Prospective Study of Low-Radiation and Low-Iodine Dose Aortic CT Angiography in Obese and Non-Obese Patients: Image Quality and Impact of Patient Characteristics
Abstract
:1. Introduction
2. Materials and Methods
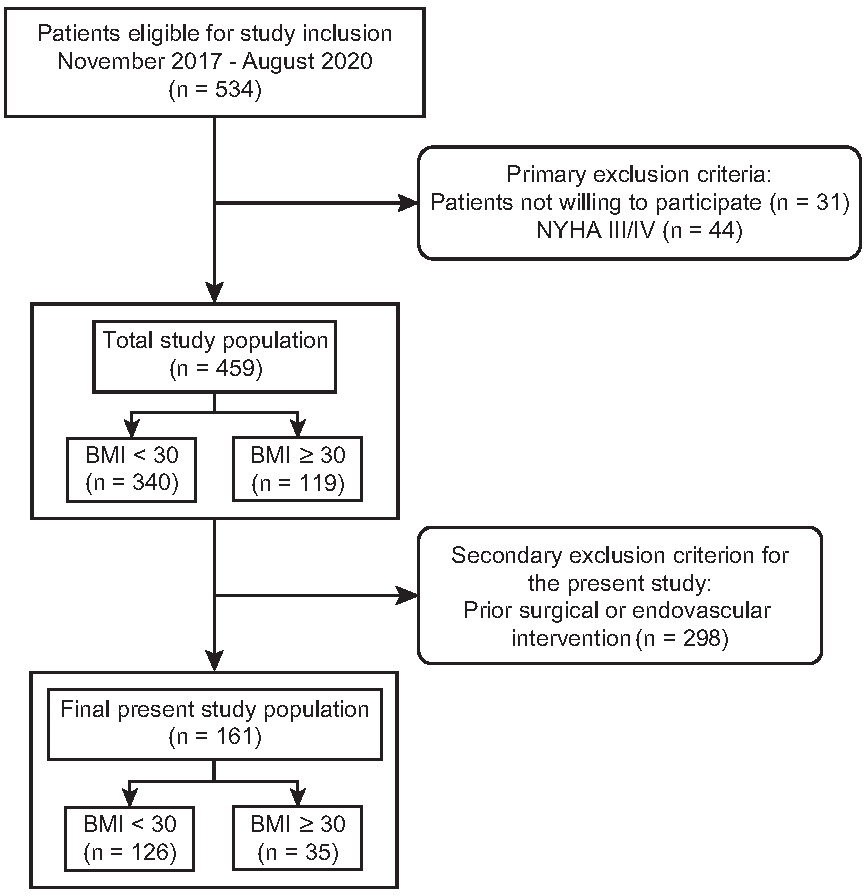
2.1. Study Design and Patients
2.2. Image Acquisition
2.3. Image Quality Evaluation
2.4. Radiation Dose Evaluation
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Image Quality Evaluation
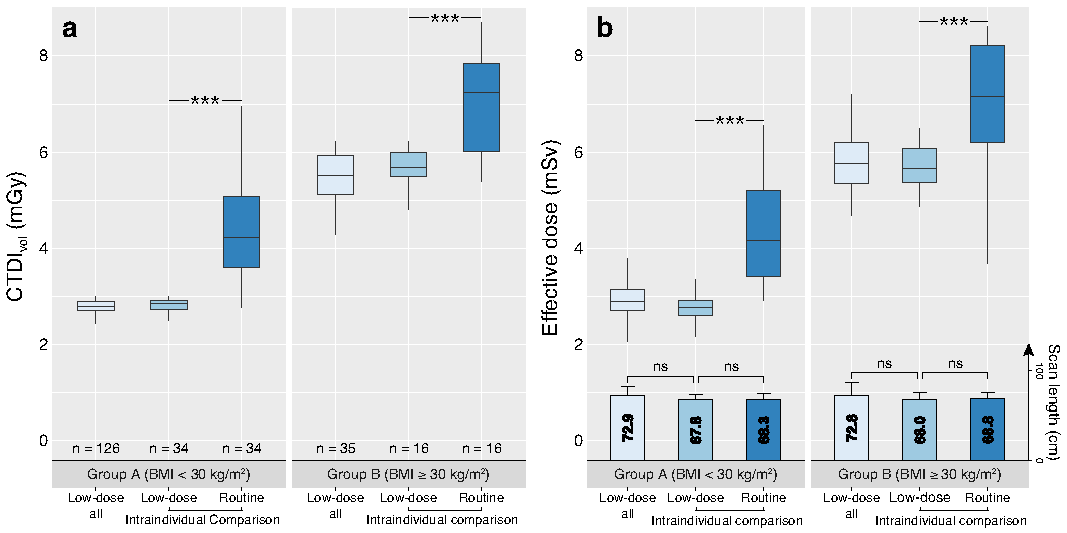
3.3. Radiation Dose Evaluation
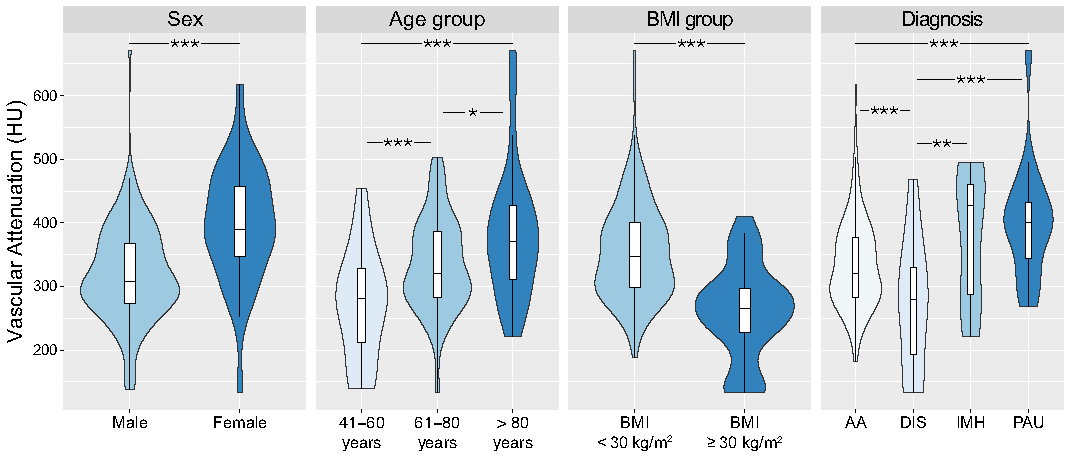
3.4. Impact of Patient Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CNR | Contrast-to-noise ratio |
| CTDIvol | Volumetric CT dose index |
| DLP | Dose-length product |
| ED | Effective dose |
| ICC | Intraclass correlation coefficient |
| IMH | Intramural haematoma |
| NYHA | New York Heart Association |
| PAU | Penetrating atherosclerotic ulcer |
| SNR | Signal-to-noise ratio |
References
- Rubin, G.D.; Leipsic, J.; Joseph Schoepf, U.; Fleischmann, D.; Napel, S. CT Angiography after 20 Years: A Transformation in Cardiovascular Disease Characterization Continues to Advance. Radiology 2014, 271, 633–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengier, F.; Geisbüsch, P.; Vosshenrich, R.; Müller-Eschner, M.; Karmonik, C.; Schoenhagen, P.; von Tengg-Kobligk, H.; Partovi, S. State-of-the-Art Aortic Imaging: Part I - Fundamentals and Perspectives of CT and MRI. Vasa 2013, 42, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Geisbüsch, P.; Schoenhagen, P.; Müller-Eschner, M.; Vosshenrich, R.; Karmonik, C.; von Tengg-Kobligk, H.; Partovi, S. State-of-the-Art Aortic Imaging: Part II—Applications in Transcatheter Aortic Valve Replacement and Endovascular Aortic Aneurysm Repair. Vasa 2014, 43, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.J.; Parfrey, P.S. Clinical Practice. Preventing Nephropathy Induced by Contrast Medium. N. Engl. J. Med. 2006, 354, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balemans, C.E.A.; Reichert, L.J.M.; van Schelven, B.I.H.; van den Brand, J.A.J.G.; Wetzels, J.F.M. Epidemiology of Contrast Material-Induced Nephropathy in the Era of Hydration. Radiology 2012, 263, 706–713. [Google Scholar] [PubMed]
- Van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-Contrast Acute Kidney Injury—Part 1: Definition, Clinical Features, Incidence, Role of Contrast Medium and Risk Factors: Recommendations for Updated ESUR Contrast Medium Safety Committee Guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [PubMed] [Green Version]
- Commission, E. RP 180 Medical Radiation Exposure of the European Population, Part 1 & 2. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/RP180.pdf (accessed on 5 February 2022).
- Euler, A.; Taslimi, T.; Eberhard, M.; Kobe, A.; Reeve, K.; Zimmermann, A.; Krauss, A.; Gutjahr, R.; Schmidt, B.; Alkadhi, H. Computed Tomography Angiography of the Aorta-Optimization of Automatic Tube Voltage Selection Settings to Reduce Radiation Dose or Contrast Medium in a Prospective Randomized Trial. Investig. Radiol. 2021, 56, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.A.; Huang, E.P.; Chen, K.T.; Chen, Y.C.; Huang, Y.L.; Chuo, C.C.; Wu, F.Z.; Wu, M.T. Comparison of Four Contrast Medium Delivery Protocols in Low-Iodine and Low-Radiation Dose CT Angiography of the Aorta. Clin. Radiol. 2020, 75, 797.e9–797.e19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Li, X.; Schoepf, U.J.; Wichmann, J.L.; Tang, C.X.; Zhou, C.S.; Lu, G.M. Non-Electrocardiogram-Triggered 70-kVp High-Pitch Computed Tomography Angiography of the Whole Aorta with Iterative Reconstruction: Initial Results. J. Comput. Assist. Tomogr. 2016, 40, 109–117. [Google Scholar] [CrossRef]
- Nijhof, W.H.; Baltussen, E.J.M.; Kant, I.M.J.; Jager, G.J.; Slump, C.H.; Rutten, M.J.C.M. Low-Dose CT Angiography of the Abdominal Aorta and Reduced Contrast Medium Volume: Assessment of Image Quality and Radiation Dose. Clin. Radiol. 2016, 71, 64–73. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, D.; Zhu, K.; Xiao, X. Clinical Application of 100 kVp Acquisition with an Iterative Reconstruction Technique in Retrospective Electrocardiogram-Gated Thoracoabdominal Aortic CT Angiography. Clin. Radiol. 2015, 70, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Talei Franzesi, C.; Fior, D.; Bonaffini, P.A.; Minutolo, O.; Sironi, S. Low kV Settings CT Angiography (CTA) with Low Dose Contrast Medium Volume Protocol in the Assessment of Thoracic and Abdominal Aorta Disease: A Feasibility Study. Br. J. Radiol. 2015, 88, 20140140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Sun, Z.; Xu, L.; Li, Y.; Zhang, N.; Yan, Z.; Fan, Z. High-Pitch, Low-Voltage and Low-Iodine-Concentration CT Angiography of Aorta: Assessment of Image Quality and Radiation Dose with Iterative Reconstruction. PLoS ONE 2015, 10, e0117469. [Google Scholar]
- Apfaltrer, P.; Hanna, E.L.; Schoepf, U.J.; Spears, J.R.; Schoenberg, S.O.; Fink, C.; Vliegenthart, R. Radiation Dose and Image Quality at High-Pitch CT Angiography of the Aorta: Intraindividual and Interindividual Comparisons with Conventional CT Angiography. Am. J. Roentgenol. 2012, 199, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.O.; Arnold, M.; Klinghammer, L.; Schuhbaeck, A.; Hell, M.M.; Muschiol, G.; Gauss, S.; Lell, M.; Uder, M.; Hoffmann, U.; et al. Contrast Volume Reduction Using Third Generation Dual Source Computed Tomography for the Evaluation of Patients Prior to Transcatheter Aortic Valve Implantation. Eur. Radiol. 2016, 26, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Defining Adult Overweight & Obesity. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 5 February 2022).
- Apfaltrer, P.; Sudarski, S.; Schneider, D.; Nance, J.W.; Haubenreisser, H.; Fink, C.; Schoenberg, S.O.; Henzler, T. Value of Monoenergetic Low-kV Dual Energy CT Datasets for Improved Image Quality of CT Pulmonary Angiography. Eur. J. Radiol. 2014, 83, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Christner, J.A.; Kofler, J.M.; McCollough, C.H. Estimating Effective Dose for CT Using Dose-Length Product Compared with Using Organ Doses: Consequences of Adopting International Commission on Radiological Protection Publication 103 or Dual-Energy Scanning. Am. J. Roentgenol. 2010, 194, 881–889. [Google Scholar]
- Shrimpton, P.; Wall, B. Assessment of Patient Dose from Computed Tomography. Radiat. Prot. Dosim. 1992, 43, 205. [Google Scholar]
- Shrimpton, P.C.; Hillier, M.C.; Lewis, M.A.; Dunn, M. Doses from Computed Tomography (CT) Examinations in the UK-2003 Review; National Radiological Protection Board (NRPB): Chilton, UK, 2005; Volume 67.
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Javor, D.; Wressnegger, A.; Unterhumer, S.; Kollndorfer, K.; Nolz, R.; Beitzke, D.; Loewe, C. Endoleak Detection Using Single-Acquisition Split-Bolus Dual-Energy Computer Tomography (DECT). Eur. Radiol. 2017, 27, 1622–1630. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.J.; Zhao, Y.E.; Schoepf, U.J.; Mangold, S.; Felmly, L.M.; Li, X.; Tang, C.X.; Zhou, C.S.; Qi, L.; Lu, G.M. Seventy-Peak Kilovoltage High-Pitch Thoracic Aortic CT Angiography without ECG Gating: Evaluation of Image Quality and Radiation Dose. Acad. Radiol. 2015, 22, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Chin, A.S.; Molvin, L.; Wang, J.; Hallett, R. Computed Tomography Angiography: A Review and Technical Update. Radiol. Clin. 2016, 54, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, L.; Gabelloni, M. Iodine Concentration and Optimization in Computed Tomography Angiography: Current Issues. Investig. Radiol. 2016, 51, 816–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, D.J.; Aghayev, A.; Steigner, M.L. Vascular CT and MRI: A Practical Guide to Imaging Protocols. Insights Into Imaging 2018, 9, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.; Vavere, A.L.; Arbab-Zadeh, A.; Miller, J.M.; Sara, L.; Cox, C.; Gottlieb, I.; Yoshioka, K.; Paul, N.; Hoe, J.; et al. Patient Characteristics as Predictors of Image Quality and Diagnostic Accuracy of MDCT Compared with Conventional Coronary Angiography for Detecting Coronary Artery Stenoses: CORE-64 Multicenter International Trial. Am. J. Roentgenol. 2010, 194, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggenland, D.; Peters, S.A.; Lemburg, S.P.; Holland-Letz, T.; Nicolas, V.; Heyer, C.M. CT Angiography in Suspected Pulmonary Embolism: Impact of Patient Characteristics and Different Venous Lines on Vessel Enhancement and Image Quality. Am. J. Roentgenol. 2008, 190, W351–W359. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Kohno, T.; Hiki, T.; Kaji, Y. CT Pulmonary Angiography and CT Venography: Factors Associated with Vessel Enhancement. Am. J. Roentgenol. 2007, 189, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Fraioli, F.; Catalano, C.; Napoli, A.; Francone, M.; Venditti, F.; Danti, M.; Pediconi, F.; Passariello, R. Low-Dose Multidetector-Row CT Angiography of the Infra-Renal Aorta and Lower Extremity Vessels: Image Quality and Diagnostic Accuracy in Comparison with Standard DSA. Eur. Radiol. 2006, 16, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Heiken, J.P.; Brink, J.A. Aortic and Hepatic Contrast Medium Enhancement at CT. Part II. Effect of Reduced Cardiac Output in a Porcine Model. Radiology 1998, 207, 657–662. [Google Scholar] [CrossRef] [PubMed]
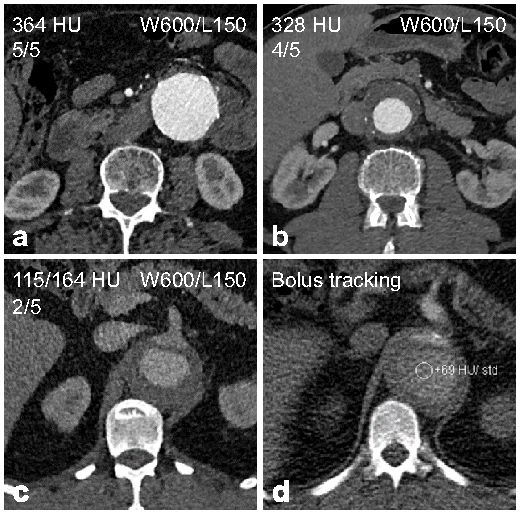
| Rating | Description |
|---|---|
| Excellent (5) | Aorta and iliac arteries with excellent enhancement and little noise. |
| Good (4) | Aorta and iliac arteries with good enhancement and little to moderate noise. |
| Moderate (3) | Aorta or iliac arteries partially with low to moderate enhancement and/or moderate to high noise. |
| Fair (2) | Aorta and iliac arteries completely with low enhancement and/or high noise. |
| Non-diagnostic (1) | Aorta or iliac arteries partially with insufficient enhancement and/or disruptive noise. |
| Parameter | All Patients | Group A (BMI < 30 kg/m2) | Group B (BMI ≥ 30 kg/m2) |
|---|---|---|---|
| Patients | 161 | 126 | 35 |
| Women | 32 (19.9) | 28 (22.2) | 4 (11.4) |
| Age (y) † | 71.1 ± 9.4 | 71.7 ± 9.3 | 69.1 ± 9.4 |
| Weight (kg) † | 82.8 ± 16.3 | 76.9 ± 11.7 | 104.3 ± 12.3 |
| Height (cm) † | 173.9 ± 8.8 | 173.6 ± 8.9 | 175.1 ± 8.4 |
| BMI (kg/m2) † | 27.3 ± 4.6 | 25.4 ± 2.8 | 34.0 ± 3.4 |
| History of cardiac disease | 72 (44.7) | 57 (45.2) | 15 (42.9) |
| Aortic disease | |||
| Aneurysm | 115 (71.4) | 92 (73.0) | 23 (65.7) |
| Dissection | 22 (13.7) | 11 (8.7) | 11 (31.4) |
| PAU | 17 (10.6) | 16 (12.7) | 1 (2.9) |
| IMH | 7 (4.3) | 7 (5.6) | 0 (0) |
| Parameter | All Patients | Group A (BMI < 30 kg/m2) | Group B (BMI ≥ 30 kg/m2) |
|---|---|---|---|
| Vascular attenuation (HU) | |||
| Average | 334.7 ± 85.9 (321.3, 348.1) | 354.9 ± 78.2 (341.1, 368.7) | 262.1 ± 73.0 (237.0, 287.2) |
| Ascending aorta | 314.8 ± 104.7 (298.5, 331.1) | 332.5 ± 101.4 (314.6, 350.4) | 249.9 ± 91.2 (218.5, 281.3) |
| Descending thoracic aorta | 346.8 ± 98.8 (331.4, 362.2) | 368.5 ± 90.6 (352.5, 384.5) | 266.9 ± 87.1 (236.9, 296.9) |
| Abdominal aorta | 346.6 ± 88.7 (332.8, 360.4) | 367.6 ± 81.5 (353.2, 382.0) | 271.1 ± 71.4 (246.5, 295.7) |
| Aortic bifurcation | 340.2 ± 89.2 (326.3, 354.1) | 360.7 ± 83.7 (345.9, 375.5) | 266.4 ± 67.5 (243.1, 289.7) |
| Common iliac artery | 324.9 ± 85.6 (311.6, 338.2) | 344.4 ± 80.8 (330.1, 358.7) | 252.8 ± 61.5 (231.6, 274.3) |
| Contrast-to-noise ratio | |||
| Average | 10.8 ± 3.8 (10.2, 11.4) | 11.7 ± 3.5 (11.1, 12.3) | 7.8 ± 3.2 (6.7, 8.9) |
| Ascending aorta | 11.1 ± 4.8 (10.4, 11.8) | 11.9 ± 4.7 (11.1, 12.7) | 8.4 ± 4.6 (6.8, 10.0) |
| Descending thoracic aorta | 12.5 ± 4.8 (11.8, 13.2) | 13.4 ± 4.4 (12.6, 14.2) | 9.1 ± 4.5 (7.5, 10.7) |
| Abdominal aorta | 10.9 ± 3.9 (10.3, 11.5) | 11.7 ± 3.8 (11, 12.4) | 8.1 ± 3.8 (7.1, 9.1) |
| Aortic bifurcation | 10.1 ± 4.2 (9.4, 10.8) | 11.0 ± 4.1 (10.3, 11.7) | 7.0 ± 2.6 (6.1, 7.9) |
| Common iliac artery | 9.6 ± 3.9 (9.0, 10.2) | 10.4 ± 3.8 (9.7, 11.1) | 6.5 ± 2.2 (5.7, 7.3) |
| Signal-to-noise ratio | |||
| Average | 12.8 ± 3.9 (12.2, 13.4) | 13.6 ± 3.6 (13.0, 14.2) | 9.6 ± 3.3 (8.5, 10.7) |
| Ascending aorta | 13.3 ± 5.0 (12.5, 14.1) | 14.1 ± 4.7 (13.3, 14.9) | 10.4 ± 4.7 (8.8, 12.1) |
| Descending thoracic aorta | 14.7 ± 4.9 (13.9, 15.5) | 15.6 ± 4.5 (14.8, 16.4) | 11.1 ± 4.7 (9.5, 12.7) |
| Abdominal aorta | 12.6 ± 4.0 (12.0, 13.2) | 13.4 ± 3.9 (12.7, 14.1) | 9.8 ± 3.1 (8.7, 10.9) |
| Aortic bifurcation | 11.9 ± 4.0 (11.3, 12.5) | 12.8 ± 3.8 (12.1, 13.5) | 8.6 ± 2.8 (7.6, 9.6) |
| Common iliac artery | 11.5 ± 4.1 (10.9, 12.1) | 12.3 ± 4.0 (11.6, 13.0) | 8.2 ± 2.3 (7.4, 9.0) |
| Rating | All Patients n = 161 | Group A (BMI < 30 kg/m2) n = 126 | Group B (BMI ≥ 30 kg/m2) n = 35 |
|---|---|---|---|
| Average * | 4.5 ± 0.6 | 4.6 ± 0.5 | 4.1 ± 0.9 |
| Excellent | 78 (48.4) | 69 (54.8) | 9 (25.7) |
| Good | 66 (41.0) | 49 (38.9) | 17 (48.6) |
| Moderate | 13 (8.1) | 8 (6.3) | 5 (14.3) |
| Fair | 3 (1.9) | 0 (0) | 3 (8.6) |
| Non-diagnostic | 1 (0.6) | 0 (0) | 1 (2.9) |
| Parameter | All Patients n = 161 | Group A (BMI < 30 kg/m2) n = 126 | Group B (BMI ≥ 30 kg/m2) n = 35 |
|---|---|---|---|
| Scan length (cm) | 72.9 ± 10.5 (71.3, 74.5) | 72.9 ± 9.4 (71.3, 74.6) | 72.8 ± 13.7 (68.1, 77.5) # |
| CTDIvol (mGy) | 3.39 ± 1.21 (3.20, 3.58) | 2.79 ± 0.28 (2.74, 2.84) | 5.55 ± 0.69 (5.31, 5.79) * |
| DLP (mGy*cm) | 246.3 ± 93.0 (231.8, 260.8) | 203.2 ± 30.6 (197.8, 208.6) | 401.5 ± 74.8 (375.8, 427.2) * |
| ED (mSv) | 3.69 ± 1.40 (3.48, 3.91) | 3.05 ± 0.46 (2.97, 3.13) | 6.02 ± 1.14 (5.63, 6.41) * |
| Parameter | All Patients Subset n = 50 | Group A(BMI < 30 kg/m²) Subset n = 34 | Group B (BMI ≥ 30 kg/m²) Subset n = 16 | |||
|---|---|---|---|---|---|---|
| Protocol | Low-Dose | Routine | Low-Dose | Routine | Low-Dose | Routine |
| Scan length (cm) | 67.9 ± 6.3 (66.1, 69.7) | 68.3 ± 5.8 (66.6, 69.9) ns | 67.9 ± 5.6 (65.9, 69.8) | 68.4 ± 5.3 (66.5, 70.2) ns | 68.0 ± 7.6 (63.9, 72.0) | 68.1 ± 6.9 (64.4, 71.8) ns |
| CTDIvol (mGy) | 3.72 ± 1.38 (3.33, 4.11) | 5.58 ± 2.37 (4.91, 6.26) * | 2.80 ± 0.19 (2.74, 2.87) | 4.44 ± 1.0 (4.09, 4.79) * | 5.67 ± 0.43 (5.44, 5.90) | 8.02 ± 2.61 (6.63, 9.41) * |
| DLP (mGy*cm) | 252.6 ± 97.3 (224.9, 280.2) | 382.0 ± 166.3 (334.7, 429.3) * | 190.3 ± 21.6 (182.8, 197.9) | 304.4 ± 76.4 (277.7, 331.0) * | 384.8 ± 49.6 (358.3, 411.2) | 546.9 ± 186.8 (447.4, 646.4) * |
| ED (mSv) | 3.78 ± 1.46 (3.37, 4.20) | 5.72 ± 2.5 (5.01, 6.43) * | 2.85 ± 0.32 (2.74, 2.97) | 4.57 ± 1.15 (4.17, 4.97) * | 5.76 ± 0.79 (5.33, 6.18) | 8.19 ± 2.83 (6.68, 9.69) * |
| CTDIvol saving (%) | 31.2 ± 14.5 (27.1, 35.3) | 34.0 ± 13.7 (29.3, 38.8) | 25.1 ± 14.8 (17.2, 33.0) | |||
| ED saving (%) | 31.5 ± 14.9 (27.3, 35.8) | 34.4 ± 14.5 (29.3, 39.5) | 25.4 ± 14.1 (17.9, 32.9) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, M.A.; Stoll, S.; Melzig, C.; Steuwe, A.; Partovi, S.; Böckler, D.; Kauczor, H.-U.; Rengier, F. Prospective Study of Low-Radiation and Low-Iodine Dose Aortic CT Angiography in Obese and Non-Obese Patients: Image Quality and Impact of Patient Characteristics. Diagnostics 2022, 12, 675. https://doi.org/10.3390/diagnostics12030675
Fink MA, Stoll S, Melzig C, Steuwe A, Partovi S, Böckler D, Kauczor H-U, Rengier F. Prospective Study of Low-Radiation and Low-Iodine Dose Aortic CT Angiography in Obese and Non-Obese Patients: Image Quality and Impact of Patient Characteristics. Diagnostics. 2022; 12(3):675. https://doi.org/10.3390/diagnostics12030675
Chicago/Turabian StyleFink, Matthias A., Sibylle Stoll, Claudius Melzig, Andrea Steuwe, Sasan Partovi, Dittmar Böckler, Hans-Ulrich Kauczor, and Fabian Rengier. 2022. "Prospective Study of Low-Radiation and Low-Iodine Dose Aortic CT Angiography in Obese and Non-Obese Patients: Image Quality and Impact of Patient Characteristics" Diagnostics 12, no. 3: 675. https://doi.org/10.3390/diagnostics12030675
APA StyleFink, M. A., Stoll, S., Melzig, C., Steuwe, A., Partovi, S., Böckler, D., Kauczor, H.-U., & Rengier, F. (2022). Prospective Study of Low-Radiation and Low-Iodine Dose Aortic CT Angiography in Obese and Non-Obese Patients: Image Quality and Impact of Patient Characteristics. Diagnostics, 12(3), 675. https://doi.org/10.3390/diagnostics12030675







