation G.M., N.K.H., D.G., M.G.G., B.T., L.B., F.V., G.P. and A.P.; writing—original draft preparation, G.M., N.K.H. and D.G.; writing—review and editing, L.B., F.V., G.P. and A.P.; manuscript supervision, A.P.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the articles cited in the References section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wren, C.; Richmond, S.; Donaldson, L. Temporal variability in birth prevalence of cardiovascular malformations. Heart 2000, 83, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, C.; Boughman, J.A. Congenital heart disease in adolescents and adults. Teratology, genetics, and recurrence risks. Cardiol. Clin. 1993, 11, 557–567. [Google Scholar] [CrossRef]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Roguin, N.; Du, Z.-D.; Barak, M.; Nasser, N.; Hershkowitz, S.; Milgram, E. High prevalence of muscular ventricular septal defect in neonates. J. Am. Coll. Cardiol. 1995, 26, 1545–1548. [Google Scholar] [CrossRef]
- Hoffman, J.I.; Kaplan, S.; Liberthson, R.R. Prevalence of congenital heart disease. Am. Heart J. 2004, 147, 425–439. [Google Scholar] [CrossRef]
- Jansen, F.; Blumenfeld, Y.; Fisher, A.; Cobben, J.; Odibo, A.; Borrell, A.; Haak, M. Array comparative genomic hybridization and fetal congenital heart defects: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 45, 27–35. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Lee, W.; Drose, J.; Wax, J.; Goldberg, J.D.; Wilkins, I.A.; Benson, C.; Frates, M.C.; Donofrio, M.T.; Eidem, B.W.; Copel, J.; et al. AIUM practice parameter for the performance of fetal echocardiography. J. Ultrasound Med. 2020, 39, E5–E16. [Google Scholar]
- Mastromoro, G.; Guadagnolo, D.; Khaleghi Hashemian, N.; Marchionni, E.; Traversa, A.; Pizzuti, A. Molecular Approaches in Fetal Malformations, Dynamic Anomalies and Soft Markers: Diagnostic Rates and Challenges—Systematic Review of the Literature and Meta-Analysis. Diagnostics 2022, 12, 575. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Mastromoro, G.; Pizzuti, A.; Ventriglia, F. Role of ductus venosus agenesis in right ventricle development. J. Matern.-Fetal Neonatal Med. 2020, 1–4, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, C. Genetic and environmental risk factors of major cardiovascular malformations: The Baltimore-Washington infant study 1981–1989. Perspect. Pediatr. Cardiol. 1997, 5, 346–347. [Google Scholar]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.J.; Van Der Veld, M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Riggs, E.R.; Andersen, E.F.; Cherry, A.M.; Kantarci, S.; Kearney, H.; Patel, A.; Raca, G.; Ritter, D.I.; South, S.T.; Thorland, E.C.; et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Reefhuis, J.; Honein, M.; Schieve, L.; Correa, A.; Hobbs, C.; Rasmussen, S.; National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum. Reprod. 2009, 24, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Costain, G.; Silversides, C.K.; Bassett, A.S. The importance of copy number variation in congenital heart disease. NPJ Genom. Med. 2016, 1, 16031. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, Q.; Zhang, L.; Wang, X.; Dan, S.; Deng, D.; Sun, L.; Yao, L.; Ma, Y.; Wang, L. Detection of submicroscopic chromosomal aberrations by array-based comparative genomic hybridization in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2014, 43, 404–412. [Google Scholar] [CrossRef]
- Dowden, L.; Tucker, D.; Morgan, S.; Uzun, O.; Syed, Y.A. Contribution of Congenital Heart Disorders Associated with Copy Number Variants in Mediating Risk for Brain Developmental Disorders: Evidence from 20-Year Retrospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 655463. [Google Scholar] [CrossRef]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef]
- Findley, T.O.; Northrup, H. The current state of prenatal detection of genetic conditions in congenital heart defects. Transl. Pediatr. 2021, 10, 2157. [Google Scholar] [CrossRef]
- Hopkins, M.K.; Dugoff, L.; Kuller, J.A. Congenital heart disease: Prenatal diagnosis and genetic associations. Obstet. Gynecol. Surv. 2019, 74, 497–503. [Google Scholar] [CrossRef]
- Oneda, B.; Rauch, A. Microarrays in Prenatal diagnosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 42, 53–63. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Sotiriadis, A.; Girardelli, S.; Spinillo, S.; Candiani, M.; Amodeo, S.; Farina, A.; Fesslova, V. Postnatal outcome and associated anomalies of prenatally diagnosed right aortic arch with concomitant right ductal arch: A systematic review and meta-analysis. Diagnostics 2020, 10, 831. [Google Scholar] [CrossRef]
- Miyake, T. A review of isolated muscular ventricular septal defect. World J. Clin. Pediatr. 2020, 16, 120–128. [Google Scholar] [CrossRef]
- Abel, J.S.; Berg, C.; Geipel, A.; Gembruch, U.; Herberg, U.; Breuer, J.; Brockmeier, K.; Gottschalk, I. Prenatal diagnosis, associated findings and postnatal outcome of fetuses with truncus arteriosus communis (TAC). Arch. Gynecol. Obstet. 2021, 304, 1455–1466. [Google Scholar] [CrossRef]
- Ayaz, R.; Göktas, E.; Turkyilmaz, G.; Resit Asoglu, M. Prenatal Identification of Aberrant Right Subclavian Artery in Isolation: The Need for Further Genetic Work-Up? Acta Clin. Croat. 2020, 59, 582–588. [Google Scholar] [CrossRef]
- Berg, C.; Bender, F.; Soukup, M.; Geipel, A.; Axt-Fliedner, R.; Breuer, J.; Herberg, U.; Gembruch, U. Right aortic arch detected in fetal life. Ultrasound Obstet. Gynecol. 2006, 28, 882–889. [Google Scholar] [CrossRef]
- Berisha, S.Z.; Shetty, S.; Prior, T.W.; Mitchell, A.L. Cytogenetic and molecular diagnostic testing associated with prenatal and postnatal birth defects. Birth Defects Res. 2020, 112, 293–306. [Google Scholar] [CrossRef]
- Chang, C.-S.; Hong, S.-Y.; Kim, S.-Y.; Kim, Y.-M.; Sung, J.-H.; Choi, S.-J.; Oh, S.-Y.; Roh, C.-R.; Song, J.; Huh, J.; et al. Prevalence of associated extracardiac anomalies in prenatally diagnosed congenital heart diseases. PLoS ONE 2021, 16, e0248894. [Google Scholar] [CrossRef]
- Findley, T.O.; Crain, A.K.; Mahajan, S.; Deniwar, A.; Davis, J.; Solis Zavala, A.S.; Corno, A.F.; Rodriguez-Buritica, D. Congenital heart defects and copy number variants associated with neurodevelopmental impairment. Am. J. Med. Genet. Part A 2022, 188, 13–23. [Google Scholar] [CrossRef]
- Galindo, A.; Gutiérrez-Larraya, F.; Martinez, J.; Del Rio, M.; Grañeras, A.; Velasco, J.; Puerto, B.; Gratacos, E. Prenatal diagnosis and outcome for fetuses with congenital absence of the pulmonary valve. Ultrasound Obstet. Gynecol. 2006, 28, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Sasaki, J.; Jayakar, P.; Sosa, L.; Welch, E. Chromosomal microarray detects genetic risks of neurodevelopmental disorders in newborns with congenital heart disease. Cardiol. Young 2021, 31, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.-D.; Cui, C.-Y.; Wu, D.; Li, T.; Fan, T.-B.; Peng, B.-T.; Zhang, L.-Z.; Wang, C.-Z. Application of array-comparative genomic hybridization in tetralogy of Fallot. Medicine 2016, 95, 49. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.O.; Callaghan, N.; Miller, O.; Simpson, J.; Sharland, G. Right aortic arch diagnosed antenatally: Associations and outcome in 98 fetuses. Heart 2014, 100, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mone, F.; Stott, B.K.; Hamilton, S.; Seale, A.N.; Quinlan-Jones, E.; Allen, S.; Hurles, M.E.; McMullan, D.J.; Maher, E.R.; Kilby, M.D. The Diagnostic Yield of Prenatal Genetic Technologies in Congenital Heart Disease: A Prospective Cohort Study. Fetal Diagn. Ther. 2021, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Binder, G.A.; Berry, R. Prenatal diagnosis of aneuploidy and deletion 22q11. 2 in fetuses with ultrasound detection of cardiac defects. Am. J. Obstet. Gynecol. 2004, 191, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Nagy, O.; Szakszon, K.; Biró, B.O.; Mogyorósy, G.; Nagy, D.; Nagy, B.; Balogh, I.; Ujfalusi, A. Copy number variants detection by microarray and multiplex ligation-dependent probe amplification in congenital heart diseases. J. Biotechnol. 2019, 299, 86–95. [Google Scholar] [CrossRef]
- Oztunc, F.; Ugan Atik, S.; Dedeoglu, R.; Yuksel, M.A.; Madazlı, R. Aortic arch anomalies detected in foetal life by echocardiography. J. Obstet. Gynecol. 2018, 38, 647–651. [Google Scholar] [CrossRef]
- Poon, L.; Huggon, I.; Zidere, V.; Allan, L. Tetralogy of Fallot in the fetus in the current era. Ultrasound Obstet. Gynecol. 2007, 29, 625–627. [Google Scholar] [CrossRef]
- Poot, M.; Stuart, A.A.V.; van Daalen, E.; van Iperen, A.; van Binsbergen, E.; Hochstenbach, R. Variable behavioural phenotypes of patients with monosomies of 15q26 and a review of 16 cases. Eur. J. Med. Genet. 2013, 56, 346–350. [Google Scholar] [CrossRef]
- Rembouskos, G.; Passamonti, U.; De Robertis, V.; Tempesta, A.; Campobasso, G.; Volpe, G.; Gentile, M.; Volpe, P. Aberrant right subclavian artery (ARSA) in unselected population at first and second trimester ultrasonography. Prenat. Diagn. 2012, 32, 968–975. [Google Scholar] [CrossRef]
- Sun, H.; Hao, X.; Wang, X.; Zhou, X.; Zhang, Y.; Liu, X.; Han, J.; Gu, X.; Sun, L.; Zhao, Y.; et al. Genetics and clinical features of noncompaction cardiomyopathy in the fetal population. Front. Cardiovasc. Med. 2021, 7, 617561. [Google Scholar] [CrossRef]
- Vesel, S.; Rollings, S.; Jones, A.; Callaghan, N.; Simpson, J.; Sharland, G. Prenatally diagnosed pulmonary atresia with ventricular septal defect: Echocardiography, genetics, associated anomalies and outcome. Heart 2006, 92, 1501–1505. [Google Scholar] [CrossRef]
- Volpe, P.; Paladini, D.; Marasini, M.; Buonadonna, A.; Russo, M.; Caruso, G.; Marzullo, A.; Vassallo, M.; Martinelli, P.; Gentile, M. Common arterial trunk in the fetus: Characteristics, associations, and outcome in a multicentre series of 23 cases. Heart 2003, 89, 1437–1441. [Google Scholar] [CrossRef]
- Volpe, P.; Paladini, D.; Marasini, M.; Buonadonna, A.; Russo, M.; Caruso, G.; Marzullo, A.; Arciprete, P.; Martinelli, P.; Gentile, M. Characteristics, associations and outcome of absent pulmonary valve syndrome in the fetus. Ultrasound Obstet. Gynecol. 2004, 24, 623–628. [Google Scholar] [CrossRef]
- Wang, B.; Liu, M.; Yan, W.; Mao, J.; Jiang, D.; Li, H.; Chen, Y. Association of SNPs in genes involved in folate metabolism with the risk of congenital heart disease. J. Matern.-Fetal Neonatal Med. 2013, 26, 1768–1777. [Google Scholar] [CrossRef]
- Wertaschnigg, D.; Jaeggi, M.; Chitayat, D.; Shannon, P.; Ryan, G.; Thompson, M.; Yoo, S.; Jaeggi, E. Prenatal diagnosis and outcome of absent pulmonary valve syndrome: Contemporary single-center experience and review of the literature. Ultrasound Obstet. Gynecol. 2013, 41, 162–167. [Google Scholar] [CrossRef]
- Wu, X.-L.; Li, R.; Fu, F.; Pan, M.; Han, J.; Yang, X.; Zhang, Y.-L.; Li, F.-T.; Liao, C. Chromosome microarray analysis in the investigation of children with congenital heart disease. BMC Pediatr. 2017, 17, 117. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Q.; Wang, L.; Han, J.; Pei, Y.; Zhi, W.; Liu, Y.; Yin, C.; Jiang, Y. Next-generation sequencing identified genetic variations in families with fetal non-syndromic atrioventricular septal defects. Int. J. Clin. Exp. Pathol. 2018, 11, 3732. [Google Scholar]
- Xue, J.; Shen, R.; Xie, M.; Liu, Y.; Zhang, Y.; Gong, L.; Li, H. 22q11. 2 recurrent copy number variation-related syndrome: A retrospective analysis of our own microarray cohort and a systematic clinical overview of ClinGen curation. Transl. Pediatr. 2021, 10, 3273. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Huang, X.; He, Z.; Lin, S.; Wang, Y.; Li, L.; Luo, Y.; Fang, Q. Chromosomal aberrations and CNV s in twin fetuses with cardiovascular anomalies: Comparison between monochorionic diamniotic and dichorionic diamniotic twins. Prenat. Diagn. 2018, 38, 318–327. [Google Scholar] [CrossRef]
- Zidere, V.; Tsapakis, E.; Huggon, I.; Allan, L. Right aortic arch in the fetus. Ultrasound Obstet. Gynecol. 2006, 28, 876–881. [Google Scholar] [CrossRef]
- Atli, E.I.; Atli, E.; Yalcintepe, S.; Demir, S.; Kalkan, R.; Akurut, C.; Ozen, Y.; Gurkan, H. Investigation of Genetic Alterations in Congenital Heart Diseases in Prenatal Period. Glob. Med. Genet. 2022, 9, 029–033. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Y.; Hu, H.; Yao, H.; Li, Y.; Tang, S.; Zheng, L.; Xu, Y.; Liang, Z. Karyotypic and molecular genetic changes associated with fetal cardiovascular abnormalities: Results of a retrospective 4-year ultrasonic diagnosis study. Int. J. Biol. Sci. 2013, 9, 463. [Google Scholar] [CrossRef][Green Version]
- Cai, M.; Huang, H.; Su, L.; Lin, N.; Wu, X.; Xie, X.; An, G.; Li, Y.; Lin, Y.; Xu, L. Fetal congenital heart disease: Associated anomalies, identification of genetic anomalies by single-nucleotide polymorphism array analysis, and postnatal outcome. Medicine 2018, 97, e13617. [Google Scholar] [CrossRef]
- Huang, H.; Cai, M.; Wang, Y.; Liang, B.; Lin, N.; Xu, L. SNP array as a tool for Prenatal diagnosis of congenital heart disease screened by echocardiography: Implications for precision assessment of fetal prognosis. Risk Manag. Healthc. Policy 2021, 14, 345. [Google Scholar] [CrossRef]
- Jansen, F.A.; Hoffer, M.J.; van Velzen, C.L.; Plati, S.K.; Rijlaarsdam, M.E.; Clur, S.-A.B.; Blom, N.A.; Pajkrt, E.; Bhola, S.L.; Knegt, A.C.; et al. Chromosomal abnormalities and copy number variations in fetal left-sided congenital heart defects. Prenat. Diagn. 2016, 36, 177–185. [Google Scholar] [CrossRef]
- Kong, C.; Cheng, Y.K.; To, W.W.; Leung, T. Prevalence of chromosomal abnormalities and 22q11. 2 deletion in conotruncal and non-conotruncal antenatally diagnosed congenital heart diseases in a Chinese population. Hong Kong Med. J. 2019, 25, 6. [Google Scholar]
- Kowalczyk, K.; Bartnik-G-laska, M.; Smyk, M.; Plaskota, I.; Bernaciak, J.; Kedzior, M.; Wi´sniowiecka-Kowalnik, B.; Jakub´ow-Durska, K.; Braun-Walicka, N.; Barczyk, A.; et al. Prenatal diagnosis by Array Comparative Genomic Hybridization in Fetuses with Cardiac Abnormalities. Genes 2021, 12, 2021. [Google Scholar] [CrossRef]
- Liao, C.; Li, R.; Fu, F.; Xie, G.; Zhang, Y.; Pan, M.; Li, J.; Li, D. Prenatal diagnosis of congenital heart defect by genome-wide high-resolution SNP array. Prenat. Diagn. 2014, 34, 858–863. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, J.; Peng, R.; Du, L.; Zheng, Q.; Lei, T.; Xie, H. Prenatal diagnosis of chromosomal aberrations in fetuses with conotruncal heart defects by genome-wide high-resolution SNP array. J. Matern.-Fetal Neonatal Med. 2020, 33, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Meng, D.; Li, Q.; Hu, X.; Chen, Y.; He, C.; Xie, B.; She, S.; Li, Y.; Fu, C. Genetic testing and pregnancy outcome analysis of 362 fetuses with congenital heart disease identified by prenatal ultrasound. Arq. Bras. Cardiol. 2018, 111, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Mademont-Soler, I.; Morales, C.; Soler, A.; MartÍnez-Crespo, J.; Shen, Y.; Margarit, E.; Clusellas, N.; Obon, M.; Wu, B.-L.; Sanchez, A. Prenatal diagnosis of chromosomal abnormalities in fetuses with abnormal cardiac ultrasound findings: Evaluation of chromosomal microarray-based analysis. Ultrasound Obstet. Gynecol. 2013, 41, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Minsart, A.-F.; Boucoiran, I.; Delrue, M.-A.; Audibert, F.; Abadir, S.; Lapierre, C.; Lemyre, E.; Raboisson, M.-J. Left superior vena cava in the fetus: A rarely isolated anomaly. Pediatr. Cardiol. 2020, 41, 230–236. [Google Scholar] [CrossRef]
- O’Mahony, E.F.; Hutchinson, D.P.; McGillivray, G.; Nisbet, D.L.; Palma-Dias, R. Right-sided aortic arch in the age of microarray. Prenat. Diagn. 2017, 37, 440–445. [Google Scholar] [CrossRef]
- Peng, R.; Xie, H.-N.; Zheng, J.; Zhou, Y.; Lin, M.-F. Fetal right aortic arch: Associated anomalies, genetic anomalies with chromosomal microarray analysis, and postnatal outcome. Prenat. Diagn. 2017, 37, 329–335. [Google Scholar] [CrossRef]
- Peng, R.; Zheng, J.; Xie, H.-N.; He, M.; Lin, M.-F. Genetic anomalies in fetuses with tetralogy of Fallot by using high-definition chromosomal microarray analysis. Cardiovasc. Ultrasound 2019, 17, 8. [Google Scholar] [CrossRef]
- Schmid, M.; Stary, S.; Blaicher, W.; Gollinger, M.; Husslein, P.; Streubel, B. Prenatal genetic diagnosis using microarray analysis in fetuses with congenital heart defects. Prenat. Diagn. 2012, 32, 376–382. [Google Scholar] [CrossRef]
- Sukenik-Halevy, R.; Sukenik, S.; Koifman, A.; Alpert, Y.; Hershkovitz, R.; Levi, A.; Biron-Shental, T. Clinical aspects of prenatally detected congenital heart malformations and the yield of chromosomal microarray analysis. Prenat. Diagn. 2016, 36, 1185–1191. [Google Scholar] [CrossRef]
- Tang, S.; Lv, J.; Chen, X.; Bai, L.; Li, H.; Chen, C.; Wang, P.; Xu, X.; Lu, J. Prenatal diagnosis of DNA copy number variations by genomic single-nucleotide polymorphism array in fetuses with congenital heart defects. Fetal. Diagn. Ther. 2016, 39, 64–73. [Google Scholar] [CrossRef]
- Tonni, G.; Palmisano, M.; Zamarian, A.C.P.; Caetano, A.C.R.; Santana, E.F.M.; Peixoto, A.B.; Armbruster-Moraes, E.; Ruano, R.; Júnior, E.A. Phenotype to genotype characterization by array-comparative genomic hydridization (a-CGH) in case of fetal malformations: A systematic review. Taiwan J. Obstet. Gynecol. 2019, 58, 15–28. [Google Scholar] [CrossRef]
- van Nisselrooij, A.E.; Lugthart, M.A.; Clur, S.-A.; Linskens, I.H.; Pajkrt, E.; Rammeloo, L.A.; Rozendaal, L.; Blom, N.A.; van Lith, J.M.; Knegt, A.C.; et al. The prevalence of genetic diagnoses in fetuses with severe congenital heart defects. Genet. Med. 2020, 22, 1206–1214. [Google Scholar] [CrossRef]
- Vigneswaran, T.V.; Allan, L.; Charakida, M.; Durward, A.; Simpson, J.M.; Nicolaides, K.H.; Zidere, V. Prenatal diagnosis and clinical implications of an apparently isolated right aortic arch. Prenat. Diagn. 2018, 38, 1055–1061. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Y.; Huang, S.; Wu, Y.; Li, P.; Zhuang, J. Clinical application of chromosomal microarray analysis for the Prenatal diagnosis of chromosomal abnormalities and copy number variations in fetuses with congenital heart disease. Prenat. Diagn. 2018, 38, 406–413. [Google Scholar] [CrossRef]
- Zeng, D.-W.; Zhang, J.-M.; Liu, Y.-R.; Dong, J.; Jiang, J.-J.; Zhu, Y.-Y. A retrospective study on the significance of liver biopsy and hepatitis B surface antigen in chronic hepatitis B infection. Medicine 2016, 95, e2503. [Google Scholar] [CrossRef]
- Huang, H.; Cai, M.; Liu, L.; Xu, L.; Lin, N. Effectiveness of Chromosomal Microarray Analysis for Prenatal diagnosis of Fetal Echogenic Intracardiac Focus: A Single-Center Experience. Int. J. Gen. Med. 2021, 14, 1991. [Google Scholar] [CrossRef]
- Balzeau, J.; Menezes, M.R.; Cao, S.; Hagan, J.P. The LIN28/let-7 pathway in cancer. Front. Genet. 2017, 8, 31. [Google Scholar] [CrossRef]
- Cao, Q.; Xu, L.; Li, R.; Han, J.; Yi, C.; Jing, X.; Zhang, L.; Li, D.; Pan, M. Prenatal diagnosis and clinical outcomes of 297 fetuses with conotruncal defects. Zhonghua Fu Chan Ke Za Zhi 2022, 57, 25–31. [Google Scholar]
- Ren, M.; Zhou, S.; Hou, L.; Zhang, W.; Wang, X.; Fan, X.; Chai, X. Prenatal diagnosis and pregnancy outcomes of sixty-three fetuses with tetralogy of Fallot. Zhonghua Fu Chan Ke Za Zhi 2019, 54, 660–665. [Google Scholar]
- Wang, J.; Zhao, Y.; Jin, H.; Tao, J.; Cao, L.; Cai, Y. Analysis of genomic copy number variations in fetuses with conotruncal defects using single nucleotide polymorphism array. Zhonghua Yi Xue Yi Chuan Xue Za Zhi = Zhonghua Yixue Yichuanxue Zazhi = Chin. J. Med. Genet. 2018, 35, 347–350. [Google Scholar]
- Gao, M.; Pang, H.; Zhao, Y.; Li-Ling, J. Analysis of genomic copy number variations in 36 fetuses with heart malformations using next-generation sequencing. Zhonghua Yi Xue Yi Chuan Xue Za Zhi = Zhonghua Yixue Yichuanxue Zazhi = Chin. J. Med. Genet. 2017, 34, 524–527. [Google Scholar]
- Liu, Y.; Xie, J.; Geng, Q.; Xu, Z.; Wu, W.; Luo, F.; Li, S.; Wang, Q.; Chen, W.; Tan, H.; et al. Combined G-banded karyotyping and multiplex ligation-dependent probe amplification for the detection of chromosomal abnormalities in fetuses with congenital heart defects. Zhonghua Yi Xue Yi Chuan Xue Za Zhi = Zhonghua Yixue Yichuanxue Zazhi = Chin. J. Med. Genet. 2017, 34, 1–5. [Google Scholar]
- Wu, X.; Fu, F.; Li, R.; Pan, M.; Han, J.; Zhen, L.; Yang, X.; Zhang, Y.; Li, F.; Liao, C. Clinical value of genome-wide high resolution chromosomal microarray analysis in etiological study of fetuses with congenital heart defects. Zhonghua Fu Chan Ke Za Zhi 2014, 49, 893–898. [Google Scholar]
- Minier, F.; Carles, D.; Pelluard, F.; Alberti, E.; Stern, L.; Saura, R. DiGeorge syndrome, a review of 52 patients. Arch. Pediatr. Organe Off. Soc. Francaise Pediatr. 2005, 12, 254–257. [Google Scholar]
- Salzer-Sheelo, L.; Polak, U.; Barg, A.; Kahana, S.; Yacobson, S.; Agmon-Fishman, I.; Klein, C.; Matar, R.; Rurman-Shahar, N.; Sagi-Dain, L.; et al. Prenatal and postnatal chromosomal microarray analysis in 885 cases of various congenital heart defects. Arch. Gynecol. Obstet. 2022, 1–7. [Google Scholar] [CrossRef]
- Topbas Selcuki, N.F.; Senol, G.; Esin, D.; Ozkose, Z.G.; Caypinar, S.S.; Bornaun, H.; Cetin, B.A.; Yuksel, M.A. Prenatal diagnosis and postnatal outcomes of right aortic arch anomalies. Arch. Gynecol. Obstet. 2022, 1–8. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Zhang, Y.; Zhao, Y.; Liu, X.; Gu, X.; Han, J.-C.; He, Y.-H. Prenatal diagnosis of isolation of aortic brachiocephalic artery. Acta Radiol. 2021, 2841851211058278. [Google Scholar] [CrossRef]
- Huang, J.; Deng, X.; Wang, Y.; Tang, N.; Zeng, D. Analysis of Copy Number Variations by Low-Depth Whole-Genome Sequencing in Fetuses with Congenital Cardiovascular Malformations. Cytogenet. Genome Res. 2020, 160, 643–649. [Google Scholar] [CrossRef]
- Fu, F.; Deng, Q.; Lei, T.-Y.; Li, R.; Jing, X.-Y.; Yang, X.; Liao, C. Clinical application of SNP array analysis in fetuses with ventricular septal defects and normal karyotypes. Arch. Gynecol. Obstet. 2017, 296, 929–940. [Google Scholar] [CrossRef]
- Gindes, L.; Salem, Y.; Gasnier, R.; Raucher, A.; Tamir, A.; Assa, S.; Weissman- Brenner, A.; Weisz, B.; Kasif, E.; Achiron, R. Prenatal diagnosis of major aortopulmonary collateral arteries (MAPCA) in fetuses with pulmonary atresia with ventricular septal defect and agenesis of ductus arteriosus. J. Matern.-Fetal Neonatal Med. 2021, 1–9, online ahead of print. [Google Scholar] [CrossRef]
- Hureaux, M.; Guterman, S.; Hervé, B.; Till, M.; Jaillard, S.; Redon, S.; Valduga, M.; Coutton, C.; Missirian, C.; Prieur, F.; et al. Chromosomal microarray analysis in fetuses with an isolated congenital heart defect: A retrospective, nationwide, multicenter study in France. Prenat. Diagn. 2019, 39, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Lazier, J.; Fruitman, D.; Lauzon, J.; Bernier, F.; Argiropoulos, B.; Chernos, J.; Caluseriu, O.; Simrose, R.; Thomas, M.A. Prenatal array comparative genomic hybridization in fetuses with structural cardiac anomalies. J. Obstet. Gynaecol. Can. 2016, 38, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-Y.; Won, H.-S.; Han, Y.J.; Ryu, H.M.; Lee, D.E.; Jeong, B.-D. Clinical value of chromosomal microarray analysis in prenatally diagnosed dextro-transposition of the great arteries. J. Matern.-Fetal Neonatal Med. 2020, 33, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Xue, P.; Zhang, B.; Wang, J.; Yu, B.; Liu, J. Estimating the frequency of causal genetic variants in foetuses with congenital heart defects: A Chinese cohort study. Orphanet J. Rare Dis. 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Maya, I.; Kahana, S.; Yeshaya, J.; Tenne, T.; Yacobson, S.; Agmon-Fishman, I.; Cohen-Vig, L.; Levi, A.; Reinstein, E.; Basel-Vanagaite, L.; et al. Chromosomal microarray analysis in fetuses with aberrant right subclavian artery. Ultrasound Obstet. Gynecol. 2017, 49, 337–341. [Google Scholar] [CrossRef]
- Maya, I.; Singer, A.; Yonath, H.; Reches, A.; Rienstein, S.; Zeligson, S.; Ben Shachar, S.; Sagi-Dain, L. What have we learned from 691 prenatal chromosomal microarrays for ventricular septal defects? Acta Obstet. Gynecol. Scand. 2020, 99, 757–764. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Jacobs, K.M.; Tessier, K.M.; Narasimhan, S.L.; Tofte, A.N.; McCarter, A.R.; Cross, S.N. Chromosomal microarray analysis in the investigation of prenatally diagnosed congenital heart disease. Am. J. Obstet. Gynecol. MFM 2020, 2, 100078. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, Y.; Zhang, C.; Zhou, R.; Wu, Y.; Wang, C.; Meng, L.; Mao, P.; Cheng, Q.; Luo, C.; et al. Comprehensive evaluation of genetic variants using chromosomal microarray analysis and exome sequencing in fetuses with congenital heart defect. Ultrasound Obstet. Gynecol. 2021, 58, 377–387. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Singer, A.; Segel, R.; Berger, R.; Kanengisser-Pines, B.; Maya, I. The yield of chromosomal microarray in pregnancies with congenital cardiac defects and normal noninvasive prenatal screening. Am. J. Obstet. Gynecol. 2021, 225, 333-e1. [Google Scholar] [CrossRef]
- Shaffer, L.G.; Rosenfeld, J.A.; Dabell, M.P.; Coppinger, J.; Bandholz, A.M.; Ellison, J.W.; Ravnan, J.B.; Torchia, B.S.; Ballif, B.C.; Fisher, A.J. Detection rates of clinically significant genomic alterations by microarray analysis for specific anomalies detected by ultrasound. Prenat. Diagn. 2012, 32, 986–995. [Google Scholar] [CrossRef]
- Song, T.; Wan, S.; Li, Y.; Xu, Y.; Dang, Y.; Zheng, Y.; Li, C.; Zheng, J.; Chen, B.; Zhang, J. Detection of copy number variants using chromosomal microarray analysis for the Prenatal diagnosis of congenital heart defects with normal karyotype. J. Clin. Lab. Anal. 2019, 33, e22630. [Google Scholar] [CrossRef]
- Svirsky, R.; Brabbing-Goldstein, D.; Rozovski, U.; Kapusta, L.; Reches, A.; Yaron, Y. The genetic and clinical outcome of isolated fetal muscular ventricular septal defect (VSD). J. Matern.-Fetal Neonatal Med. 2019, 32, 2837–2841. [Google Scholar] [CrossRef]
- Turan, S.; Asoglu, M.R.; Benziv, R.G.; Doyle, L.; Harman, C.; Turan, O.M. Yield rate of chromosomal microarray analysis in fetuses with congenital heart defects. Eur. J. Obstet. Gynecol. 2018, 221, 172–176. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Liang, D.; Meng, L.; Wu, Y.; Qiao, F.; Ji, X.; Luo, C.; Zhang, J.; Xu, T.; et al. Prenatal chromosomal microarray analysis in fetuses with congenital heart disease: A prospective cohort study. Am. J. Obstet. Gynecol. 2018, 218. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Su, L.; Xie, X.; Cai, M.; Lin, N.; Huang, H.; Lin, Y.; Xu, L. Chromosomal microarray analysis for the fetuses with aortic arch abnormalities and normal karyotype. Mol. Diagn. Ther. 2020, 24, 611–619. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Ru, T.; Wang, Y.; Xu, Y.; Yang, Y.; Wu, X.; Cram, D.S.; Hu, Y. Identification of copy number variations associated with congenital heart disease by chromosomal microarray analysis and next-generation sequencing. Prenat. Diagn. 2016, 36, 321–327. [Google Scholar] [CrossRef]
- Piacentini, G.; Mastromoro, G.; Romano, V.; Riccardi, R.; Orfeo, L. Fetal echocardiographic features of absent pulmonary valve syndrome. Am. J. Obstet. Gynecol. 2022, in press. [Google Scholar] [CrossRef]
- Evangelidou, P.; Alexandrou, A.; Moutafi, M.; Ioannides, M.; Antoniou, P.; Koumbaris, G.; Kallikas, I.; Velissariou, V.; Sismani, C.; Patsalis, P.C. Implementation of high resolution whole genome array CGH in the prenatal clinical setting: Advantages, challenges, and review of the literature. BioMed Res. Int. 2013, 2013, 346762. [Google Scholar] [CrossRef]
- Jelin, A.C.; Sagaser, K.G.; Wilkins-Haug, L. Prenatal genetic testing options. Pediatr. Clin. 2019, 66, 281–293. [Google Scholar] [CrossRef]
- Kang, J.U.; Koo, S.H. Clinical implementation of chromosomal microarray technology in Prenatal diagnosis. Mol. Med. Rep. 2012, 6, 1219–1222. [Google Scholar] [CrossRef][Green Version]
- Leavitt, K.; Goldwaser, T.; Bhat, G.; Kalia, I.; Klugman, S.D.; Dolan, S.M. Chromosomal microarray in Prenatal diagnosis: Case studies and clinical challenges. Per. Med. 2016, 13, 249–255. [Google Scholar] [CrossRef]
- Hillman, S.C.; McMullan, D.; Williams, D.; Maher, E.; Kilby, M. Microarray comparative genomic hybridization in Prenatal diagnosis: A review. Ultrasound Obstet. Gynecol. 2012, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal microarray versus karyotyping for Prenatal diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians. Committee opinion no. 581: The use of chromosomal microarray analysis in Prenatal diagnosis. Obstet. Gynecol. 2013, 122, 1374–1377. [Google Scholar] [CrossRef]
- Brady, P.D.; Vermeesch, J.R. Genomic microarrays: A technology overview. Prenat. Diagn. 2012, 32, 336–343. [Google Scholar] [CrossRef]
- Rajcan-Separovic, E. Chromosome microarrays in human reproduction. Hum. Reprod. Update 2012, 18, 555–567. [Google Scholar] [CrossRef]
- Levy, B.; Wapner, R. Prenatal diagnosis by chromosomal microarray analysis. Fertil. Steril. 2018, 109, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, B.A.; Soucier, D.; Hanson, K.; Savage, M.S.; Jackson, L.; Wapner, R.J. Women’s experiences receiving abnormal prenatal chromosomal microarray testing results. Genet. Med. 2013, 15, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.; Digilio, M.C.; Toscano, A.; Anaclerio, S.; Giannotti, A.; Feltri, C.; De Ioris, M.A.; Angioni, A.; Dallapiccola, B. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet. Med. 2001, 3, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, D.; Mastromoro, G.; Di Palma, F.; Pizzuti, A.; Marchionni, E. Prenatal exome sequencing: Background, current practice and future perspectives—A systematic review. Diagnostics 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K.; et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Li, R.; Fu, F.; Yu, Q.; Wang, D.; Jing, X.; Zhang, Y.; Li, F.; Li, F.; Han, J.; Pan, M.; et al. Prenatal exome sequencing in fetuses with congenital heart defects. Clin. Genet. 2020, 98, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Mone, F.; Eberhardt, R.Y.; Morris, R.K.; Hurles, M.E.; McMullan, D.J.; Maher, E.R.; Lord, J.; Chitty, L.; Giordano, J.; Wapner, R.J.; et al. COngenital heart disease and the Diagnostic yield with Exome sequencing (CODE) study: Prospective cohort study and systematic review. Ultrasound Obstet. Gynecol. 2021, 57, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Olshaker, H.; Ber, R.; Hoffman, D.; Derazne, E.; Achiron, R.; Katorza, E. Volumetric brain MRI study in fetuses with congenital heart disease. Am. J. Neuroradiol. 2018, 39, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Masoller, N.; Sanz-Cortés, M.; Crispi, F.; Gómez, O.; Bennasar, M.; Egaña-Ugrinovic, G.; Bargalló, N.; Martínez, J.M.; Gratacós, E. Severity of fetal brain abnormalities in congenital heart disease in relation to the main expected pattern of in utero brain blood supply. Fetal. Diagn. Ther. 2016, 39, 269–278. [Google Scholar] [CrossRef]
- Sadhwani, A.; Wypij, D.; Rofeberg, V.; Gholipour, A.; Mittleman, M.; Rohde, J.; Velasco-Annis, C.; Calderon, J.; Friedman, K.G.; Tworetzky, W.; et al. Fetal brain volume predicts neurodevelopment in congenital heart disease. Circulation 2022, 145, 1108–1119. [Google Scholar] [CrossRef]
- Dovjak, G.O.; Hausmaninger, G.; Zalewski, T.; Schmidbauer, V.; Weber, M.; Worda, C.; Seidl-Mlczoch, E.; Berger-Kulemann, V.; Prayer, D.; Kasprian, G.J.; et al. Brainstem and cerebellar volumes at magnetic resonance imaging are smaller in fetuses with congenital heart disease. Am. J. Obstet. Gynecol. 2022, in press. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).