An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer?
Abstract
1. Introduction
2. iOCT in Anterior Segment Surgeries
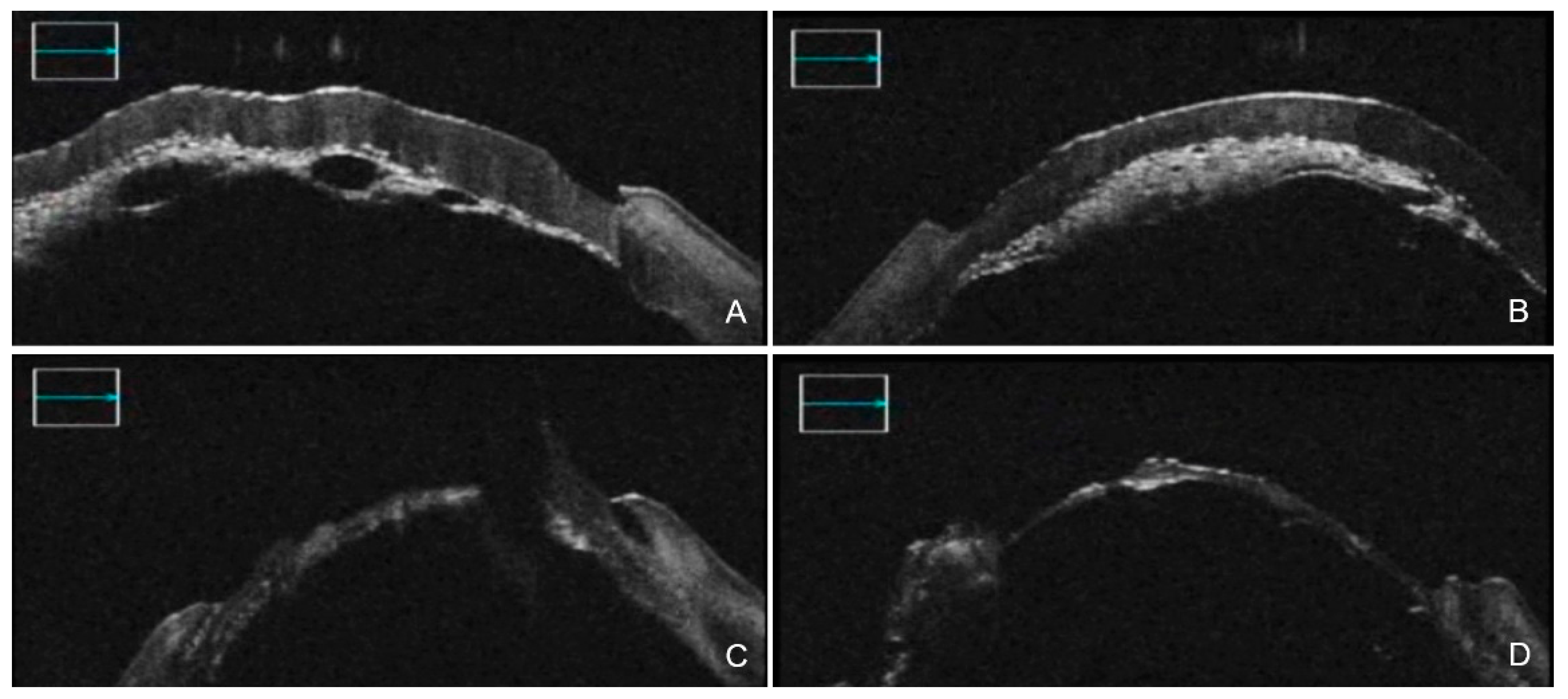
3. iOCT-Assisted DALK
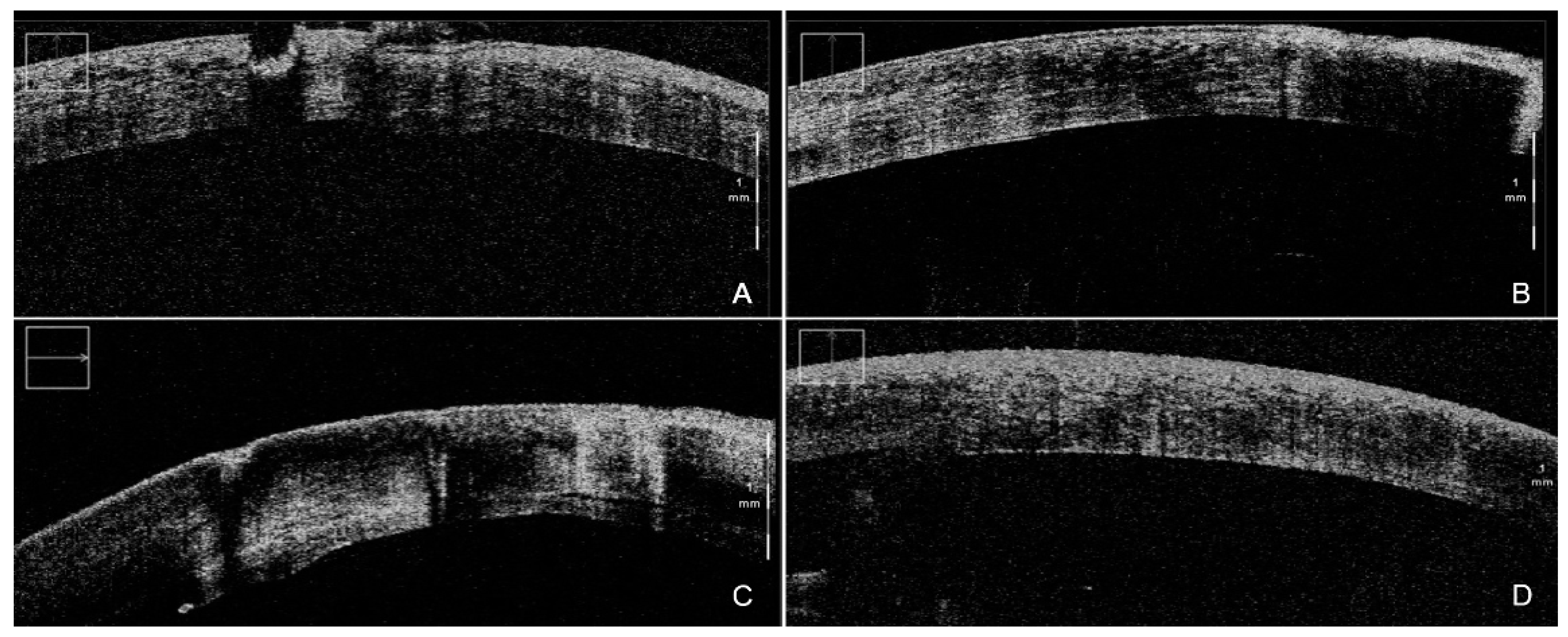
4. iOCT-Assisted Endothelial Keratoplasty
4.1. iOCT-Aided DSAEK
4.2. iOCT-Aided DMEK
5. Limitations, Strengths and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.B.; Jacobs, D.S.; Musch, D.C.; Kaufman, S.C.; Reinhart, W.J.; Shtein, R.M. Descemet’s stripping endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology 2009, 116, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology 2013, 120, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Wang, P.; Li, B.; Wang, W.; Su, Y.; Sheng, M. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: A meta-analysis. PLoS ONE 2015, 10, e0113332. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, CD012097. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch. Ophthalmol. Chic. 2001, 119, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Muller, M.; Winter, C.; Hoerauf, H.; Oelckers, S.; Laqua, H.; Birngruber, R. Intraoperative 2-dimensional optical coherence tomography as a new tool for anterior segment surgery. Arch. Ophthalmol. Chic. 2005, 123, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Wang, J.H.; Tao, A.Z.; Leng, T.; Kymionis, G.D.; O’Brien, T.P.; Yoo, S.H. Intraoperative Use of Three-Dimensional Spectral-Domain Optical Coherence Tomography. Ophthal. Surg. Las. Imaging Retin. 2010, 41, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Knecht, P.B.; Kaufmann, C.; Menke, M.N.; Watson, S.L.; Bosch, M.M. Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am. J. Ophthalmol. 2010, 150, 360–365.e2. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am. J. Ophthalmol. 2014, 158, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Goshe, J.; Dupps, W.J.; Kaiser, P.K.; Singh, R.P.; Gans, R.; Eisengart, J.; Srivastava, S.K. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015, 133, 1124–1132. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. Chic. 1994, 112, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Just, T.; Lankenau, E.; Huttmann, G.; Pau, H.W. Intra-operative application of optical coherence tomography with an operating microscope. J. Laryngol. Otol. 2009, 123, 1027–1030. [Google Scholar] [CrossRef]
- Siebelmann, S.; Steven, P.; Cursiefen, C. Intraoperative Optical Coherence Tomography Ocular Surgery on a Higher Level or Just Nice Pictures? JAMA Ophthalmol. 2015, 133, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.K.; Ehlers, J.P.; Toth, C.A.; Izatt, J.A. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt. Lett 2010, 35, 3315–3317. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Tao, Y.K.; Srivastava, S.K. The value of intraoperative OCT imaging in vitreoretinal surgery. Curr. Opin. Ophthalmol. 2014, 25, 221. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Berrocal, A.M.; Schefler, A.C.; Uhhorn, S.R.; Ruggeri, M.; Hess, D. Intraoperative OCT of a Full-Thickness Macular Hole Before and After Internal Limiting Membrane Peeling. Ophthal. Surg. Las. Imaging Retina 2010, 41, 7–11. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Ohr, M.P.; Kaiser, P.K.; Srivastava, S.K. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina 2013, 33, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Kusaka, S.; Arimura-Koike, E.; Tachibana, K.; Tsujioka, D.; Fukuda, M.; Shimomura, Y. Intraoperative optical coherence tomography (RESCAN (R) 700) for detecting iris incarceration and iridocorneal adhesion during keratoplasty. Int. Ophthalmol. 2017, 37, 761–765. [Google Scholar] [CrossRef] [PubMed]
- AlTaan, S.L.; Termote, K.; Elalfy, M.S.; Hogan, E.; Werkmeister, R.; Schmetterer, L.; Holland, S.; Dua, H.S. Optical coherence tomography characteristics of different types of big bubbles seen in deep anterior lamellar keratoplasty by the big bubble technique. Eye 2016, 30, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Le Blanc, C.; Velten, K.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huttmann, G.; Cursiefen, C. Optimizing Descemet Membrane Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. JAMA Ophthalmol. 2013, 131, 1135–1142. [Google Scholar] [CrossRef]
- Steven, P.; Le Blanc, C.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huettmann, G.; Cursiefen, C. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). Br. J. Ophthalmol. 2014, 98, 900–904. [Google Scholar] [CrossRef]
- Au, J.; Goshe, J.; Dupps, W.J., Jr.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography for Enhanced Depth Visualization in Deep Anterior Lamellar Keratoplasty from the PIONEER Study. Cornea 2015, 34, 1039–1043. [Google Scholar] [CrossRef][Green Version]
- Sharma, N.; Aron, N.; Kakkar, P.; Titiyal, J.S. Continuous intraoperative OCT guided management of post-deep anterior lamellar keratoplasty descemet’s membrane detachment. Saudi J. Ophthalmol. 2016, 30, 133–136. [Google Scholar] [CrossRef]
- Titiyal, J.S.; Kaur, M.; Falera, R.; Jose, C.P.; Sharma, N. Evaluation of Time to Donor Lenticule Apposition Using Intraoperative Optical Coherence Tomography in Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2016, 35, 477–481. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yokogawa, H.; Mori, N.; Sugiyama, K. Visualization of precut DSAEK and pre-stripped DMEK donor corneas by intraoperative optical coherence tomography using the RESCAN 700. BMC Ophthalmol. 2016, 16, 135. [Google Scholar] [CrossRef]
- Saad, A.; Guilbert, E.; Grise-Dulac, A.; Sabatier, P.; Gatinel, D. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea 2015, 34, 802–807. [Google Scholar] [CrossRef]
- Xu, D.; Dupps, W.J.; Srivastava, S.K.; Ehlers, J.P. Automated Volumetric Analysis of Interface Fluid in Descemet Stripping Automated Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. Investig. Ophth. Vis. Sci. 2014, 55, 5610–5615. [Google Scholar] [CrossRef]
- Titiyal, J.S.; Kaur, M.; Falera, R. Intraoperative optical coherence tomography in anterior segment surgeries. Indian J. Ophthalmol. 2017, 65, 116–121. [Google Scholar] [CrossRef]
- Heindl, L.M.; Siebelmann, S.; Dietlein, T.; Huttmann, G.; Lankenau, E.; Cursiefen, C.; Steven, P. Future prospects: Assessment of intraoperative optical coherence tomography in ab interno glaucoma surgery. Curr. Eye Res. 2015, 40, 1288–1291. [Google Scholar] [CrossRef]
- Han, D.C.; Mehta, J.S.; Por, Y.M.; Htoon, H.M.; Tan, D.T. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am. J. Ophthalmol. 2009, 148, 744–751.e741. [Google Scholar] [CrossRef] [PubMed]
- Riss, S.; Heindl, L.M.; Bachmann, B.O.; Kruse, F.E.; Cursiefen, C. Pentacam-based big bubble deep anterior lamellar keratoplasty in patients with keratoconus. Cornea 2012, 31, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Teichmann, K.D. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J. Cataract Refract. Surg. 2002, 28, 398–403. [Google Scholar] [CrossRef]
- Dua, H.S.; Katamish, T.; Said, D.G.; Faraj, L.A. Differentiating type 1 from type 2 big bubbles in deep anterior lamellar keratoplasty. Clin. Ophthalmol. 2015, 9, 1155–1157. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, X.Y.; Wang, Y.; Zhang, H.; Hu, A.D.; Gao, N. Outcomes of Deep Anterior Lamellar Keratoplasty Using the Big-Bubble Technique in Various Corneal Diseases. Am. J. Ophthalmol. 2012, 154, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M. Common complications of deep lamellar keratoplasty in the early phase of the learning curve. Clin. Ophthalmol. 2011, 5, 791–795. [Google Scholar] [CrossRef][Green Version]
- Siebelmann, S.; Steven, P.; Cursiefen, C. Intraoperative Optical Coherence Tomography in Deep Anterior Lamellar Keratoplasty. Klin. Monbl. Augenheilkd. 2016, 233, 717–721. [Google Scholar] [PubMed]
- Braun, J.M.; Hofmann-Rummelt, C.; Schlotzer-Schrehardt, U.; Kruse, F.E.; Cursiefen, C. Histopathological changes after deep anterior lamellar keratoplasty using the “big-bubble technique”. Acta Ophthalmol. 2013, 91, 78–82. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wittwer, V.V.; Yusoff, N.Z.M.; Lwin, C.N.; Seah, X.Y.; Mehta, J.S.; Seiler, T. Intraoperative Optical Coherence Tomography-Guided Femtosecond Laser-Assisted Deep Anterior Lamellar Keratoplasty. Cornea 2019, 38, 648–653. [Google Scholar] [CrossRef]
- Buzzonetti, L.; Laborante, A.; Petrocelli, G. Standardized big-bubble technique in deep anterior lamellar keratoplasty assisted by the femtosecond laser. J. Cataract Refract. Surg. 2010, 36, 1631–1636. [Google Scholar] [CrossRef]
- Santorum, P.; Yu, A.C.; Bertelli, E.; Busin, M. Microscope-Integrated Intraoperative Optical Coherence Tomography-Guided Big-Bubble Deep Anterior Lamellar Keratoplasty. Cornea 2022, 41, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahay, P.; Maharana, P.K.; Kumar, P.; Ahsan, S.; Titiyal, J.S. Microscope Integrated Intraoperative Optical Coherence Tomography-Guided DMEK in Corneas with Poor Visualization. Clin. Ophthalmol. 2020, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study: First 100 Cases. Am. J. Ophthalmol. 2020, 210, 167–173. [Google Scholar] [CrossRef]
- Hsu, M.; Hereth, W.L.; Moshirfar, M. Double-pass microkeratome technique for ultra-thin graft preparation in Descemet’s stripping automated endothelial keratoplasty. Clin. Ophthalmol. 2012, 6, 425–432. [Google Scholar] [PubMed]
- Sharma, N.; Sharma, V.K.; Arora, T.; Singh, K.R.; Agarwal, T.; Vajpayee, R.B. Novel Technique for Descemet Membrane Remnant Stripping in Hazy Cornea During DSAEK. Cornea 2016, 35, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Juthani, V.V.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. The association between transient interface fluid on intraoperative OCT and textural interface opacity following DSAEK surgery in the PIONEER Study. Cornea 2014, 33, 887. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, A.; Ozaki, H.; Otsuka, M.; Hayashi, A. Efficacy of Intraoperative Anterior Segment Optical Coherence Tomography during Descemet’s Stripping Automated Endothelial Keratoplasty. ISRN Ophthalmol. 2014, 2014, 562062. [Google Scholar] [CrossRef][Green Version]
- Wylegala, E.; Nowinska, A.K.; Wroblewska-Czajka, E.; Janiszewska, D. Donor disc attachment assessment with intraoperative spectral optical coherence tomography during descemet stripping automated endothelial keratoplasty. Indian J. Ophthalmol. 2013, 61, 511–513. [Google Scholar] [PubMed]
- Hallahan, K.M.; Cost, B.; Goshe, J.M.; Dupps, W.J., Jr.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Interface Fluid Dynamics and Clinical Outcomes for Intraoperative Optical Coherence Tomography-Assisted Descemet Stripping Automated Endothelial Keratoplasty from the PIONEER Study. Am. J. Ophthalmol. 2017, 173, 16–22. [Google Scholar] [CrossRef][Green Version]
- Steverink, J.G.; Wisse, R.P.L. Intraoperative optical coherence tomography in descemet stripping automated endothelial keratoplasty: Pilot experiences. Int. Ophthalmol. 2017, 37, 939–944. [Google Scholar] [CrossRef]
- Asif, M.I.; Bafna, R.K.; Sharma, N.; Kaginalkar, A.; Sinha, R.; Agarwal, T.; Maharana, P.K.; Kaur, M.; Taank, P.; Titiyal, J.S. Microscope Integrated Optical Coherence Tomography Guided Descemet Stripping Automated Endothelial Keratoplasty in Congenital Hereditary Endothelial Dystrophy. Clin. Ophthalmol. 2021, 15, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, M.; Panda, A.; Vengayil, S.; Chaudhuri, Z.; Dada, T. Pediatric keratoplasty. Surv. Ophthalmol. 2009, 54, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Pineda, R.; Jain, V.; Shome, D.; Hunter, D.C.; Natarajan, S. Descemet’s stripping endothelial keratoplasty: Is it an option for congenital hereditary endothelial dystrophy? Int. Ophthalmol. 2010, 30, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Melles, G.R.; Ong, T.S.; Ververs, B.; van der Wees, J. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006, 25, 987–990. [Google Scholar]
- Tourtas, T.; Laaser, K.; Bachmann, B.O.; Cursiefen, C.; Kruse, F.E. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am. J. Ophthalmol. 2012, 153, 1082–1090.e1082. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Giebel, A.W.; Fairchild, K.M.; Price, F.W., Jr. Descemet’s membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Anshu, A.; Price, M.O.; Price, F.W. Risk of Corneal Transplant Rejection Significantly Reduced with Descemet’s Membrane Endothelial Keratoplasty. Ophthalmology 2012, 119, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.A. Endothelial keratoplasty: Why aren’t we all doing Descemet membrane endothelial keratoplasty? Cornea 2012, 31, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Cost, B.; Goshe, J.M.; Srivastava, S.; Ehlers, J.P. Intraoperative optical coherence tomography-assisted descemet membrane endothelial keratoplasty in the DISCOVER study. Am. J. Ophthalmol. 2015, 160, 430–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bachmann, B.O.; Laaser, K.; Cursiefen, C.; Kruse, F.E. A method to confirm correct orientation of descemet membrane during descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2010, 149, 922–925.e922. [Google Scholar] [CrossRef] [PubMed]
- Veldman, P.B.; Dye, P.K.; Holiman, J.D.; Mayko, Z.M.; Sales, C.S.; Straiko, M.D.; Galloway, J.D.; Terry, M.A. The S-stamp in Descemet Membrane Endothelial Keratoplasty Safely Eliminates Upside-down Graft Implantation. Ophthalmology 2016, 123, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sáles, C.S.; Terry, M.A.; Veldman, P.B.; Mayko, Z.M.; Straiko, M.D. Relationship between tissue unscrolling time and endothelial cell loss. Cornea 2016, 35, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Lee, W.B.; Hammersmith, K.M.; Kuo, A.N.; Li, J.Y.; Shen, J.F.; Weikert, M.P.; Shtein, R.M. Descemet Membrane Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology 2018, 125, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Muijzer, M.B.; Soeters, N.; Godefrooij, D.A.; van Luijk, C.M.; Wisse, R.P.L. Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty: Toward More Efficient, Safer Surgery. Cornea 2020, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.J.; Alward, W.L.; Kwon, Y.H.; Bettis, D.I.; Fingert, J.H.; Provencher, L.M.; Goins, K.M.; Wagoner, M.D.; Greiner, M.A. Glaucoma-associated corneal endothelial cell damage: A review. Surv. Ophthalmol. 2018, 63, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Fortune, B.; Yang, H.; Strouthidis, N.G.; Cull, G.A.; Grimm, J.L.; Downs, J.C.; Burgoyne, C.F. The effect of acute intraocular pressure elevation on peripapillary retinal thickness, retinal nerve fiber layer thickness, and retardance. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Agarwal, A.; Agarwal, A.; Narasimhan, S.; Kumar, D.A.; Sivagnanam, S. Endoilluminator-assisted transcorneal illumination for Descemet membrane endothelial keratoplasty: Enhanced intraoperative visualization of the graft in corneal decompensation secondary to pseudophakic bullous keratopathy. J. Cataract Refract. Surg. 2014, 40, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hayashi, T.; Yuda, K.; Tsuchiya, A.; Oyakawa, I.; Mizuki, N.; Kato, N. Chandelier Illumination for Descemet Membrane Endothelial Keratoplasty. Cornea 2017, 36, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Yokogawa, H.; Yamazaki, N.; Masaki, T.; Sugiyama, K. The use of endoillumination probe-assisted Descemet membrane endothelial keratoplasty for bullous keratopathy secondary to argon laser iridotomy. Clin. Ophthalmol. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Haddad, M.T.; Tao, Y.K. Automated stereo vision instrument tracking for intraoperative OCT guided anterior segment ophthalmic surgical maneuvers. Biomed. Opt. Express 2015, 6, 3014–3031. [Google Scholar] [CrossRef]
- Siebelmann, S.; Bachmann, B.; Lappas, A.; Dietlein, T.; Hermann, M.; Roters, S.; Cursiefen, C.; Steven, P. Intraoperative optical coherence tomography in corneal and glaucoma surgical procedures. Ophthalmologe 2016, 113, 646–650. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Gambini, G.; Caporossi, T.; De Vico, U.; Mosca, L.; Guccione, L.; Baldascino, A.; Rizzo, C.; et al. An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer? Diagnostics 2022, 12, 727. https://doi.org/10.3390/diagnostics12030727
Carlà MM, Boselli F, Giannuzzi F, Gambini G, Caporossi T, De Vico U, Mosca L, Guccione L, Baldascino A, Rizzo C, et al. An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer? Diagnostics. 2022; 12(3):727. https://doi.org/10.3390/diagnostics12030727
Chicago/Turabian StyleCarlà, Matteo Mario, Francesco Boselli, Federico Giannuzzi, Gloria Gambini, Tomaso Caporossi, Umberto De Vico, Luigi Mosca, Laura Guccione, Antonio Baldascino, Clara Rizzo, and et al. 2022. "An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer?" Diagnostics 12, no. 3: 727. https://doi.org/10.3390/diagnostics12030727
APA StyleCarlà, M. M., Boselli, F., Giannuzzi, F., Gambini, G., Caporossi, T., De Vico, U., Mosca, L., Guccione, L., Baldascino, A., Rizzo, C., Kilian, R., & Rizzo, S. (2022). An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer? Diagnostics, 12(3), 727. https://doi.org/10.3390/diagnostics12030727






