Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers
Abstract
:1. Introduction
2. Methodology
3. Clinical Diagnosis and Definition
3.1. Concussion, TBI, and RMHI
3.2. Post-Concussion Syndrome
3.3. Chronic Traumatic Encephalopathy
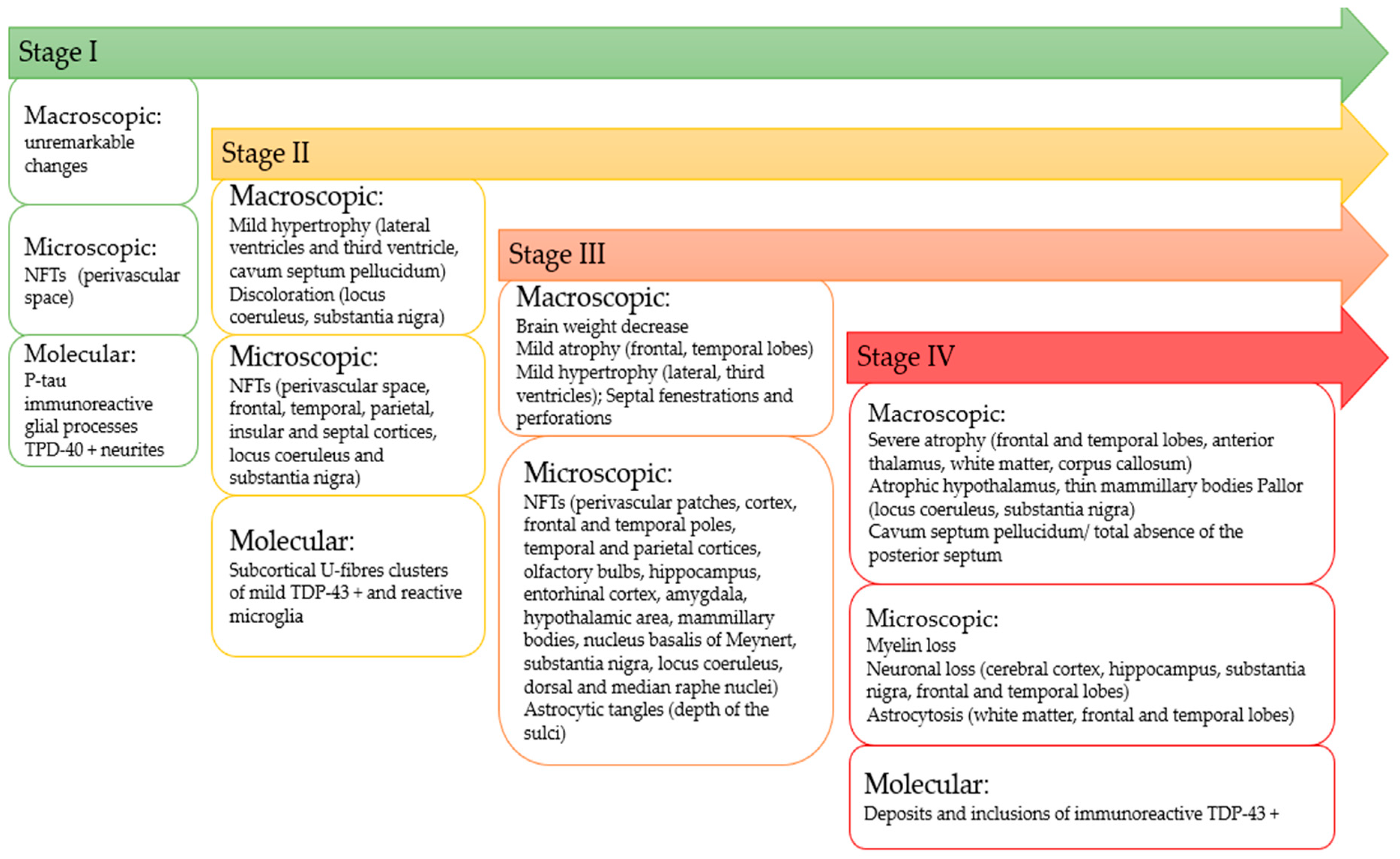
3.3.1. Staging of CTE
3.3.2. Neurophysiological Pathological Description
4. Clinical Biomarkers
4.1. Neuroimaging
4.1.1. Magnetic Resonance Imaging (MRI)
4.1.2. Diffusion MRI
4.1.3. Functional MRI
4.1.4. Magnetic Spectroscopy (MRS)
4.1.5. Susceptibility Weighted Imaging (SWI)
4.1.6. Positron Emission Tomography (PET)—Metabolic and Molecular Neuroimaging
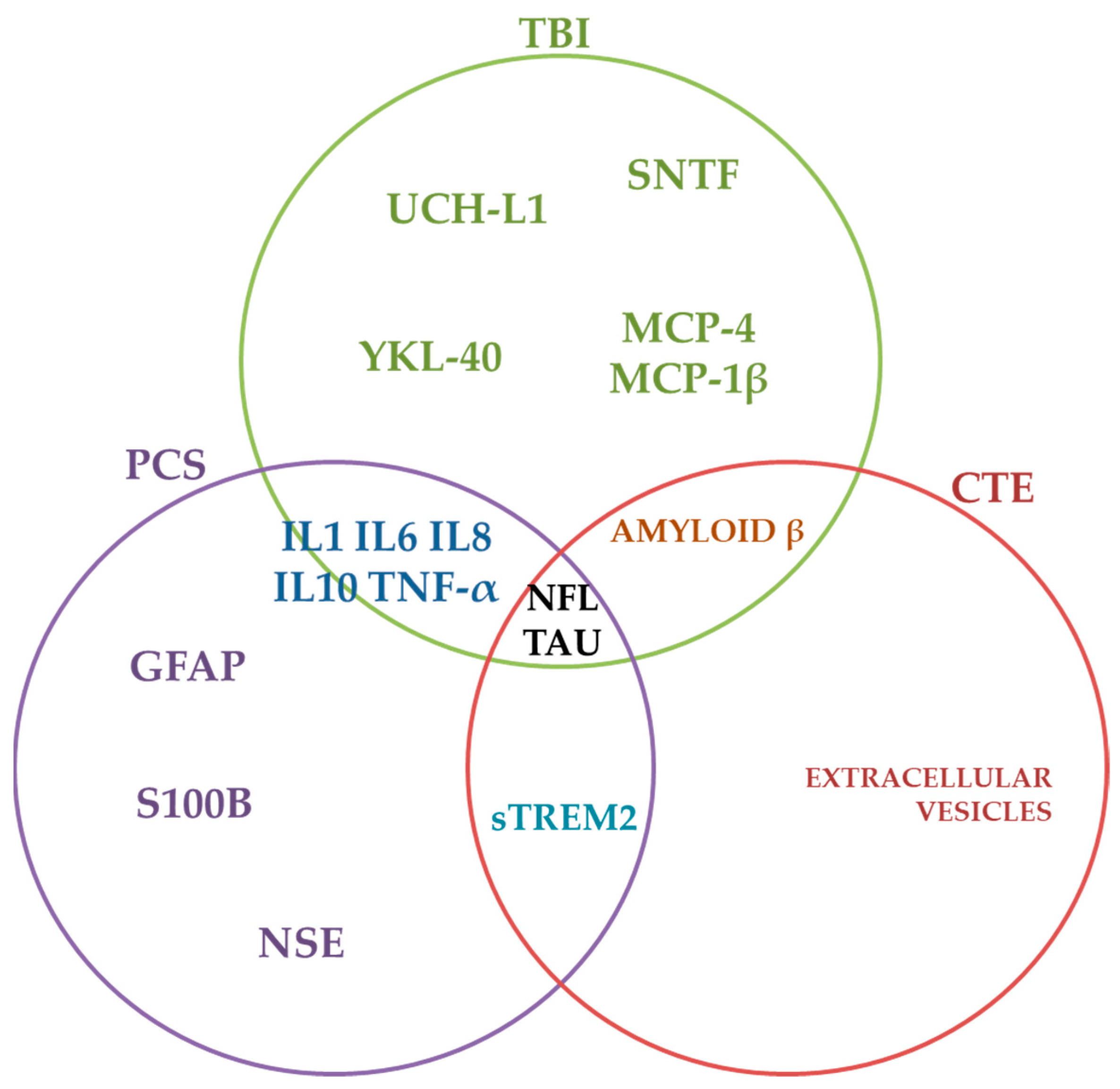
4.2. Fluid Biomarkers
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. JNS 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazarian, J.J.; Wong, T.; Harris, M.; Leahey, N.; Mookerjee, S.; Dombovy, M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999, 13, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Jenkins, P.O. Concussion is confusing us all. Pract. Neurol. 2015, 15, 172–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.K.; Alavinia, S.M.; Lawrence, D.W.; Munce, S.E.P.; Kam, A.; Tam, A.; Ruttan, L.; Comper., P.; Bayley, M.T. Prediction of risk of prolonged post-concussion symptoms: Derivation and validation of the TRICORDRR (Toronto Rehabilitation Institute Concussion Outcome Determination and Rehab Recommendations) score. PLoS Med. 2021, 18, e1003652. [Google Scholar] [CrossRef]
- Permenter, C.M.; Fernández-de Thomas, R.J.; Sherman, A. Postconcussive Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534786/ (accessed on 3 March 2022).
- Saatman, K.; Duhaime, A.; Bullock, R.; Maas, A.; Valadka, A. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [Green Version]
- McAllister, T.; McCrea, M. Long-Term Cognitive and Neuropsychiatric Consequences of Repetitive Concussion and Head-Impact Exposure. J. Athl. Train. 2017, 52, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Roberts, G.W.; Allsop, D.; Bruton, C. The occult aftermath of boxing. J. Neurol. Neurosurg. Psychiatry 1990, 53, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Omalu, B.I.; DeKosky, S.T.; Minster, R.L.; Kamboh, M.I.; Hamilton, R.L.; Wecht, C.H. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005, 57, 128–134. [Google Scholar] [CrossRef]
- Omalu, B.I.; DeKosky, S.T.; Hamilton, R.L.; Minster, R.L.; Kamboh, M.I.; Shakir, A.M.; Wecht, C.H. Chronic traumatic encephalopathy in a national football league player: Part II. Neurosurgery 2006, 59, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Omalu, B.I.; Fitzsimmons, R.P.; Hammers, J.; Bailes, J. Chronic traumatic encephalopathy in a professional American wrestler. J. Forensic Nurs. 2010, 6, 130–136. [Google Scholar] [CrossRef]
- Omalu, B.I.; Hamilton, R.L.; Kamboh, M.I.; DeKosky, S.T.; Bailes, J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J. Forensic Nurs. 2010, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Omalu, B.I.; Bailes, J.; Hammers, J.L.; Fitzsimmons, R.P. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: The role of the forensic pathologist. Am. J. Forensic Med. Pathol. 2010, 31, 130–132. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Gavett, B.E.; Stern, R.A.; Nowinski, C.J.; Cantu, R.C.; Kowall, N.W.; Perl, D.P.; Hedley-Whyte, E.T.; Price, B.; Sullivan, C.; et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 918–929. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Stern, R.A.; Nowinski, C.J.; Stein, T.D.; Alvarez, V.E.; Daneshvar, D.H.; Lee, H.S.; Wojtowicz, S.M.; Hall, G.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136 Pt 1, 43–64. [Google Scholar] [CrossRef]
- Hof, P.R.; Knabe, R.; Bovier, P.; Bouras, C. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol. 1991, 82, 321–326. [Google Scholar] [CrossRef]
- Geddes, J.F.; Vowles, G.H.; Nicoll, J.A.; Revesz, T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999, 98, 171–178. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012, 4, 134–160. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.C.; Stein, T.D.; Kiernan, P.T.; Alvarez, V.E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015, 25, 350–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, X.; Castro-Obregón, S.; Gutiérrez-Becker, B.; Gutiérrez-Ospina, G.; Karalis, N.; Khalil, A.A.; Lopez-Noguerola, J.S.; Rodríguez, L.L.; Martínez-Martínez, E.; Perez-Cruz, C.; et al. Re-thinking the Etiological Framework of Neurodegeneration. Front. Neurosci. 2019, 13, 728. [Google Scholar] [CrossRef] [Green Version]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Victoroff, J. Traumatic encephalopathy: Review and provisional research diagnostic criteria. Neuro Rehabil. 2013, 32, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Reams, N.; Eckner, J.T.; Almeida, A.A.; Aagesen, A.L.; Giordani, B.; Paulson, H.; Lorincz, M.T.; Kutcher, J.S. A Clinical Approach to the Diagnosis of Traumatic Encephalopathy Syndrome: A Review. JAMA Neurol. 2016, 73, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenigro, P.H.; Bernick, C.; Cantu, R.C. Clinical features of repetitive traumatic brain injury and chronic traumatic encephalopathy. Brain Pathol. 2015, 25, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, S.C.; Beaton, C.S.; Welton, T.; Grieve, S.M. Characterizing the Risk of Depression Following Mild Traumatic Brain Injury: A Meta-Analysis of the Literature Comparing Chronic mTBI to Non-mTBI Populations. Front. Neurol. 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Jain, S.; Giacino, J.T.; Levin, H.; Dikmen, S.; Nelson, L.D.; Vassar, M.J.; Okonkwo, D.O.; Diaz-Arrastia, R.; Robertson, C.S.; et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients after Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA Psychiatr. 2019, 76, 249–258. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Levin, H.S.; Contant, C.F.; Song, J.X. Postconcussional disorder following mild to moderate traumatic brain injury: Anxiety, depression, and social support as risk factors and comorbidities. J. Clin. Exp. Neuropsychol. 2001, 23, 792–808. [Google Scholar] [CrossRef]
- Hammond, F.M.; Corrigan, J.D.; Ketchum, J.M.; Malec, J.F.; Dams-O’Connor, K.; Hart, T.; Novack, T.A.; Bogner, J.; Dahdah, M.N.; Whiteneck, G.G. Prevalence of Medical and Psychiatric Comorbidities Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2019, 34, E1–E10. [Google Scholar] [CrossRef]
- Hai, T.; Agimi, Y.; Stout, K. Prevalence of Comorbidities in Active and Reserve Service Members Pre and Post Traumatic Brain Injury, 2017–2019. Mil. Med. 2021, usab342. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Snoek, J.; Bond, M.R.; Brooks, N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 1981, 44, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Hardy, J.; Zetterberg, H. Neurological consequences of traumatic brain injuries in sports. Mol. Cell. Neurosci. 2015, 66 Pt B, 114–122. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Blennow, K.; Ikonomovic, M.D.; Gandy, S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2013, 9, 192–20023. [Google Scholar] [CrossRef] [Green Version]
- Cantu, R. Concussion Classification: Ongoing Controversy. In Foundations of Sport-Related Brain Injuries; Sebastianelli, W.J., Slobounov, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 87–110. ISBN 978-0-387-32564-4. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Nakase-Richardson, R.; Sherer, M.; Seel, R.T.; Hart, T.; Hanks, R.; Arango-Lasprilla, J.C.; Yablon, S.; Sander, A.; Barnett, S.; Walker, W.; et al. Utility of post-traumatic amnesia in predicting 1-year productivity following traumatic brain injury: Comparison of the Russell and Mississippi PTA classification intervals. J. Neurol. Neurosurg. Psychiatry 2011, 82, 494–499. [Google Scholar] [CrossRef]
- Greenwald, B.D.; Ambrose, A.F.; Armstrong, G.P. Mild brain injury. Rehabil. Res. Pract. 2012, 2012, 469475. [Google Scholar] [CrossRef]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The Mayo Classification System for Traumatic Brain Injury Severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef]
- Russell, W.R.; Smith, A. Post-traumatic amnesia in closed head injury. Arch. Neurol. 1961, 5, 4–17. [Google Scholar] [CrossRef]
- Ontario Neurotrauma Foundation. Guideline For Concussion/Mild Traumatic Brain Injury & Prolonged Symptoms (3rd Edition), For Adults Over 18 Years of Age. Available online: https://braininjuryguidelines.org/concussion/ (accessed on 19 January 2022).
- Reuben, A.; Sampson, P.; Harris, A.R.; Williams, H.; Yates, P. Postconcussion syndrome (PCS) in the emergency department: Predicting and pre-empting persistent symptoms following a mild traumatic brain injury. Emerg. Med. J. 2014, 31, 72–77. [Google Scholar] [CrossRef]
- Jordan, B.D. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 222–230. [Google Scholar] [CrossRef]
- Omalu, B.; Bailes, J.; Hamilton, R.L.; Kamboh, M.I.; Hammers, J.; Case, M.; Fitzsimmons, R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 2011, 69, 173–183. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Martland, H.S. Punch drunk. JAMA 1928, 91, 1103–1107. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G. Dementia Pugilistica Revisited. J. Alzheimers Dis. 2017, 60, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyburn, L.; Sajja, V.S.S.S.; Long, J.B. The Role of TDP-43 in Military-Relevant TBI and Chronic Neurodegeneration. Front. Neurol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, W.L.; Povlishock, J.T.; Graham, D.L. A mechanistic analysis of nondisruptive axonal injury: A review. J. Neurotrauma 1997, 14, 419–440. [Google Scholar] [CrossRef]
- Medana, I.; Esiri, M. Axonal damage: A key predictor of outcome in human CNS diseases. Brain 2003, 126, 515–530. [Google Scholar] [CrossRef] [Green Version]
- Zanier, E.R.; Bertani, I.; Sammali, E.; Pischiutta, F.; Chiaravalloti, M.A.; Vegliante, G.; Masone, A.; Corbelli, A.; Smith, D.H.; Menon, D.K.; et al. Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018, 141, 2685–2699. [Google Scholar] [CrossRef]
- Moisse, K.; Mepham, J.; Volkening, K.; Welch, I.; Hill, T.; Strong, M.J. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL(−/−) mice: Support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009, 1296, 176–186. [Google Scholar] [CrossRef]
- Blaylock, R.L.; Maroon, J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—A unifying hypothesis. Surg. Neurol. Int. 2011, 2, 107. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.P.; Tetzlaff, J.E.; Bonis, J.M.; Nelson, L.D.; Mayer, A.; Huber, D.L.; Harezlak, J.; Mathews, V.P.; Ulmer, J.L.; Sinson, G.P.; et al. Prevalence of potentially clinically significant MRI findings in athletes with and without sport-related concussion. J. Neurotrauma 2019, 36, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Asken, B.M.; DeKosky, S.T.; Clugston, J.R.; Jaffee, M.S.; Bauer, R.M. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): A systematic critical review. Brain Imaging Behav. 2011, 12, 585–612. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Muehlmann, M.; Tripodis, Y.; Hufschmidt, J.; Stamm, J.; Green, K.; Wrobel, P.; Schultz, V.; Weir, I.; Alosco, M.L.; et al. Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. 2019, 13, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Champagne, A.A.; Peponoulas, E.; Terem, I.; Ross, A.; Tayebi, M.; Chen, Y.; Coverdale, N.S.; Nielsen, P.; Wang, A.; Shim, V.; et al. Novel strain analysis informs about injury susceptibility of the corpus callosum to repeated impacts. Brain Commun. 2019, 1, fcz021. [Google Scholar] [CrossRef] [Green Version]
- Weis, S.; Sonnberger, M.; Dunzinger, A.; Voglmayr, E.; Aichholzer, M.; Kleiser, R.; Strasser, P. Imaging Brain Diseases A Neuroradiology, Nuclear Medicine, Neurosurgery, Neuropathology and Molecular Biology-Based Approach; Springer: Wien, Austria, 2019; pp. 427–442. [Google Scholar]
- Schultz, V.; Stern, R.A.; Tripodis, Y.; Stamm, J.; Wrobel, P.; Lepage, C.; Weir, I.; Guenette, J.P.; Chua, A.; Alosco, M.L.; et al. Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional american football players. J. Neurotrauma 2018, 35, 278–285. [Google Scholar] [CrossRef]
- Lee, J.K.; Wu, J.; Banks, S.; Bernick, C.; Massand, M.G.; Modic, M.T.; Ruggieri, P.; Jones, S.E. Prevalence of traumatic findings on routine MRI in a large cohort of professional fighters. AJNR Am. J. Neuroradiol. 2017, 38, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koerte, I.K.; Mayinger, M.; Muehlmann, M.; Kaufmann, D.; Lin, A.P.; Steffinger, D.; Fisch, B.; Rauchmann, B.S.; Immler, S.; Karch, S.; et al. Cortical thinning in former professional soccer players. Brain Imaging Behav. 2016, 10, 792–798. [Google Scholar] [CrossRef]
- Stone, J.R.; Avants, B.B.; Tustison, N.J.; Wassermann, E.M.; Gill, J.; Polejaeva, E.; Dell, K.C.; Carr, W.; Yarnell, A.M.; LoPresti, M.L.; et al. Functional and structural neuroimaging correlates of repetitive low-level blast exposure in career breachers. J. Neurotrauma 2020, 37, 2468–2481. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zhu, T.; Zhong, J.; Janigro, D.; Rozen, E.; Roberts, A.; Javien, H.; Merchant-Borna, K.; Abar, B.; Blackman, E.G. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS ONE 2014, 9, e94734. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Wu, J.; Bullen, J.; Banks, S.; Bernick, C.; Modic, M.T.; Ruggieri, P.; Bennett, L.; Jones, S.E. Association of cavum septum pellucidum and cavum vergae with cognition mood; and brain volumes in professional fighters. JAMA Neurol. 2020, 77, 35–42. [Google Scholar] [CrossRef]
- Koerte, I.K.; Hufschmidt, J.; Muehlmann, M.; Tripodis, Y.; Stamm, J.M.; Pasternak, O.; Giwerc, M.Y.; Coleman, M.J.; Baugh, C.M.; Fritts, N.G.; et al. Cavum septi pellucidi in symptomatic former professional football players. J. Neurotrauma 2016, 33, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Multani, N.; Goswami, R.; Khodadadi, M.; Ebraheem, A.; Davis, K.D.; Tator, C.H.; Wennberg, R.; Mikulis, D.J.; Ezerins, L.; Tartaglia, M.C. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J. Neurol. 2016, 263, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Herweh, C.; Hess, K.; Meyding-Lamadé, U.; Bartsch, A.J.; Stippich, C.; Jost, J.; Friedmann-Bette, B.; Heiland, S.; Bendszus, M.; Hähnel, S. Reduced white matter integrity in amateur boxers. Neuroradiology 2016, 58, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Koerte, I.K.; Ertl-Wagner, B.; Reiser, M.; Zafonte, R.; Shenton, M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 2012, 1859, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchill, N.W.; Caverzasi, E.; Graham, S.J.; Hutchison, M.G.; Schweizer, T.A. White matter during concussion recovery: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging NODDI. Hum. Brain Mapp. 2019, 40, 1908–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettwiler, A.; Murugavel, M.; Putukian, M.; Cubon, V.; Furtado, J.; Osherson, D. Persistent differences in patterns of brain activation after sports-related concussion: A longitudinal functional magnetic resonance imaging study. J. Neurotrauma 2014, 31, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Militana, A.R.; Donahue, M.J.; Sills, A.K.; Solomon, G.S.; Gregory, A.J.; Strother, M.K.; Morgan, V.L. Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: A pilot study. Brain Imaging Behav. 2016, 10, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Disrupted intrinsic connectivity among default, dorsal attention, and frontoparietal control networks in individuals with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 2016, 22, 263–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, L.E.; Möller, M.C.; Julin, P.; Bartfai, A.; Hashim, F.; Li, T.-Q. Post mTBI fatigue is associated with abnormal brain functional connectivity. Sci. Rep. 2016, 6, 21183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amyot, F.; Kenney, K.; Moore, C.; Haber, M.; Turtzo, L.C.; Shenouda, C.; Silverman, E.; Gong, Y.; Qu, B.X.; Harburg, L.; et al. Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 2018, 35, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Tripodis, Y.; Rowland, B.; Chua, A.S.; Liao, H.; Martin, B.; Jarnagin, J.; Chaisson, C.E.; Pasternak, O.; Karmacharya, S.; et al. A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging Behav. 2020, 14, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Ramadan, S.; Stern, R.A.; Box, H.C.; Nowinski, C.J.; Ross, B.D.; Mountford, C.E. Changes in the neurochemistry of athletes with repetitive brain trauma: Preliminary results using localized correlated spectroscopy. Alzheimers Res. Ther. 2015, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, M.H.; Beare, R.; Ditchfield, M.; Coleman, L.; Babl, F.E.; Kean, M.; Crossley, L.; Catroppa, C.; Yeates, K.O.; Anderson, V. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex 2013, 49, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, F.A.; Jordan, B.; Tikofsky, R.S.; Saxena, C.; Van Heertum, R.L.; Ichise, M. F-18 FDG PET imaging of chronic traumatic brain injury in boxers: A statistical parametric analysis. Nucl. Med. Commun. 2010, 31, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.A.; Song, Y.S.; Moon, B.S.; Lee, B.C.; Lee, H.Y.; Kim, J.M.; Kim, S.E. Neuropsychological, metabolic, and GABAA receptor studies in subjects with repetitive traumatic brain injury. J. Neurotrauma 2016, 33, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Meabon, J.S.R.; Huber, B.R.; Cross, D.J.; Richards, T.L.; Minoshima, S.; Pagulayan, K.F.; Li, G.; Meeker, K.D.; Kraemer, B.C.; Petrie, E.C.; et al. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 2016, 8, 321ra6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesman-Segev, O.H.; La Joie, R.; Stephens, M.L.; Sonni, I.; Tsai, R.; Bourakova, V.; Visani, A.V.; Edwards, L.; O’Neil, J.P.; Baker, S.L.; et al. Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. NeuroImage Clin. 2019, 24, 102025. [Google Scholar] [CrossRef] [PubMed]
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.D.; Montenigro, P.H.; Alvarez, V.E.; Xia, W.; Crary, J.F.; Tripodis, Y.; Daneshvar, D.H.; Mez, J.; Solomon, T.; Meng, G.; et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015, 130, 21–34. [Google Scholar] [CrossRef]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Thompson, P.W.; Ye, L.; Morgenstern, J.L.; Sue, L.; Beach, T.G.; Judd, D.J.; Shipley, N.J.; Libri, V.; Lockhart, A. Interaction of the amyloid imaging tracer FDDNP with hallmark Alzheimer’s disease pathologies. J. Neurochem. 2009, 109, 623–630. [Google Scholar] [CrossRef]
- Leung, K. 2-(4-(2-[(18)F]Fluoroethyl)piperidin-1-yl)benzo[4;5]imidazo[1,2-a]pyrimidine. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Agdeppa, E.D.; Kepe, V.; Liu, J.; Flores-Torres, S.; Satyamurthy, N.; Petric, A.; Cole, G.M.; Small, G.W.; Huang, S.C.; Barrio, J.R. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer’s disease. J. Neurosci. 2001, 21, Rc189. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Okamura, N.; Furumoto, S.; Tago, T.; Yanai, K.; Arai, H.; Kudo, Y. Characteristics of tau and its ligands in PET Imaging. Biomolecules 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Lejbman, N.; Jeromin, A.; French, L.M.; Kim, H.S.; Cashion, A.; Mysliwiec, V.; Diaz-Arrastia, R.; Gill, J. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015, 72, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Shahim, P.; Tegner, Y.; Marklund, N.; Höglund, K.; Portelius, E.; Brody, D.L.; Blennow, K.; Zetterberg, H. Astroglial activation and altered amyloid metabolism in human repetitive concussion. Neurology 2017, 88, 1400–1407. [Google Scholar] [CrossRef] [Green Version]
- Shahim, P.; Tegner, Y.; Gustafsson, B.; Gren, M.; Ärlig, J.; Olsson, M.; Lehto, N.; Engström, Å.; Höglund, K.; Portelius, E.; et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. 2016, 73, 1308–1315. [Google Scholar] [CrossRef] [Green Version]
- Sundman, M.; Doraiswamy, P.M.; Morey, R.A. Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: Implications for CTE. Front. Neurosci. 2015, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Karantali, E.; Kazis, D.; McKenna, J.; Chatzikonstantinou, S.; Petridis, F.; Mavroudis, I. Neurofilament light chain in patients with a concussion or head impacts: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2021. [Google Scholar] [CrossRef]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42, and IL-10 are associated with mild TBIs andchronic symptoms in military personnel. Brain Inj. 2018, 32, 1277–1284. [Google Scholar] [CrossRef]
- Stern, R.A.; Tripodis, Y.; Baugh, C.M.; Fritts, N.G.; Martin, B.M.; Chaisson, C.; Cantu, R.C.; Joyce, J.A.; Shah, S.; Ikezu, T.; et al. Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J. Alzheimers Dis. 2016, 51, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Alosco, M.L.; Tripodis, Y.; Jarnagin, J.; Baugh, C.M.; Martin, B.; Chaisson, C.E.; Estochen, N.; Song, L.; Cantu, R.C.; Jeromin, A.; et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2016, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kenney, K.; Qu, B.X.; Lai, C.; CENC Multisite Observational Study Investigators. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 2018, 32, 1276–1284. [Google Scholar] [CrossRef]
- Alosco, M.L.; Tripodis, Y.; Fritts, N.G.; Heslegrave, A.; Baugh, C.M.; Conneely, S.; Mariani, M.; Martin, B.M.; Frank, S.; Mez, J.; et al. Cerebrospinal fluid tau, Aβ, and sTREM2 in former National Football League players: Modelling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement. 2018, 14, 1159–1170. [Google Scholar] [CrossRef]
- Edwards, K.A.; Greer, K.; Leete, J.; Lai, C.; Devoto, C.; Qu, B.X.; Yarnell, A.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.L.; et al. Neuronally-derived tau is increased in experienced breachers and is associated with neurobehavioral symptoms. Sci. Rep. 2021, 11, 19527. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Baker, A.J.; Hare, G.M.T.; Reinders, F.X.; Schlichter, L.C.; Moulton, R.J. Association between Cerebrospinal Fluid Interleukin-6 Concentrations and Outcome after Severe Human Traumatic Brain Injury. J. Neurotrauma 2002, 19, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Hensler, T.; Sauerland, S.; Riess, P.; Hess, S.; Helling, H.J.; Andermahr, J.; Bouillon, B.; Neugebauer, E.A. The effect of additional brain injury on systemic interleukin (IL)-10 and IL-13 levels in trauma patients. Inflamm. Res. 2000, 49, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.M.; Lindell, A.; Murdock, K.R.; Kufera, J.A.; Menaker, J.; Keledjian, K.; Bochicchio, G.V.; Aarabi, B.; Scalea, T.M. Relationship of serum and cerebrospinal fluid biomarkers with intracranial hypertension and cerebral hypoperfusion after severe traumatic brain injury. J. Trauma 2011, 70, 1096–1103. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar] [CrossRef]
- Heyser, C.J.; Masliah, E.; Samimi, A.; Campbell, I.L.; Gold, L.H. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA 1997, 94, 1500–1505. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.J.; Tweedie, D.; Scerba, M.T.; Greig, N.H. Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments. Front. Cell Dev. Biol. 2019, 7, 313. [Google Scholar] [CrossRef]
- Cheng, Y.; Pereira, M.; Raukar, N.; Reagan, J.L.; Queseneberry, M.; Goldberg, L.; Borgovan, T.; LaFrance, W.C.; Dooner, M.; Deregibus, M.; et al. Potential biomarkers to detect traumatic brain injury by the profiling of salivary extracellular vesicles. J. Cell. Physiol. 2019, 234, 14377–14388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.M.; Jones, M.T.; Kirk, K.M.; Gable, D.A.; Repshas, J.T.; Johnson, T.A.; Andréasson, U.; Norgren, N.; Blennow, K.; Zetterberg, H. Serum Neurofilament Light in American Football Athletes over the Course of a Season. J. Neurotrauma 2016, 33, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Shahim, P.; Tegner, Y.; Wilson, D.H.; Randall, J.; Skillbäck, T.; Pazooki, D.; Kallberg, B.; Blennow, K.; Zetterberg, H. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014, 71, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Tanriverdi, F.; Unluhizarci, K.; Selcuklu, A.; Kelestimur, F.; Blennow, K. Sustained release of neuron-specific enolase to serum in amateur boxers. Brain Inj. 2009, 23, 723–726. [Google Scholar] [CrossRef]
- Siman, R.; Toraskar, N.; Dang, A.; McNeil, E.; McGarvey, M.; Plaum, J.; Maloney, E.; Grady, M.S. A panel of neuronenriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma 2009, 26, 1867–1877. [Google Scholar] [CrossRef]
- Siman, R.; Shahim, P.; Tegner, Y.; Blennow, K.; Zetterberg, H.; Smith, D.H.D. Serum SNTF increases in concussed professional ice hockey players and relates to the severity of post-concussion symptoms. J. Neurotrauma 2014, 32, 1294–1300. [Google Scholar] [CrossRef] [Green Version]
- McMahon, P.J.; Panczykowski, D.M.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Sorani, M.D.; Lingsma, H.F.; Maas, A.I.; Valadka, A.B.; Yuh, E.L.; et al. Measurement of the glial fibrillary acidic protein and its breakdown products GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging. J. Neurotrauma 2015, 32, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.M.; Dhawan, S.; Obirieze, A.C.; Sarno, B.; Akers, J.; Heller, M.J.; Chen, C.C. Plasma Biomarker for Post-concussive Syndrome: A Pilot Study Using an Alternating Current Electro-Kinetic Platform. Front. Neurol. 2020, 11, 685. [Google Scholar] [CrossRef]
- McCrea, M.; Broglio, S.P.; McAllister, T.W.; Gill, J.; Giza, C.C.; Huber, D.L.; Harezlak, J.; Cameron, K.L.; Houston, M.N.; McGinty, G.; et al. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: Findings From the NCAA and department of defense CARE consortium. JAMA Netw. Open 2020, 3, e1919771. [Google Scholar] [CrossRef]
- Siman, R.; Giovannone, N.; Hanten, G.; Wilde, E.A.; McCauley, S.R.; Hunter, J.V.; Li, X.; Levin, H.S.; Smith, D.H. Evidence That the Blood Biomarker SNTF Predicts Brain Imaging Changes and Persistent Cognitive Dysfunction in Mild TBI Patients. Front. Neurol. 2013, 4, 190. [Google Scholar] [CrossRef] [Green Version]
- Di Battista, A.P.; Churchill, N.; Rhind, S.G.; Richards, D.; Hutchison, M.G. Evidence of a distinct peripheral inflammatory profile in sport-related concussion. J. Neuroinflamm. 2019, 16, 17. [Google Scholar] [CrossRef] [PubMed]
| Disorder | Classification System | Stages | Diagnostic Criteria |
|---|---|---|---|
| Concussion [36] | Nelson Grading system | • Grade 0 | not stunned or dazed; headache, difficult concentration; |
| • Grade 1 | stunned or dazed; no LOC or PTA; sensorium recovery < 1 min; | ||
| • Grade 2 | headache; sensorium recovery > 1 min; no LOC; tinnitus, amnesia, irritability, hyperexcitability, confusion, dizziness; | ||
| • Grade 3 | LOC < 1 min; no coma; grade 2 symptoms during recovery; | ||
| • Grade 4 | LOC > 1 min; no coma; demonstrates grade 2 symptoms during recovery | ||
| Ommaya grading system | • Grade 1 | Confusion; no PTA; | |
| • Grade 2 | PTA without coma; | ||
| • Grade 3 | Coma < 6 h | ||
| • Grade 4 | Coma = 6–24 h | ||
| • Grade 5 | Comas > 24 h | ||
| • Grade 6 | Coma, death within 24 h | ||
| Jordan grading system | • Grade 1 | Confusion; no PTA, LOC; | |
| • Grade 2 | Confusion; PTA < 24 h; no LOC; | ||
| • Grade 3 | LOC (altered level of consciousness < 2–3 min); PTA < 24 h; | ||
| • Grade 4 | LOC (altered level of consciousness > 2–3 min); | ||
| Torg grading system | • Grade 1 | Tinnitus; short-term confusion; dazed; no PTA; | |
| • Grade 2 | PTA; vertigo; no LOC; | ||
| • Grade 3 | PTA retrograde; vertigo; no LOC; | ||
| • Grade 4 | Immediate transient LOC; | ||
| • Grade 5 | Paralytic coma; cardiorespiratory arrest; | ||
| • Grade 6 | Death | ||
| Colorado Medical Society guidelines | • Mild | Confusion; no PTA, LOC; | |
| • Moderate | Confusion; PTA; no LOC; | ||
| • Severe | LOC. | ||
| Cantu grading system | • Mild | No LOC; PTA < 30 min; | |
| • Moderate | LOC < 5 min; PTA > 30 min; | ||
| • Severe | LOC > 5 min or PTA > 24 h. | ||
| Roberts grading system | • Bell ringer | No LOC, PTA; recovery < 10 min; | |
| • Mild | No LOC; PTA < 30 min; recovery > 10 min; | ||
| • Moderate | LOC < 5 min; PTA > 30 min; | ||
| • Severe | LOC > 5 min; PTA > 24 h. | ||
| Kelly and Rosenberg grading system | • Mild | Transient confusion; no LOC; symptoms resolve in < 15 min; | |
| • Moderate | Transient confusion; no LOC; symptoms last > 15 min; | ||
| • Severe | Brief or prolonged LOC. | ||
| Traumatic brain injury (TBI) | Glasgow Coma Scale (GCS) [37] | • Mild | CGS score = 13–15 |
| • Moderate | CGS score = 9–12 | ||
| • Severe | CGS score = 3–8 | ||
| PTA Mississippi intervals [38,39] | • Moderate | PTA 1–24 h | |
| • Severe | PTA > 24 h | ||
| Mayo system [40] | • Possible | Blurred vision, confusion (mental state changes), dazed, dizziness, focal neurologic symptoms, headache, nausea | |
| • Probable—mild | Loss of consciousness < 30 min, PTA < 24 h, skull fracture (dura intact) | ||
| • Definite—moderate/severe | Loss of consciousness > 30 min, PTA > 24 h, CGS score (first 24 h) < 13, skull fracture (with hematoma, hemorrhage, concussion, or brain stem injury), death | ||
| Glasgow Outcome Scale [33] | • Dead | ||
| • Vegetative state | Lack of function in the cerebral cortex, although structurally intact | ||
| • Severe disability | Conscious, total dependency on caregiver (severe physical and mental disability) | ||
| • Moderate disability | Independent, but disabled (physical and mental disability) | ||
| • Good recovery | Minor physical and mental disability | ||
| Russell and Smith’s classification system [41] | • Severe | PTA = 1–7 days | |
| • Very severe | PTA = +7 days | ||
| Nakase–Richardson classification system [38] | • Moderate | PTA = 0–14 days | |
| • Moderately severe | PTA = 15–28 days | ||
| • Severe | PTA = 29–70 days | ||
| Post-concussion syndrome (PCS) | Ontario Neurotrauma Foundation [42,43] | Symptoms according to ICD10 or DSM-V | |
| • Minor | Symptoms duration = 1–6 months | ||
| • Persistent | Symptoms duration > 6 months | ||
| Traumatic encephalopathy syndrome (TES)/Chronic Traumatic Encephalopathy (CTE) | Jordan classification system [44] | • Improbable CTE | Pathophysiological process unrelated to brain trauma; |
| • Possible CTE | CTE clinical description also seen in other neuropathologies; | ||
| • Probable CTE | Cognitive and/or behavioral impairment; morpho-functional changes in central nervous system; | ||
| • Definite CTE | CTE clinical presentation and pathological confirmation. | ||
| Montenigro clasiffication system [27] | • Behavioural/mood variant | Behavioural and mood features; | |
| • Cognitive variant | Cognitive impairment; | ||
| • Mixed variant | Both behavioural and cognitive impairments; | ||
| • Dementia variant | Progressive cognitive decline dependent or independent of Alzheimer’s disease diagnostic. | ||
| Omalu neuropathological classification [45] | • Phenotype I | +NFTs and neuritic threads (cerebral cortex and brainstem) −diffuse amyloid plaques | |
| • Phenotype II | +NFTs and neuritic threads (cerebral cortex and brainstem) | ||
| • Phenotype III | −diffuse amyloid plaques (cerebral cortex) | ||
| • Phenotype IV | +NFTs and neuritic threads (brainstem) −diffuse amyloid plaques −NFTs and neuritic threads −diffuse amyloid plaques |
| Method | Disorder | Observable Features | Clinical Studies |
|---|---|---|---|
| Magnetic Resonance Imaging (MRI) | PCS, CTE | White matter injury, focal concussions, haemorrhages | • 86 symptomatic former NFL players—the decreased amygdala, hippocampus, and cingulate gyrus volumes [57,60] • 33 male Canadian football players—changes in the microstructural integrity of the white matter (anterior and posterior regions of the corpus callosum) [58] • 476 active and retired professional fighters and 63 controls—the presence of cavum septum pellucidum and cavum vergae, lower brain volumes in the supratentorium [61] • 15 former male professional soccer players and 15 male, age-matched former professional non-contact sport athletes-right inferolateral-parietal, temporal, and occipital cortex thinning [62] • 20 current or previous military or civilian law enforcement breachers and 14 controls—increased cortical thickness (occipital lobes) [63] • 10 Division III college football players and five non-athlete controls-changes in fractional anisotropy and mean diffusivity (white matter) [64] • 499 fighters (boxers, mixed martial artists, and martial artists) and 62 controls-increased prevalence of cavum septum pellucidum and cerebral microhemorrhages among fighters [65] • 72 symptomatic former professional football players and 14 former professional noncontact sports athletes-presence of cavum septum pellucidum [66] |
| Diffusion tensor imaging (DTI) | TBI, RMHI | Asymptomatic head trauma concussion, mild traumatic brain injuries (cortical and subcortical microstructures) | • 18 retired professional football players and 17 healthy controls’chronic axonal degeneration (superior longitudinal fasciculus, corticospinal tract, and anterior thalamic radiations) [67] |
| Diffusion tensor imaging | TBI, RMHI, CTE | Random Brownian motion of water molecules within a voxel of tissue, cellular swelling, grey matter status (cerebral cortex nuclei) | • 31 amateur boxers and 31 control individuals—reduced fractional anisotropy, increased diffusivity along central white matter tracts [68] • 12 soccer players and 11 swimmers—increased radial diffusivity and axial diffusivity in soccer players [69] • 10 Division III college football players and five non-athlete controls—up to 6 months persistent white matter changes [64] • 18 retired professional football players and 17 healthy controls—changes in axial diffusivity [67] |
| Neurite orientation dispersion and density imaging (NODDI) | PCS | Acute alterations in microstructure (neurite density and orientation, axons and dendrites) | • 31 concussed athletes and 27 matched controls - reductions in fractional anisotropy and increased axial and radial diffusivity, increased neurite dispersion [70] |
| Functional MRI | TBI | brain activity (changes associated with blood flow) | • 15 varsity level college students who sustained a sports-related concussion and 15 age and sex-matched controls—increased activation of prefrontal area (BA46, BA10) and left inferior parietal (supramarginal) gyrus (BA40) [71] • Seven college athletes and 11 healthy controls—increased cerebrovascular reactivity in all evaluated regions and independently in anterior cingulate and the right thalamus and increased focal connectivity in left and right hippocampus, precuneus and ventromedial prefrontal cortex [72] • 40 chronic TBI individuals and 17 healthy individuals—reduced connectivity in TBI individuals in the default mode network, dorsal attention network, and frontoparietal control network and in between interactions [73] • 10 mild TBI patients and 10 healthy controls—decreased functional connectivity networks in thalamus, dorsal anterior cingulate and medial frontal gyri [74] • 27 TBI patients and 15 healthy controls—asymmetry in cerebrovascular reactivity and cerebral blood flow maps in TBI (multifocal pattern of deficits) [75] |
| Magnetic Spectroscopy (MRS) | TBI, RMHI | Human brain metabolism in vivo | • 77 symptomatic former NFL players and 23 asymptomatic individuals without a head trauma history—significantly lower N-acetyl aspartate level in the parietal white matter [76] • Five former professional male athletes and five healthy men—increased levels of glutamine/glutamate, choline, fucosylated molecules, and phenylalanine [77] |
| Susceptibility weighted imaging (SWI) | TBI | Hemorrhage, microbleeds in the brain | • 106 children with TBI and 43 healthy controls—increased number and volume of lesions in TBI group, predominantly in the frontal, extra frontal, deep grey, and cerebellum regions [78] |
| Positron Emission Tomography (PET) FDG-PET Tau-PET Aβ-PET | TBI, CTE | Severity and distribution of brain changes (altered synaptic activity) | • 19 boxers and 17 controls—altered activity in the posterior cingulate cortex, parieto-occipital, frontal lobes (Broca’s area) bilaterally, and the cerebellum [79] • Five retired professional boxers and four age-matched controls—neuronal deficits in angular gyrus and temporal cortical regions [80] • 33 veterans with mild TBI—lower cerebellar metabolism [81] • 11 Traumatic Encephalopathy Syndrome patients—elevated tau-PET binding, lower gray matter volumes in frontotemporal areas [82] • 202 football players—p-tau clusters in medial temporal lobe, cerebral cortex, diencephalon, and brain stem [83] • 114 CTE-diagnosed deceased athletes and military veterans—diffuse or neuritic Aβ deposition present in 52 % of CTE subjects [84] |
| Biomarker | Disorder | Observable Features | Clinical Studies |
|---|---|---|---|
| Serum neurofilament light polypeptide (NFL) | TBI | axonal injury | • 19 American football athletes and 19 swim athletes—increased levels of NFL in football athletes, not normalizing even after nine months following TBI [108] |
| Serum S-100 calcium-binding protein B and neuron-specific enolase (NSE) | PCS | astroglial injury general neuronal injury | • 47 preseason and 28 PCS professional ice hockey players—increased S100B in PCS, no changes in NSE levels; levels of S-100B and NSE also increasing following physical effort [109] • 44 amateur boxers and 23 healthy controls—increased serum NSE in amateur boxers, no changes in S100B levels [110] |
| Serum neurofilament H and SNTF | TBI | stretch injury of neuronal axons | • Nine TBI and three controls—increased SNTF and NFH in sera of TBI patients receiving surgical brain pressure release [111] • 28 concussed and 45 non-concussed professional ice hockey players—diagnostic accuracy increased levels of SNTF in concussed athletes [112] |
| Serum GFAP | TBI | astrocytic response to neuronal damage | • 215 acute TBI patients—increased serum GFAP levels following acute TBI [113] |
| Serum IL6, IL8 and TNF-α | TBI | neuroinflammation | • 24 TBI patients—increased serum IL6, IL8, and TNF- α in possible correlation with poor outcome and subsequent additional insults (brain damage) [103] |
| Exosomal tau | TBI | neuronal loss and neurodegeneration | • 98 veterans with mild TBI with PTA or LOC, 52 with mild TBI without PTA or LOC and 45 without TBI—increased plasma and exosomal tau, p-tau significantly correlated to post-concussive symptoms [98] |
| PCS | • 20 current or previous military or civilian law enforcement breachers ad 14 controls—neuronal-derived extracellular vesicles (serum) tau levels increased and correlated Neurobehavioral Symptom Inventory score, in experienced breachers [100] • 42 PCS military personnel and 22 without PCS—increased concentrations of exosomal tau, Aβ42, and IL-10 [95] | ||
| CTE | • 78 former National Football League players and 16 controls—the increased presence of tau-positive extracellular vesicles in former NFL players, as compared to controls [96] | ||
| Plasma total tau | PCS | axonal injury | • 70 participants with self-reported TBI compared with the 28 controls—increased plasmatic total tau levels [90] • 47 preseason and 28 PCS professional ice hockey players—increased PCS levels, as compared to preseason evaluation [109] |
| RHI | • 96 former NFL players and 25 same-age controls - total-tau plasma concentration ≥ 3.56 pg/mL was specific to repetitive head impact individuals [97] | ||
| Plasma UCH-L1, GFAP, Tau | TBI | neuronal damage | • 27 TBI and six controls—increased UCH-L1 in oen TBI patient with abnormal CT scan [114] • 264 contact sport and 138 non-contact sport controls—increased plasma UCH-L1 levels in concussed athletes [115] |
| Plasmatic calpain-cleaved SNTF | TBI | acute brain damage, neurodegeneration | • 38 participants—increased plasma calpain-cleaved SNTF in TBI and some orthopedic cases, as compared to uninjured controls [116] |
| Plasma MCP-4 and MCP-1β | TBI | neuroinflammation | • 43 TBI athletes and 102 control athletes—increased blood levels of MCP-4 and MIP-1β [117] |
| Plasma IL1 and IL6 | TBI | neuroinflammation | • 37 severe TBI patients—increased CSF IL1 and IL6 levels in correlation to TBI severity (Glasgow Outcome Scale) [101] |
| Plasma IL10 | TBI | neuroinflammation | • 82 severe head trauma patients, 39 multiple injuries patients, and 37 healthy donors—increased systemic levels of IL10 following multiple injuries, without possibility to discriminate between head and non-head trauma [102] |
| CSF GFAP, YKL-40, and amyloid β40, β42 | PCS | astroglia injury | • 28 professional athletes with PCS and 19 controls—increased glial fibrillary acidic protein (GFAP) and YKL-40, lower Aβ40 and Aβ42 levels [91] |
| CSF NF light protein, amyloid β | PCS | axonal injury | • 16 ice hockey players with PCS and 15 control individuals—increased NF light protein and decreased amyloid β CSF levels [92] |
| CSF total tau level | TBI | axonal injury | • 68 former NFL players and 21 controls—higher CSF t-tau levels correlated with cumulative head impact index in NFL players [99] |
| CSF sTREM2 | TBI | microglial activation | • 68 former NFL players and 21 controls—increased sTREM2 levels in repeatedly concussed individuals, significantly associated with increased CSF T-tau levels [99] |
| CSF IL1 and IL6 | TBI | neuroinflammation | • 37 severe TBI patients—increased CSF IL1 and IL6 levels in correlation to TBI severity (Glasgow Outcome Scale) [101] |
| Salivary extracelluar vesicles | TBI/CTE | cell membrane damage | • 31 concussion trauma patients and 23 controls—many Alzheimer’s disease relevant salivary biomarkers isolated from extracellular vesicles were found to be expressed in concussed patients [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.-M.; Ciobica, A.; Luca, A.-C.; Radu, I.; Dobrin, R.P.; et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics 2022, 12, 740. https://doi.org/10.3390/diagnostics12030740
Mavroudis I, Kazis D, Chowdhury R, Petridis F, Costa V, Balmus I-M, Ciobica A, Luca A-C, Radu I, Dobrin RP, et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics. 2022; 12(3):740. https://doi.org/10.3390/diagnostics12030740
Chicago/Turabian StyleMavroudis, Ioannis, Dimitrios Kazis, Rumana Chowdhury, Foivos Petridis, Vasiliki Costa, Ioana-Miruna Balmus, Alin Ciobica, Alina-Costina Luca, Iulian Radu, Romeo Petru Dobrin, and et al. 2022. "Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers" Diagnostics 12, no. 3: 740. https://doi.org/10.3390/diagnostics12030740
APA StyleMavroudis, I., Kazis, D., Chowdhury, R., Petridis, F., Costa, V., Balmus, I.-M., Ciobica, A., Luca, A.-C., Radu, I., Dobrin, R. P., & Baloyannis, S. (2022). Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics, 12(3), 740. https://doi.org/10.3390/diagnostics12030740










