Urethral Sphincter Length but Not Prostatic Apex Shape in Preoperative MRI Is Associated with Mid-Term Continence Rates after Radical Prostatectomy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
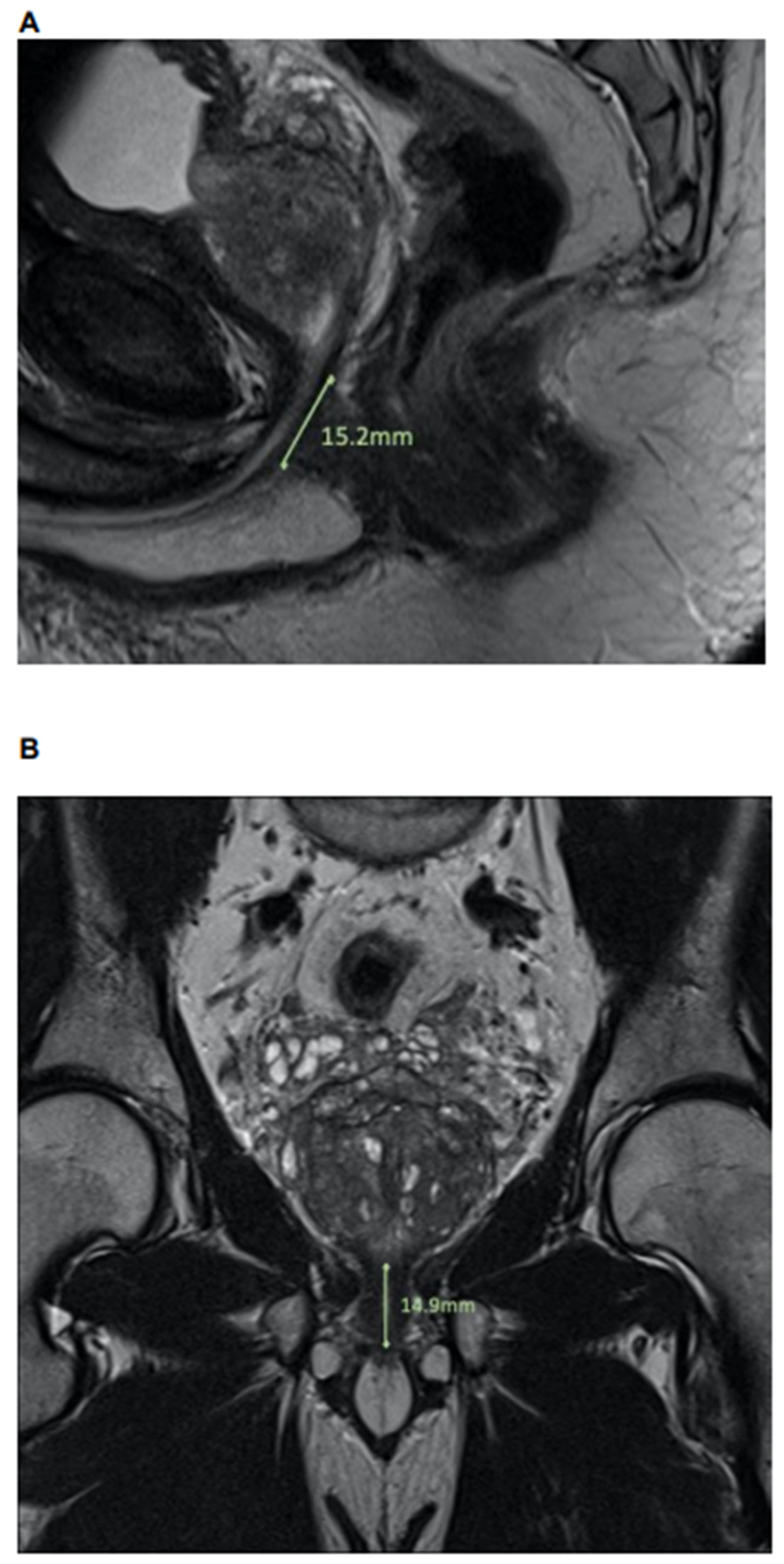
2.2. MpMRI: Lee-Type Definition and Urethral Sphincter Length
2.3. Outcome Measurements
2.4. Statistical Analyses
3. Results
3.1. Descriptive Characteristics of the Study Population
3.2. Continence Rates and Urethral Sphincter Length and Lee-Type in mpMRI
3.3. Univariable and Multivariable Logistic Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pompe, R.S.; Tian, Z.; Preisser, F.; Tennstedt, P.; Beyer, B.; Michl, U.; Graefen, M.; Huland, H.; Karakiewicz, P.I.; Tilki, D. Short- and Long-term Functional Outcomes and Quality of Life after Radical Prostatectomy: Patient-reported Outcomes from a Tertiary High-volume Center. Eur. Urol. Focus 2017, 3, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Theissen, L.; Preisser, F.; Wenzel, M.; Humke, C.; Roos, F.C.; Kluth, L.A.; Becker, A.; Banek, S.; Bodelle, B.; Köllermann, J.; et al. Very Early Continence After Radical Prostatectomy and Its Influencing Factors. Front. Surg. 2019, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Moore, T.H.; Jameson, C.M.; Davies, P.; Rowlands, M.-A.; Burke, M.; Beynon, R.; Savovic, J.; Donovan, J.L. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: A systematic review. Br. J. Urol. 2016, 118, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.C.; Tobias-Machado, M.; Gabriotti, E.N.; Figueiredo, F.W.D.S.; Bezerra, C.A.; Glina, S. Post-radical prostatectomy urinary incontinence: Is there any discrepancy between medical reports and patients’ perceptions? BMC Urol. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Byun, S.-S.; Lee, H.J.; Song, S.H.; Chang, I.H.; Kim, Y.J.; Gill, M.C.; Hong, S.K. Impact of variations in prostatic apex shape on early recovery of urinary continence after radical retropubic prostatectomy. Urology 2006, 68, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Paparel, P.; Akin, O.; Sandhu, J.S.; Otero, J.R.; Serio, A.M.; Scardino, P.T.; Hricak, H.; Guillonneau, B. Recovery of Urinary Continence after Radical Prostatectomy: Association with Urethral Length and Urethral Fibrosis Measured by Preoperative and Postoperative Endorectal Magnetic Resonance Imaging. Eur. Urol. 2009, 55, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Marenco, J.; Orczyk, C.; Collins, T.; Moore, C.; Emberton, M. Role of MRI in planning radical prostatectomy: What is the added value? World J. Urol. 2019, 37, 1289–1292. [Google Scholar] [CrossRef]
- Mungovan, S.F.; Sandhu, J.; Akin, O.; Smart, N.; Graham, P.; Patel, M.I. Preoperative Membranous Urethral Length Measurement and Continence Recovery Following Radical Prostatectomy: A Systematic Review and Meta-analysis. Eur. Urol. 2017, 71, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colarieti, A.; Thiruchelvam, N.; Barrett, T. Evaluation of image-based prognostic parameters of post-prostatectomy urinary incontinence: A literature review. Int. J. Urol. 2021, 28, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Preisser, F.; Mueller, M.; Theissen, L.H.; Welte, M.N.; Hoeh, B.; Humke, C.; Bernatz, S.; Bodelle, B.; Würnschimmel, C.; et al. Effect of prostatic apex shape (Lee types) and urethral sphincter length in preoperative MRI on very early continence rates after radical prostatectomy. Int. Urol. Nephrol. 2021, 53, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; China, T.; Kanayama, M.; Nagata, M.; Isotani, S.; Wakumoto, Y.; Muto, S.; Ide, H.; Horie, S. Significant association between urethral length measured by magnetic resonance imaging and urinary continence recovery after robot-assisted radical prostatectomy. Prostate Int. 2019, 7, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kim, C.K.; Park, B.K.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Lee, H.M. Impact of preoperative and postoperative membranous urethral length measured by 3 Tesla magnetic resonance imaging on urinary continence recovery after robotic-assisted radical prostatectomy. Can. Urol. Assoc. J. 2017, 11, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coakley, F.V.; Eberhardt, S.; Kattan, M.W.; Wei, D.C.; Scardino, P.T.; Hricak, H. Urinary Continence After Radical Retropubic Prostatectomy: Relationship with Membranous Urethral Length on Preoperative Endorectal Magnetic Resonance Imaging. J. Urol. 2002, 168, 1032–1035. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Ramaswamy, A.; Yu, M.; Margolis, D.J.; Hu, J.C. Racial Variation in Membranous Urethral Length and Postprostatectomy Urinary Function. Eur. Urol. Open Sci. 2021, 27, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Ehdaie, B.; Sandhu, J.; Sjoberg, D.D.; Carlsson, S.V.; Tzeng, M.; Vickers, A.J. Asian-American Race and Urinary Continence After Radical Prostatectomy. Eur. Urol. Open Sci. 2020, 22, 51–53. [Google Scholar] [CrossRef]
- Preisser, F.; Theissen, L.; Wild, P.; Bartelt, K.; Kluth, L.; Köllermann, J.; Graefen, M.; Steuber, T.; Huland, H.; Tilki, D.; et al. Implementation of Intraoperative Frozen Section During Radical Prostatectomy: Short-term Results from a German Tertiary-care Center. Eur. Urol. Focus 2021, 7, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Weinreb, J.C.; Verma, S.; Thoeny, H.C.; Tempany, C.M.; Shtern, F.; Padhani, A.; Margolis, D.; Macura, K.J.; Haider, M.A.; et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur. Urol. 2016, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- RCT. R: A Language and Environment for Statistical Computing. 2017. Available online: https://wwwr-projectorg2017 (accessed on 13.12.2021).
- Kim, L.H.; Patel, A.; Kinsella, N.; Sharabiani, M.T.; Ap Dafydd, D.; Cahill, D. Association Between Preoperative Magnetic Resonance Imaging–based Urethral Parameters and Continence Recovery Following Robot-assisted Radical Prostatectomy. Eur. Urol. Focus 2020, 6, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.S.; Choo, S.H.; Kim, S.J.; Shim, K.H.; Park, S.G.; Kim, S.I. Postoperative membranous urethral length is the single most important surgical factor predicting recovery of postoperative urinary continence. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 930-e7. [Google Scholar] [CrossRef] [PubMed]
- Satake, Y.; Kaiho, Y.; Saito, H.; Yamada, T.; Kawamorita, N.; Yamashita, S.; Mitsuzuka, K.; Yamada, S.; Ito, A.; Arai, Y. Estimated Minimal Residual Membranous Urethral Length on Preoperative Magnetic Resonance Imaging Can Be a New Predictor for Continence After Radical Prostatectomy. Urology 2018, 112, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Tennstedt, P.; Berliner, C.; Well, L.; Huland, H.; Budäus, L.; Adam, G.; Beyersdorff, D. Predictors of Short and Long Term Urinary Incontinence after Radical Prostatectomy in Prostate MRI: Significance and Reliability of Standardized Measurements. Eur. J. Radiol. 2019, 120, 108668. Available online: https://www.ejradiology.com/article/S0720-048X(19)30318-3/fulltext (accessed on 13.12.2021). [CrossRef] [PubMed]
- Matsushita, K.; Kent, M.T.; Vickers, A.; Von Bodman, C.; Bernstein, M.; Touijer, K.A.; Coleman, J.; Laudone, V.T.; Scardino, P.T.; Eastham, J.A.; et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. Br. J. Urol. 2015, 116, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Choi, S.-K.; Park, S. Randomized clinical trial of a bladder neck plication stitch during robot-assisted radical prostatectomy. Asian J. Androl. 2015, 17, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Haese, A.; Knipper, S.; Isbarn, H.; Heinzer, H.; Tilki, D.; Salomon, G.; Michl, U.; Steuber, T.; Budäus, L.; Maurer, T.; et al. A comparative study of robot-assisted and open radical prostatectomy in 10 790 men treated by highly trained surgeons for both procedures. Br. J. Urol. 2019, 123, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Hoeh, B.; Wenzel, M.; Hohenhorst, L.; Köllermann, J.; Graefen, M.; Haese, A.; Tilki, D.; Walz, J.; Kosiba, M.; Becker, A.; et al. Anatomical Fundamentals and Current Surgical Knowledge of Prostate Anatomy Related to Functional and Oncological Outcomes for Robotic-Assisted Radical Prostatectomy. Front. Surg. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Michl, U.; Tennstedt, P.; Feldmeier, L.; Mandel, P.; Oh, S.J.; Ahyai, S.; Budäus, L.; Chun, F.K.; Haese, A.; Heinzer, H.; et al. Nerve-sparing Surgery Technique, Not the Preservation of the Neurovascular Bundles, Leads to Improved Long-term Continence Rates After Radical Prostatectomy. Eur. Urol. 2016, 69, 584–589. [Google Scholar] [CrossRef] [PubMed]
| Overall, N = 68 (100%) | Robotic-Assisted RP, N = 37 (55%) | Open RP, N = 31 (45%) | p-Value | |
|---|---|---|---|---|
|
Length of urethral sphincter, coronal, in mm Median (IQR) | 14.7 (13.0, 16.7) | 15.0 (13.7, 17.1) | 14.5 (12.2, 16.2) | 0.2 |
|
Length of urethral sphincter, sagital, in mm Median (IQR) | 15.1 (12.8, 16.8) | 15.1 (14.1, 17.0) | 15.3 (10.8, 16.7) | 0.4 |
|
Length of urethral sphincter, axial, in mm Median (IQR) | 10.2 (9.2, 11.2) | 9.9 (9.2, 11.1) | 10.2 (9.1, 11.2) | 0.8 |
|
Diameter of urethral sphincter, coronal, in mm Median (IQR) | 9.1 (7.9, 10.1) | 9.4 (8.2, 10.1) | 8.7 (7.6, 9.8) | 0.2 |
|
Lee-type, n (%) | 0.3 | |||
| A | 11 (16%) | 5 (14%) | 6 (19%) | |
| B | 5 (7%) | 1 (3%) | 4 (13%) | |
| C | 5 (7%) | 4 (11%) | 1 (3%) | |
| D | 47 (69%) | 27 (73%) | 20 (65%) | |
|
Age in years, Median (IQR) | 66 (58, 72) | 66 (58, 70) | 66 (60, 72) | 0.6 |
|
PSA, in ng/mL, Median (IQR) | 7 (5, 10) | 6 (5, 8) | 8 (6, 15) | 0.005 |
|
Body-Mass Index in kg/m2, Median (IQR) | 25.6 (24.0, 27.2) | 25.7 (23.9, 27.1) | 25.4 (24.2, 27.7) | 0.6 |
|
Prostate volume in mL, Median (IQR) | 35 (28, 45) | 35 (29, 44) | 36 (28, 49) | 0.7 |
|
D’Amico Score, n (%) | <0.001 | |||
| Low | 5 (8%) | 4 (11%) | 1 (3%) | |
| Intermediate | 39 (59%) | 28 (78%) | 11 (37%) | |
| High | 22 (33%) | 4 (11%) | 18 (60%) | |
|
ISUP Score at biopsy, n (%) | <0.001 | |||
| ISUP1 | 7 (10%) | 4 (11%) | 3 (10%) | |
| ISUP2 | 26 (38%) | 21 (57%) | 5 (16%) | |
| ISUP3 | 17 (25%) | 8 (22%) | 9 (29%) | |
| ISUP4 | 10 (15%) | 4 (11%) | 6 (19%) | |
| ISUP5 | 8 (12%) | 0 (0%) | 8 (26%) | |
|
Pathological ISUP Score, n (%) | <0.001 | |||
| ISUP1 | 8 (12%) | 7 (19%) | 1 (3%) | |
| ISUP2 | 30 (46%) | 23 (62%) | 7 (26%) | |
| ISUP3 | 14 (22%) | 5 (14%) | 9 (32%) | |
| ISUP4 | 2 (3%) | 0 (0%) | 2 (7%) | |
| ISUP5 | 11 (17%) | 2 (5%) | 9 (32%) | |
|
Nerve sparing approach, n (%) | 0.003 | |||
| None | 6 (9%) | 0 (0%) | 6 (19%) | |
| Yes | 62 (91%) | 37 (100%) | 25 (81%) | |
|
pT-stage, n (%) | 0.02 | |||
| pT2 | 41 (60%) | 27 (73%) | 14 (45%) | |
| >pT2 | 27 (40%) | 10 (27%) | 17 (55%) | |
|
pN-stage, n (%) | 0.2 | |||
| pN0 | 60 (88%) | 34 (92%) | 26 (84%) | |
| pN1 | 3 (4%) | 0 (0%) | 3 (10%) | |
| pNx | 5 (8%) | 3 (8%) | 2 (6%) |
| Overall, N = 68 (100%) | Continent, N = 55 (81%) | Incontinent, N = 13 (19%) | p-Value | |
|---|---|---|---|---|
|
Length of urethral sphincter, coronal, in mm Median (IQR) | 14.7 (13.0, 16.7) | 15.1 (13.8, 16.9) | 12.5 (11.9, 14.2) | 0.009 |
|
Length of urethral sphincter, sagital, in mm Median (IQR) | 15.1 (12.8, 16.8) | 15.4 (14.4, 17.4) | 11.1 (9.8, 13.7) | <0.001 |
|
Length of urethral sphincter, axial, in mm Median (IQR) | 10.2 (9.2, 11.2) | 9.7 (9.1, 11.2) | 10.5 (10.1, 11.1) | 0.5 |
|
Diameter of urethral sphincter, coronal, in mm Median (IQR) | 9.1 (7.9, 10.1) | 9.1 (7.9, 10.1) | 9.4 (8.9, 9.9) | 0.5 |
|
Lee-type, n (%) | 0.4 | |||
| A | 11 (16%) | 8 (15%) | 3 (23%) | |
| B | 5 (7%) | 3 (6%) | 2 (15%) | |
| C | 5 (7%) | 4 (8%) | 1 (7.7%) | |
| D | 47 (69%) | 40 (73%) | 7 (54%) | |
|
Age in years, Median (IQR) | 66 (58, 72) | 64 (58, 69) | 72 (68, 74) | 0.006 |
|
PSA, in ng/mL, Median (IQR) | 7 (5, 10) | 7 (5, 10) | 7 (6, 11) | 0.7 |
|
Body-Mass Index, Median (IQR) | 25.6 (24.0, 27.2) | 25.8 (24.0, 27.4) | 24.6 (24.1, 27.1) | 0.5 |
|
Prostate volume, in mL Median (IQR) | 35 (28, 45) | 35 (26, 45) | 39 (30, 55) | 0.5 |
| D’Amico Score, n (%) | 0.030 | |||
| Low | 5 (8%) | 5 (9%) | 0 (0%) | |
| Intermediate | 39 (59%) | 35 (65%) | 4 (33%) | |
| High | 22 (33%) | 14 (26%) | 8 (67%) | |
|
ISUP Score at biopsy, n (%) | 0.08 | |||
| ISUP1 | 7 (10%) | 6 (11%) | 1 (8%) | |
| ISUP2 | 26 (38%) | 24 (44%) | 2 (15%) | |
| ISUP3 | 17 (25%) | 14 (25%) | 3 (23%) | |
| ISUP4 | 10 (15%) | 7 (12%) | 3 (23%) | |
| ISUP5 | 8 (12%) | 4 (8%) | 4 (31%) | |
| Pathological ISUP Score, n (%) | 0.008 | |||
| ISUP1 | 8 (12%) | 6 (11%) | 2 (18%) | |
| ISUP2 | 30 (46%) | 27 (50%) | 3 (27%) | |
| ISUP3 | 14 (22%) | 14 (26%) | 0 (0%) | |
| ISUP4 | 2 (3%) | 1 (2%) | 1 (9.1%) | |
| ISUP5 | 11 (17%) | 6 (11%) | 5 (45%) | |
| Nerve sparing approach, n (%) | 0.019 | |||
| None | 6 (9%) | 2 (4%) | 4 (31%) | |
| Yes | 62 (91%) | 53 (96%) | 9 (31%) | |
| pT-stage, n (%) | 0.2 | |||
| pT2 | 41 (60%) | 35 (64%) | 6 (46%) | |
| >pT2 | 27 (40%) | 20 (36%) | 7 (54%) | |
|
pN-stage, n (%) | 0.2 | |||
| pN0 | 60 (88%) | 50 (91%) | 10 (77%) | |
| pN1 | 3 (4%) | 2 (4%) | 1 (8%) | |
| pNx | 5 (8%) | 3 (6%) | 2 (15%) | |
| Surgical approach, n (%) | 0.012 | |||
| Robotic-assisted | 37 (54%) | 34 (62%) | 3 (23%) | |
| Open | 31 (46%) | 21 (38%) | 10 (77%) |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | CI 2.5 % | CI 97.5 % | p-Value | Odds Ratio | CI 2.5 % | CI 97.5 % | p-Value | |
| Urethral sphincter length, coronal | 1.42 | 1.09 | 1.96 | 0.02 | - | - | - | - |
| Urethral sphincter length, sagital | 1.69 | 1.31 | 2.33 | >0.001 | 1.67 | 1.22 | 2.52 | 0.005 |
| Urethral sphincter length, axial | 0.94 | 0.62 | 1.43 | 0.77 | - | - | - | - |
| Diameter of urethral sphincter, coronal | 0.88 | 0.57 | 1.33 | 0.54 | - | - | - | - |
| Lee-type | ||||||||
| A | Ref. | - | - | - | - | |||
| B | 0.56 | 0.06 | 5.91 | 0.61 | - | - | - | - |
| C | 1.50 | 0.13 | 35.90 | 0.76 | - | - | - | - |
| D | 2.14 | 0.40 | 9.72 | 0.34 | - | - | - | - |
| Age | 0.88 | 0.79 | 0.97 | 0.02 | 0.87 | 0.73 | 0.99 | 0.04 |
| Prostate volume | 0.99 | 0.95 | 1.03 | 0.63 | - | - | - | - |
| pT-stage | ||||||||
| pT2 | Ref. | - | - | - | - | |||
| >pT2 | 0.49 | 0.14 | 1.67 | 0.25 | - | - | - | - |
| Surgical approach | ||||||||
| Open RP | Ref. | |||||||
| Robotic-assisted RP | 5.40 | 1.46 | 26.17 | 0.02 | 3.03 | 0.38 | 34.13 | 0.31 |
| Nerve sparing approach | ||||||||
| None | Ref. | Ref. | ||||||
| Yes | 11.78 | 2.01 | 94.71 | 0.01 | 0.79 | 0.01 | 45.67 | 0.90 |
| Pathological ISUP | ||||||||
| 1/2/3 | Ref. | Ref. | ||||||
| 4/5 | 0.12 | 0.03 | 0.51 | 0.004 | 0.18 | 0.02 | 1.92 | 0.15 |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | CI 2.5 % | CI 97.5 % | p-Value | Odds Ratio | CI 2.5 % | CI 97.5 % | p-Value | |
| Urethral sphincter length, coronal | 1.42 | 1.09 | 1.96 | 0.02 | 1.35 | 1.01 | 1.96 | 0.045 |
| Urethral sphincter length, sagital | 1.69 | 1.31 | 2.33 | >0.001 | - | - | - | - |
| Urethral sphincter length, axial | 0.94 | 0.62 | 1.43 | 0.77 | - | - | - | - |
| Diameter of urethral sphincter, coronal | 0.88 | 0.57 | 1.33 | 0.54 | - | - | - | - |
| Lee-type | ||||||||
| A | Ref. | - | - | - | - | |||
| B | 0.56 | 0.06 | 5.91 | 0.61 | - | - | - | - |
| C | 1.50 | 0.13 | 35.90 | 0.76 | - | - | - | - |
| D | 2.14 | 0.40 | 9.72 | 0.34 | - | - | - | - |
| Age | 0.88 | 0.79 | 0.97 | 0.02 | 0.87 | 0.75 | 0.98 | 0.04 |
| Prostate volume | 0.99 | 0.95 | 1.03 | 0.63 | - | - | - | - |
| pT-stage | ||||||||
| pT2 | Ref. | - | - | - | - | |||
| >pT2 | 0.49 | 0.14 | 1.67 | 0.25 | - | - | - | - |
| Surgical approach | ||||||||
| Open RP | Ref. | |||||||
| Robotic-assisted RP | 5.40 | 1.46 | 26.17 | 0.02 | 2.35 | 0.36 | 18.01 | 0.37 |
| Nerve sparing approach | ||||||||
| None | Ref. | Ref. | ||||||
| Yes | 11.78 | 2.01 | 94.71 | 0.01 | 0.65 | 0.03 | 22.08 | 0.79 |
| Pathological ISUP | ||||||||
| 1/2/3 | Ref. | Ref. | ||||||
| 4/5 | 0.12 | 0.03 | 0.51 | 0.004 | 0.22 | 0.02 | 1.83 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeh, B.; Wenzel, M.; Müller, M.; Wittler, C.; Schlenke, E.; Hohenhorst, J.L.; Köllermann, J.; Steuber, T.; Graefen, M.; Tilki, D.; et al. Urethral Sphincter Length but Not Prostatic Apex Shape in Preoperative MRI Is Associated with Mid-Term Continence Rates after Radical Prostatectomy. Diagnostics 2022, 12, 701. https://doi.org/10.3390/diagnostics12030701
Hoeh B, Wenzel M, Müller M, Wittler C, Schlenke E, Hohenhorst JL, Köllermann J, Steuber T, Graefen M, Tilki D, et al. Urethral Sphincter Length but Not Prostatic Apex Shape in Preoperative MRI Is Associated with Mid-Term Continence Rates after Radical Prostatectomy. Diagnostics. 2022; 12(3):701. https://doi.org/10.3390/diagnostics12030701
Chicago/Turabian StyleHoeh, Benedikt, Mike Wenzel, Matthias Müller, Clarissa Wittler, Eva Schlenke, Jan L. Hohenhorst, Jens Köllermann, Thomas Steuber, Markus Graefen, Derya Tilki, and et al. 2022. "Urethral Sphincter Length but Not Prostatic Apex Shape in Preoperative MRI Is Associated with Mid-Term Continence Rates after Radical Prostatectomy" Diagnostics 12, no. 3: 701. https://doi.org/10.3390/diagnostics12030701
APA StyleHoeh, B., Wenzel, M., Müller, M., Wittler, C., Schlenke, E., Hohenhorst, J. L., Köllermann, J., Steuber, T., Graefen, M., Tilki, D., Bernatz, S., Karakiewicz, P. I., Preisser, F., Becker, A., Kluth, L. A., Mandel, P., & Chun, F. K. H. (2022). Urethral Sphincter Length but Not Prostatic Apex Shape in Preoperative MRI Is Associated with Mid-Term Continence Rates after Radical Prostatectomy. Diagnostics, 12(3), 701. https://doi.org/10.3390/diagnostics12030701






