Abstract
Gene fusions involving NTRK1, NTRK2, and NTRK3 are rare drivers of cancer that can be targeted with histology-agnostic inhibitors. This study aimed to determine the nationwide landscape of NTRK/TRK testing in the Netherlands and the usage of pan-TRK immunohistochemistry (IHC) as a preselection tool to detect NTRK fusions. All pathology reports in 2017–2020 containing the search term ‘TRK’ were retrieved from the Dutch Pathology Registry (PALGA). Patient characteristics, tumor histology, NTRK/TRK testing methods, and reported results were extracted. NTRK/TRK testing was reported for 7457 tumors. Absolute testing rates increased from 815 (2017) to 3380 (2020). Tumors were tested with DNA/RNA-based molecular assay(s) (48%), IHC (47%), or in combination (5%). A total of 69 fusions involving NTRK1 (n = 22), NTRK2 (n = 6) and NTRK3 (n = 41) were identified in tumors from adult (n = 51) and pediatric (n = 18) patients. In patients tested with both IHC and a molecular assay (n = 327, of which 29 NTRK fusion-positive), pan-TRK IHC had a sensitivity of 77% (95% confidence interval (CI), 56–91) and a specificity of 84% (95% CI, 78–88%). These results showed that pan-TRK IHC has a low sensitivity in current routine practice and warrants the introduction of quality guidelines regarding the implementation and interpretation of pan-TRK IHC.
1. Introduction
Gene fusions that involve the neurotrophin tyrosine/tropomyosin receptor kinases 1, 2, and 3 genes (NTRK1, NTRK2, and NTRK3, which encode TRKA, TRKB, and TRKC proteins, respectively) result in constitutively active fusion proteins with an active TRK kinase domain that drives the development of cancer [1]. NTRK fusions are pathognomonic in rare types of cancer, such as the ETV6-NTRK3 fusions in secretory carcinoma of the breast or salivary gland [2,3], congenital mesoblastic nephroma [4], and infantile fibrosarcoma (IFS) [5]. In addition, NTRK fusions drive subsets of numerous other tumor types, including Spitzoid neoplasms (10–25%) [6], thyroid cancers (2–3%) [7,8], and rare cases of colorectal cancers (CRC) (<1%), primary brain tumors (<1%), sarcomas (<1%), and non-small cell lung cancers (NSCLC) (<1%) [8].
In recent years, multiple small molecule inhibitors targeting the kinase domain of TRKA, TRKB, and TRKC have been developed and tested in clinical trials. TRK-specific inhibitor larotrectinib and the multi-kinase inhibitor entrectinib (which also targets the tyrosine-protein kinases ALK and ROS) have both yielded remarkable responses in these patients [9,10]. Between 2018 and 2020, these inhibitors received histology-agnostic approvals by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for adult and pediatric patients with solid cancers harboring fusions in NTRK1, NTRK2, and NTRK3 [11,12,13,14].
The availability of effective inhibitors has sparked interest in testing methods that can screen for or confirm the presence of an NTRK fusion in tumor tissue. Currently available methods include pan-TRK immunohistochemistry (IHC) [15], and molecular assays such as fluorescence in situ hybridization (FISH) [16], fusion gene-specific reverse-transcriptase polymerase chain reaction (RT-PCR) [17], targeted DNA- or RNA-based sequencing [18], and molecular RNA counting [19]. International guidelines from both the American Society of Clinical Oncology and the European Society for Medical Oncology agree that molecular assays are necessary to show the presence of the actual NTRK fusion, and pan-TRK IHC may be used as a screening tool [20,21,22,23]. The latter recommendation was based on three studies that reported a sensitivity of 95–100% [15,24,25]. However, more recent studies report a lower sensitivity (75–88%) for pan-TRK IHC, especially for NTRK3 (55–79%) [26,27].
The objective of this study was to determine the testing landscape and incidence of NTRK fusions reported in routine diagnostics in the Netherlands and to determine the concordance between IHC and molecular assays.
2. Materials and Methods
2.1. Patient Selection
The nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA, Houten, The Netherlands) maintain a database with excerpts of all pathology reports from pathology departments in the Netherlands [28]. For this study, all pathology reports of which the microscopy and/or conclusion text contained the search term ‘TRK’ between 2017 and 2020 were retrieved. These reports were screened for assays testing TRK expression and/or the presence of NTRK fusions. Patients were excluded if the test failed or if the result was not reported (Figure S1). The data request was approved by the scientific and privacy committee of PALGA (application number LZV2019-119) and made accessible in accordance with General Data Protection Regulation (EU) 2016/679.
2.2. Data Extraction and Handling
Variables were extracted from the pathology reports by a dedicated researcher (BK) and included patient age and sex, tumor histology, mutations that were detected in other (driver) genes, whether TRK expression or NTRK fusions were, the type of test(s) used to test for TRK/NTRK, and the reported TRK expression and/or NTRK fusion status. Data processing was performed in accordance with the General Data Protection Regulation (EU) 2016/679.
2.3. Interpretation of Reported TRK/NTRK Testing Results
The reported TRK/NTRK testing results were interpreted in accordance with (inter) national consensus guidelines [20,21,22,23]. These state that a positive or inconclusive IHC result should be confirmed with a molecular assay such as FISH or an RNA analysis (Figure S2). If all test results were negative, it was interpreted as “no NTRK fusion.” A positive molecular result was interpreted as “NTRK fusion positive.” In case of a positive or inconclusive IHC staining, the result was interpreted as “unconfirmed” when no subsequent confirmatory molecular assay was performed and “NTRK fusion positive” if a subsequent molecular assay did demonstrate presence of an NTRK fusion. If a molecular test yielded an inconclusive result, or if there were discrepancies between two or more molecular tests, the result was interpreted as “inconclusive”.
2.4. Statistics
Statistical analysis was performed with SPSS version 23 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used, proportional differences were assessed using Fisher’s exact tests. The performance of IHC as a screening tool in the routine setting for the detection of NTRK fusions was determined by calculating sensitivity and specificity for positive IHC results with the molecular result as a reference. This calculation was performed for cases with only positive IHC and repeated for cases with either positive or inconclusive results. A nominal p-value of 0.05 or less was considered statistically significant.
3. Results
3.1. Patients Included in the Analysis
A total of 14,025 pathology reports contained the search term ‘TRK’ in PALGA between 2017 and 2020, representing 9595 unique malignant and benign tumors. TRK expression and/or NTRK fusion testing results were reported in 7457 tumors from 7434 patients (for 23 patients, two unique tumors were tested for NTRK). The number of tested tumors gradually increased from 2017 (n = 815) to 2020 (n = 3380) (Figure 1A).
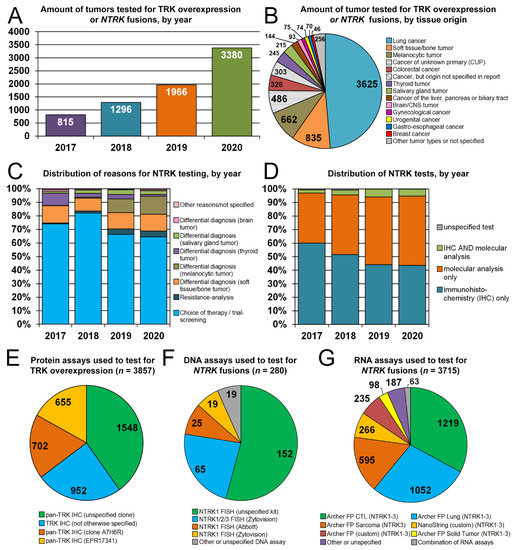
Figure 1.
Indications and number of TRK overexpression and/or NTRK fusions testing in the Netherlands, 2017–2020. (A,B) Number of tumors tested for TRK overexpression or NTRK fusions, by year (A) and tissue origin (B). (C) Distribution of reasons for NTRK/TRK testing, by year. (D) Distribution of NTRK tests, by year. (E–G) Protein (E), DNA (F) and RNA (G) assays used to test for TRK overexpression or NTRK fusions. Abbreviations: CNS, central nervous system; CTL, comprehensive thyroid and lung; FISH, fluorescence in situ hybridization; FP, FusionPlex.
3.2. TRK Expression or NTRK Fusion Testing: Types of Tumors and Reasons for Testing
Lung cancer was the most frequent type of tumor tested for NTRK (n = 3625; 48.6%). Less frequent types of tumors included soft tissue/bone tumors (n = 835; 11.2%), melanocytic tumors (n = 662; 8.9%), cancers of unknown primary (n = 486; 6.5%), CRC (n = 328; 4.4%), thyroid tumors (n = 245; 3.3%) and salivary gland tumors (n = 215; 2.9%), among others (Figure 1B). Tumor biopsies were tested for TRK expression or NTRK fusions for choice of therapy (n = 5147; 69.0%), differential diagnosis (n = 2056; 27.6%) or resistance analysis after therapy progression (n = 250; 3.4%) (Figure 1C). Indications that proportionally increased in 2019–2020 as opposed to 2017–2018 were the differential diagnosis of melanocytic tumors (increase from 0.5% to 12.1%; p < 0.001) and resistance analysis at progression on targeted therapy (increase from 1.1% to 4.2%; p < 0.001). The latter category included 248 lung cancer patients progressing on ALK, BRAF or EGFR inhibitors and two patients with a gastro-intestinal stromal tumor progressing on imatinib.
3.3. Assays Used to Test for TRK Overexpression and NTRK Fusions
Tumors were tested with a molecular analysis only (n = 3587; 48.1%), IHC only (n = 3496; 46.9%), IHC and a molecular analysis (n = 352; 4.7%), or with an unspecified test (n = 22; 0.3%) (Figure 1D). In total, 3848 tumors were tested with IHC, but for 2536 tumors (66%), the clone was not specified. The pan-TRK rabbit monoclonal antibodies specified in reports were EPR17341 (Abcam, Cambridge, MA, USA; n = 655) and A7H6R (Cell Signaling Technology, Danvers, MA, USA; n = 701; Figure 1E). The EPR17341 antibody was only used as part of a laboratory-developed test: the recently approved CE-IVD kit with this antibody (Ventana Medical Systems, Oro Valley, AZ, USA) was not reported.
FISH analysis was the most used DNA-based molecular test (277/280) (Figure 1F). The proportion of tumors tested only with a DNA assay decreased in 2019–2020 as opposed to 2017–2018 (4.8% to 1.4%), whereas the proportion tested with only multiplex RNA analysis increased (35% to 49%). Nearly all (>98%) of RNA-based molecular assay (n = 3715) were multiplex RNA analyses, and the vast majority of tumor biopsies were tested with Archer FusionPlex kits (Archer DX, Boulder, CO, USA; n = 3273) (Figure 1G).
Of note, the FDA-approved companion diagnostic FoundationOne CDx was not used by any laboratory. This is in part because this assay was approved only at the end of the inclusion period of the current study (October 2020) [29]. In addition, Dutch and European guidelines currently do not specify which RNA assay is most suitable for the detection of NTRK fusions [21,22].
3.4. NTRK Fusions or TRK Expression Detected in Routine Diagnostics
Between 2017 and 2020, a total of 7457 tumors (7434 patients) were tested for TRK expression and/or NTRK fusions, and an NTRK1-3 fusion was detected with a molecular assay in 69 (0.93%) tumors (Table 1 and Table S1). This proportion was 1.8% when counting only the patients tested with a molecular assay (n = 3939). These percentages do not reflect the true prevalence of NTRK fusions in the population as there is a selection bias in routine diagnostics (patients will only be tested when clinically relevant). For this reason, frequencies could not be calculated for individual tumor types. In addition, there were nine patients with inconclusive molecular results or with discrepancies between two or more molecular tests.

Table 1.
Characteristics of patients with a molecular-confirmed NTRK1-3 fusion.
The detected NTRK fusions involved NTRK1 (n = 22), NTRK2 (n = 6) and NTRK3 (n = 41). Frequently identified NTRK fusion genes included TMP3-NTRK1 (n = 7), LMNA-NTRK1 (n = 4), ETV6-NTRK3 (n = 28), and MYO5A-NTRK3 (n = 4). In addition to these four fusions, 18 other unique fusion genes were reported (Figure 2A). NTRK fusion-positive patients had varying types of malignant and benign tumors (Figure 2B).
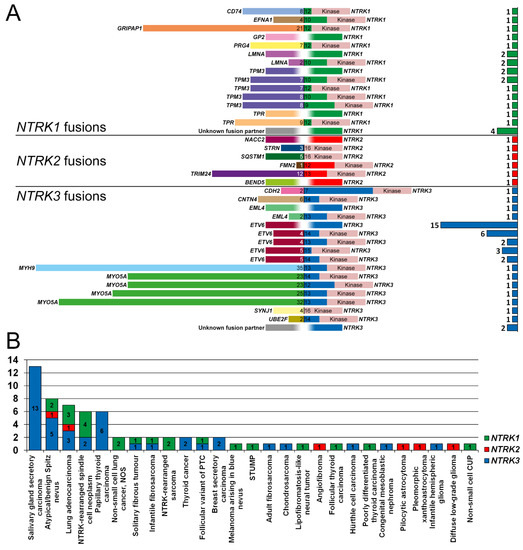
Figure 2.
NTRK1-3 fusions identified in the Dutch population, 2017–2020. (A) Fusion partners and exonic breakpoints were identified in 69 patients with NTRK1 (green), NTRK2 (red), and NTRK3 (blue) fusions. The kinase domain is annotated and shown in pink. A blurred white breakpoint indicates that the exact breakpoint was not known or specified. At the right, a bar graph depicts the number of times the specific fusion gene was detected. (B) Frequency of NTRK1 (green), NTRK2 (red), and NTRK3 (blue) fusions and types of malignant or benign tumors. Abbreviations: CUP, cancer of unknown primary; NOS, not otherwise specified; PTC, papillary thyroid carcinoma, STUMP, Spitzoid Tumor of Uncertain Malignant Potential.
In addition to the 69 molecular-positive patients, there were 130 patients (1.7%) with a positive (n = 67) or inconclusive IHC result (n = 63) for whom molecular assay was not performed to confirm (or exclude) the presence of an NTRK fusion. Reasons for not confirming the IHC result varied but were not specified in the vast majority of cases (n = 113; 87%).
3.5. IHC as a Preselection Tool to Detect Fusions
A total of 352 tumors were tested with both IHC and a molecular assay. After excluding patients for whom either test failed (n = 15), with inconclusive molecular results (n = 4) or insufficient information (n = 6), 327 were eligible for a sensitivity/specificity analysis, of which 29 patients had a molecular-confirmed NTRK fusion. There were 114 patients with either a positive (n = 61) or inconclusive (n = 53) IHC result. An NTRK fusion was confirmed in 20% (23 of 114); the positivity rate was 33% (20 of 61) for IHC-positive patients and 6% (3 of 53) for IHC-inconclusive patients. On the other hand, there were six patients who tested negative with IHC, but who nevertheless harbored an NTRK fusion using a molecular test (representing 21% of all NTRK fusion-positive patients tested with both IHC and a molecular assay). This included multiple well-described NTRK3 fusions, including ETV6-NTRK3, MYO5A-NTRK3, and TPM3-NTRK1. These six patients with IHC-negative NTRK fusion-positive tumors were tested in five different laboratories, and the antibody clone was only reported in one (EPR17431, laboratory-developed). Pan-TRK IHC was found to have a sensitivity of 77% (95% CI, 56–91) and a specificity of 83% (95% CI, 78–88%) when excluding patients with an inconclusive IHC result. When patients with an inconclusive IHC result were also included, sensitivity raised slightly to 79% (95% CI, 60–92%), but specificity declined to 70% (95% CI, 64–74%) (Table 2).

Table 2.
Sensitivity and specificity of pan-TRK IHC as a screening tool for NTRK fusions with molecular assay as a reference.
4. Discussion
In this study, the incidence of NTRK fusions in tumors was investigated in the Dutch population between 2017 and 2020 reported in the nationwide PALGA database of pathology reports. Over time, tumor biopsies were increasingly tested for TRK expression or NTRK fusions, with most tumors being tested with IHC and/or multiplex RNA analysis. In this four-year period, 69 NTRK fusion-positive tumors were reported in 7434 patients tested. In addition, the data were used to assess the performance of pan-TRK IHC as a pre-screening tool to detect NTRK fusions. The analysis of real-world data indicated a low sensitivity for pan-TRK IHC to preselect patients for molecular NTRK testing in routine practice.
4.1. Testing Rates for TRK Expression and NTRK Fusion in The Netherlands
Testing for NTRK fusions was not standard-of-care for any type of cancer until 2020, when NTRK became part of the required molecular markers that any advanced NSCLC had to be tested for according to the Dutch guideline for NSCLC [30]. However, NTRK fusions have been of interest since 2017, as diagnostic markers of disease (for example, for secretory carcinoma) or as predictive markers of response to TRK inhibitors, which were then available within clinical trials and in named patient programs. The FDA and EMA have since approved both entrectinib and larotrectinib for the treatment of advanced cancers with an NTRK fusion [11,12,13,14]. This is reflected by the increasing national testing rate (from 815 tumors in 2017 to 3380 tumors in 2020), resulting in an increase from 10 NTRK fusions diagnosed in 2017 (1.2% of tumors tested) to 31 NTRK fusions diagnosed in 2020 (0.9% of tumors tested). As larotrectinib and entrectinib have been approved for use in the Netherlands recently, the testing rate will likely further increase in the coming years.
4.2. Types of Tumors Harboring NTRK Fusions
NTRK fusions are highly enriched in secretory carcinomas (of the salivary gland or breast), congenital mesoblastic nephroma, and IFS (>90%) [2,3,4,5]. In the current dataset, these cancers were all reported, with NTRK fusions detected in similar proportions, though the absolute numbers were relatively low. For example, there were only two NTRK fusion-positive secretory carcinomas of the breast, whereas the expected number in the Netherlands between 2017–2020 is approximately 10–11 (estimated incidence 0.015% of approximately 17,000 annual breast cancer diagnoses) [31,32,33]. The low incidence in the current dataset is likely because these cancers are not yet routinely tested for the presence of NTRK fusions but rather are diagnosed clinicopathologically and/or immunophenotypically, or that patients often present with early-stage disease and are therefore not eligible for treatment with TRK inhibitors [32].
Spitzoid melanocytic tumors also commonly harbor NTRK fusions (2–25%) [6,7,8]. Indeed, these tumors were among the most reported NTRK fusion-positive tumors in the Dutch population (n = 10). In Spitzoid tumors, NTRK1 and NTRK3 fusions have frequently been reported, and these fusions are a diagnostic marker for this category of melanocytic tumors [34,35]. NTRK1 and NTRK3 fusions were indeed more common in the Dutch population (n = 4 and n = 5, respectively), mostly in benign tumors, and all of these patients were tested for the differential diagnosis. Of note, one patient with a benign Spitz nevus harbored a previously reported SQSTM1-NTRK2 fusion, which confirms findings from a recent case report that Spitz/Reed nevi can harbor fusions in any of the three NTRK genes [36].
In thyroid tumors, NTRK fusions are also relatively common (2–25%) [7,8], though some studies report that this is mostly true for papillary thyroid carcinoma (PTC) [37]. In the Dutch population (13 NTRK fusion-positive thyroid cancers), PTC was indeed the most frequent subtype of NTRK fusion-positive thyroid cancer (6/13), but NTRK fusions were also detected in follicular variants of PTC, a Hürthle cell carcinoma, and a poorly differentiated thyroid carcinoma. Thus, the histological subtype of thyroid cancer does not exclude the presence of an NTRK fusion.
In addition, NTRK fusions are rare (<1%) drivers of other types of cancer, such as NSCLC, CRC, primary brain tumors, and soft tissue/bone tumors [8]. In NSCLC and CRC, the prevalence is estimated at 0.23% [38,39]. In our dataset, a similar prevalence of 0.25% was found in NSCLC (9/3625), representing fusions in all three NTRK genes. In CRC, 89% of NTRK fusion-positive patients have mismatch repair deficiency (MMRd) and lack mutations in BRAF (BRAFwt); when testing only MMRd/BRAFwt CRC patients, the prevalence increases from 0.23% to 5.3% [40]. In our dataset, NTRK fusion-positive CRC was not identified despite a sample size of 328 patients.
Furthermore, NTRK fusions were detected in varying types of non-IFS soft tissue/bone tumors (n = 14), of which the majority (n = 8) were classified as NTRK-rearranged spindle cell neoplasm/sarcoma in accordance with the 2020 World Health Organization Classification of Tumors of Soft Tissue and Bone [41]. However, NTRK fusion-positive soft tissue/bone tumors diagnosed prior to 2020 (which include solitary fibrous tumors, an angiofibroma, and chondrosarcoma) may have been classified similarly had they been diagnosed according to the 2020 WHO classification. Considering NTRK fusions have been recommended by the WHO guideline as diagnostic markers for sarcomas, the annual incidence of NTRK fusion-positive sarcomas is expected to further increase in the coming years.
4.3. Fusion Partners of NTRK1–3
A range of different NTRK1, NTRK2 and NTRK3 fusions were reported in the Dutch population between 2017 and 2020. A total of 22 unique RNA-confirmed fusion genes were identified. ETV6-NTRK3 fusions, which are among the most well-described NTRK fusions in human tumors [3], were the most common in this study (41%; 28/69). This prevalence was higher than reported in other studies (26–29%) [42], and may be due to an overrepresentation as this has been a known diagnostic marker and thus was the only fusion for which a targeted RT-PCR was used. Aside from ETV6-NTRK3, 10 other fusion genes identified in our study had been previously reported: CD74-NTRK1 [43], LMNA-NTRK1 [44], TPM3-NTRK1 [44], TPR-NTRK1 [45], NACC2-NTRK2 [46], STRN-NTRK2 [47], SQSTM1-NTRK2 [48], EML4-NTRK3 [49], MYH9-NTRK3 [35], MYO5A-NTRK3 [35]. Three other fusions were novel, although the fusion partner has been reported in other driver genes: GRIPAP1-NTRK1 (fusion partner previously reported for TFE3) [50], TRIM24-NTRK2 (BRAF) [51], and BEND5-NTRK2 (ALK) [52]. The remaining eight fusion partners were novel: EFNA1-NTRK1, GP2-NTRK1, PRG4-NTRK1, FMN2-NTRK2, CDH2-NTRK3, CNTN4-NTRK3, SYNJ1-NTRK3, and UBE2F-NTRK3. Although all these fusions harbored the TRK kinase domain (Figure 2A), their pathogenicity is not determined. Tumors harboring EFNA-NTRK1 and CNTN4-NTRK3 fusions also had a positive pan-TRK IHC, and tumors harboring the GP2-NTRK1 fusion had both positive IHC and FISH results, which reinforces the notion that these fusions result in increased expression of the TRK kinase domain. In contrast, the tumor harboring a CDH2-NTRK3 fusion, which had a large portion of intact TRKC protein (fused at exon 7 of TRKC), had no TRK expression with IHC. The other novel fusions were not tested with IHC.
4.4. Co-Occurrence of Other Driver Mutations
Previous studies have demonstrated that NTRK fusions are typically mutually exclusive with other driver mutations [16,26]. However, studies have reported co-occurrence of NTRK fusions with well-known drivers such as oncogenic BRAF, EGFR, or KRAS mutations [53,54]. In our dataset, 53 of the 69 NTRK fusion-positive patients were tested for concurrent driver mutations and/or fusions (Table S1), of which all but three patients did not harbor another driver mutation. Two of these three patients, all of whom had lung adenocarcinoma, were treatment naïve. One EGFR p.(L858R)-mutant patient harbored the novel PRG4-NTRK1 fusion (TRK expression was not tested), and one a KRAS p.(A146T)-mutant patient harbored a CD74-NTRK1 fusion, with TRK expression. The third patient, who harbored an EGFR p.(E746_A750del) mutation, acquired the novel EFNA1-NTRK1 fusion as a resistance mechanism against EGFR inhibitor treatment; in this patient, TRK expression was confirmed with IHC. This finding is in line with previous reports that NTRK fusions can induce resistance to EGFR inhibitors [55]. It is unknown how co-occurrence of NTRK fusions with other driver mutations would impact TRK inhibitor sensitivity.
4.5. Performance of Immunohistochemistry as a Screening Tool for the Detection of NTRK Fusions
Guidelines and consensus recommendations indicate that the presence of NTRK fusions can be most reliably detected using molecular assays [20,21,22,23]. In addition, they suggest that IHC is a reliable screening tool to exclude the presence of NTRK fusions. In general, IHC is of interest as a screening tool because it is relatively inexpensive, efficient, and easily implemented in pathology laboratories [21]. For some markers, such as ALK, IHC has proven to be a valuable alternative to predict molecular marker-based treatment response [56], whereas, in other markers, such as MET [57], IHC expression correlates poorly with the presence of a molecular deficit. For a screening tool to qualify as useful, its ability to exclude the presence of a condition, its sensitivity, needs to be near to 100% [58]. However, a high specificity is also desirable to ensure the cost-effectiveness of the test (the lower the specificity, the more patients will require both the screening test and the confirmatory test). Guidelines recommending pan-TRK IHC as a screening tool were mostly based on three studies from 2017 and 2018 (Table 3) [15,24,25]. However, subsequently, two more recent studies demonstrated lower sensitivity to screen for NTRK fusions, especially for NTRK3 [26,27], which was reflected in the current nationwide study based on reports from routine testing. Multiple TRK IHC-negative cases with well-described NTRK3 fusions, including ETV6-NTRK3 and MYO5A-NTRK3 were reported.

Table 3.
Sensitivity of TRK immunohistochemistry as a screening tool to detect NTRK fusions, according to published studies.
It is uncertain whether these fusions really do not lead to IHC positivity or whether the IHC procedure or the choice of antibodies requires optimization and/or standardization. For the predictive biomarker PD-L1, it was demonstrated previously that preanalytical variability in immunostaining procedures could lead to inter-rater variability in scoring among pathologists [59]. Similarly, there are currently no quality guidelines regarding preanalytical requirements for immunostaining with pan-TRK IHC antibodies. Introducing such quality guidelines may improve the sensitivity of pan-TRK IHC to pre-screen tumors for the presence of NTRK fusions.
A multitude of antibodies are available, but most laboratories use a pan-TRK antibody, which targets the C-terminus of TRKA, TRKB, and TRKC [60]. Two antibodies are utilized especially: EPR17341 and A7H6R. It has been demonstrated that EPR17341 and A7H6R result in highly different staining patterns [61]. In our study, the antibody used to test for TRK expression was not reported in the majority of reports. The EPR17341 antibody is the only antibody that is available in a CE-IVD-approved kit but demonstrated insufficient sensitivity in the studies by Solomon et al. (88%) and Galatica et al. (75%). A false-negative IHC result was also identified in the current dataset (in melanoma harboring a TPM3-NTRK1 fusion), which indicates that the current immunohistochemical procedure may not be adequate to screen for NTRK fusions. The A7H6R antibody is not available in a CE-IVD-approved kit and has not been thoroughly investigated in the literature. Future studies should elucidate whether this antibody is more sensitive, and/or focus on the development of novel antibodies that may have improved sensitivity compared to the currently available antibodies.
There are no standardized scoring criteria to determine the positivity of TRK IHC, including for the CE-IVD approved assay [62]. In the current study, this resulted in a nearly equal number of positive and inconclusive patients. Pathologists reported an inconclusive result when they were uncertain whether the percentage of cells with positive staining or the intensity of staining could be classified as positive. Indeed, in 53 tumors with an inconclusive IHC that were also tested with a molecular assay, only three (6%) were found to harbor an NTRK fusion. In addition, depending on the fusion present, TRK IHC can stain differently (cytoplasmic, perinuclear, nuclear, or membranous) [15]. This underscores the necessity to develop internationally standardized criteria for scoring TRK IHC positivity, which is currently lacking.
4.6. Future Role of pan-TRK Immunohistochemistry in Diagnostic Algorithms
Targeted RNA analysis is considered the gold standard for detecting NTRK fusions, and all international guidelines recommend confirmation of positive pan-TRK IHC with a targeted RNA analysis [20,21,22,23]. The findings in this as well as other recent studies that address sensitivity and specificity of pan-TRK IHC [26,27], indicate that up-front testing with a targeted RNA analysis should be preferred in some scenarios. In case of a high prevalence of NTRK fusions in cancer types with a low incidence, such as the ETV6-NTRK3 fusions in secretory carcinomas, using a suboptimal pre-screening tool that requires confirmation is not practical. This is also true for tumors with high endogenous TRK expression, such as gliomas and some types of soft tissue tumors. Pre-screening with pan-TRK IHC will yield a high rate of false-positivity. Up-front RNA testing may also be feasible for patients with a frequently occurring type of cancer and a low prevalence of NTRK fusions. For example, patients with NSCLC are routinely tested for the presence of fusions in ALK, RET, ROS1, and MET exon 14 skipping in a single RNA-based assay that can often easily be expanded with the NTRK1–3 fusions. In spite of a 100% sensitivity of TRK IHC in the five lung cancer cases in the current study, it is more efficient from a tissue management and cost perspective to test NSCLC patients with an up-front, panel-based, targeted RNA assay rather than adding an IHC screening for TRK expression. For some types of cancer, such as CRC and non-Spitz melanoma, it is not cost-effective to test all patients using a costly RNA-based technique as less than 1% actually harbor an NTRK fusion, and they are not routinely tested using an RNA-based analysis for other reasons. For these types of cancer, a pre-screening test such as pan-TRK IHC may still have its value when procedures (analytical and interpretation) are optimized.
Altogether, the results of the current study support findings from other recent studies that pan-TRK IHC with currently used antibodies is not yet adequate to screen tumors for the presence of NTRK fusions in routine diagnostics. Quality guidelines regarding the implementation of validated pan-TRK IHC antibodies and standardized staining and scoring criteria are required.
5. Conclusions
This nationwide, longitudinal analysis shows that routine TRK testing is gradually increasing, with NTRK fusions detected in a variety of 69 benign and malignant tumors in 2017–2020. A performance analysis demonstrated low sensitivity and specificity of immunohistochemistry as a preselection tool for the detection of NTRK fusions in current routine practice. Recommendations by international guidelines that pan-TRK IHC is a reliable screening tool should, therefore, be reconsidered in light of the current study and recently published real-world evidence. These results warrant the introduction of quality guidelines regarding the implementation of validated pan-TRK IHC antibodies and standardized staining and scoring criteria for interpreting results in a routine diagnostic setting. More sensitive pan-TRK antibodies may be needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12030668/s1, Figure S1: Patient selection procedure; Figure S2: Interpretation of TRK testing results in accordance with various (inter)national guidelines; Table S1: List of patients with a molecular-confirmed NTRK1-3 fusion (n = 69).
Author Contributions
Conceptualization, B.K., H.J.M.G., E.S., S.M.W. and L.C.v.K. Collection and assembly of data: B.K. and C.C.H.J.K. Data analysis: B.K. Data interpretation: B.K., C.C.H.J.K., H.J.M.G., W.T., E.S., S.M.W. and L.C.v.K. Manuscript writing: B.K., C.C.H.J.K., H.J.M.G., W.T., E.S., S.M.W. and L.C.v.K. Final approval of manuscript: B.K., C.C.H.J.K., H.J.M.G., W.T., E.S., S.M.W. and L.C.v.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Bayer AG [grant number MEDSP00017], but the funder had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the data request was approved by the scientific and privacy committee of PALGA (application number LZV2019-119) and made accessible in accordance with General Data Protection Regulation (EU) 2016/679.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its Supplementary Information Files].
Acknowledgments
We thank participating laboratories for providing additional information to complement data retrieved from the pathology reports.
Conflicts of Interest
H.J.M.G. reports advisory board presence for Bayer, Lilly, Merck, and Novartis, outside the submitted work. W.T. reports consulting fees to the institution from Merck Sharp Dohme, and Bristol-Myers-Squibb, board membership for Dutch Society of Pathology, and the Council for Research and Innovation of the Federation of Medical Specialists, outside the submitted work. E.S. reports providing lectures for Bio-Rad, Novartis, Roche, Biocartis, Illumina, Pfizer, AstraZeneca, and Agena Bioscience, advisory board presence for AstraZeneca, Roche, Pfizer, Novartis, Bayer, Lilly, BMS, Amgen, Biocartis, Illumina, Agena Bioscience, and MSD/Merck, and research grants from Pfizer, Biocartis, Invitae-ArcherDX, AstraZeneca, Agena Bioscience, BMS, Bio-Rad, Roche, Boehringer Ingelheim, all outside the submitted work and all financial supports transferred to Institute. S.M.W. reports unrestricted research grants from Bayer, MSD, Roche, Pfizer, Novartis, Amgen and AstraZeneca, outside the submitted work. L.C.v.K reports grants and non-financial support from Roche, advisory board presence for AstraZeneca, Novartis, Merck, Janssen-Cilag, Bayer, BMS, nanoString and Pfizer, grants and non-financial support from Invitae, non-financial support from Biocartis, grants from Bayer, non-financial support from nanoString, all outside the submitted work. All remaining authors (B.K., C.C.H.J.K.) have declared no conflict of interest.
References
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.W.; Passador-Santos, F.; et al. Mammary Analogue Secretory Carcinoma of Salivary Glands, Containing the ETV6-NTRK3 Fusion Gene: A Hitherto Undescribed Salivary Gland Tumor Entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 Gene Fusion as a Primary Event in Human Secretory Breast Carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef]
- Rubin, B.P.; Chen, C.J.; Morgan, T.W.; Xiao, S.; Grier, H.E.; Kozakewich, H.P.; Perez-Atayde, A.R.; Fletcher, J.A. Congenital Mesoblastic Nephroma t(12;15) Is Associated with ETV6-NTRK3 Gene Fusion: Cytogenetic and Molecular Relationship to Congenital (Infantile) Fibrosarcoma. Am. J. Pathol. 1998, 153, 1451–1458. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A Novel ETV6-NTRK3 Gene Fusion in Congenital Fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, T.; He, J.; Yelensky, R.; Esteve-Puig, R.; Botton, T.; Yeh, I.; Lipson, D.; Otto, G.; Brennan, K.; Murali, R.; et al. Kinase Fusions Are Frequent in Spitz Tumours and Spitzoid Melanomas. Nat. Commun. 2014, 5, 3116. [Google Scholar] [CrossRef]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The Landscape of Kinase Fusions in Cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef]
- Okamura, R.; Boichard, A.; Kato, S.; Sicklick, J.K.; Bazhenova, L.; Kurzrock, R. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis. Oncol. 2018, 2, 1–20. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration FDA. Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. Available online: https://www.fda.gov/drugs/fda-approves-larotrectinib-solidtumors-ntrk-gene-fusions (accessed on 26 October 2021).
- U.S. Food & Drug Administration FDA. Approves Entrectinib for NTRK Solid Tumors and ROS-1 NSCLC 2019. Available online: https://www.fda.gov/drugs/fda-approves-entrectinib-ntrk-solidtumors-and-ros-1-nsclc (accessed on 26 October 2021).
- European Medicines Agency. Vitrakvi (Larotrectinib): An Overview of Vitrakvi and Why It Is Authorised in the EU; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- European Medicines Agency. Rozlytrek (Entrectinib): An Overview of Rozlytrek and Why It Is Authorised in the EU; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Rudzinski, E.R.; Sepulveda, A.R. Testing Algorithm for Identification of Patients with TRK Fusion Cancer. J. Clin. Pathol. 2019, 72, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, N.; Kirchner, M.; Lehmann, U.; Leichsenring, J.; Merkelbach-Bruse, S.; Glade, J.; Hummel, M.; Stögbauer, F.; Lehmann, A.; Trautmann, M.; et al. Testing NTRK Testing: Wet-Lab and in Silico Comparison of RNA-Based Targeted Sequencing Assays. Genes Chromosomes Cancer 2020, 59, 178–188. [Google Scholar] [CrossRef]
- Zito Marino, F.; Alì, G.; Facchinetti, F.; Righi, L.; Fontanini, G.; Rossi, G.; Franco, R. Fusion Proteins in Lung Cancer: Addressing Diagnostic Problems for Deciding Therapy. Expert Rev. Anticancer Ther. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Pentheroudakis, G.; Mishima, S.; Overman, M.J.; Yeh, K.-H.; Baba, E.; Naito, Y.; Calvo, F.; Saxena, A.; Chen, L.-T.; et al. JSCO-ESMO-ASCO-JSMO-TOS: International Expert Consensus Recommendations for Tumour-Agnostic Treatments in Patients with Solid Tumours with Microsatellite Instability or NTRK Fusions. Ann. Oncol. 2020, 31, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.-Y.; et al. ESMO Recommendations on the Standard Methods to Detect NTRK Fusions in Daily Practice and Clinical Research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Van Kempen, L.C.; van Wezel, T.; Morreau, H.; Cohen, D.; Timens, W.; Willems, S.M.; Schuuring, E. De Rol van Moleculaire Diagnostiek in Het Identificeren van Patiënten Die Baat Hebben Bij TRK-Remmer-Therapie. Ned. Tijdschr. Oncol. 2020, 17, 266–273. [Google Scholar]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovée, J.V.M.G.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and Management of Tropomyosin Receptor Kinase (TRK) Fusion Sarcomas: Expert Recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Lockwood, C.M.; Stohr, B.A.; Vargas, S.O.; Sheridan, R.; Black, J.O.; Rajaram, V.; Laetsch, T.W.; Davis, J.L. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol. 2018, 42, 927–935. [Google Scholar] [CrossRef]
- Murphy, D.A.; Ely, H.A.; Shoemaker, R.; Boomer, A.; Culver, B.P.; Hoskins, I.; Haimes, J.D.; Walters, R.D.; Fernandez, D.; Stahl, J.A.; et al. Detecting Gene Rearrangements in Patient Populations through a 2-Step Diagnostic Test Comprised of Rapid IHC Enrichment Followed by Sensitive Next-Generation Sequencing. Appl. Immunohistochem. Mol. Morphol. AIMM 2017, 25, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular Characterization of Cancers with NTRK Gene Fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef]
- Casparie, M.; Tiebosch, A.T.M.G.; Burger, G.; Blauwgeers, H.; van de Pol, A.; van Krieken, J.H.J.M.; Meijer, G.A. Pathology Databanking and Biobanking in The Netherlands, a Central Role for PALGA, the Nationwide Histopathology and Cytopathology Data Network and Archive. Anal. Cell. Oncol. 2007, 29, 19–24. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration FDA. Approves Companion Diagnostic to Identify NTRK Fusions in Solid Tumors for Vitrakvi. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-companion-diagnostic-identify-ntrk-fusions-solid-tumors-vitrakvi (accessed on 21 October 2021).
- Nederlandse Vereniging voor Artsen voor Longziekten en Tuberculose Behandeling Patiënten Met Een Zeldzame Mutatie Bij NSCLC. Available online: https://richtlijnendatabase.nl/richtlijn/niet_kleincellig_longcarcinoom/systemische_behandeling_stadium_iv_nsclc/behandeling_pati_nten_met_een_zeldzame_mutatie_bij_nsclc.html (accessed on 23 April 2020).
- Integraal Kankercentrum Nederland NKR Cijfers. Available online: https://iknl.nl/nkr-cijfers (accessed on 3 August 2021).
- Horowitz, D.P.; Sharma, C.S.; Connolly, E.; Gidea-Addeo, D.; Deutsch, I. Secretory Carcinoma of the Breast: Results from the Survival, Epidemiology and End Results Database. Breast 2012, 21, 350–353. [Google Scholar] [CrossRef]
- Jacob, J.D.; Hodge, C.; Franko, J.; Pezzi, C.M.; Goldman, C.D.; Klimberg, V.S. Rare Breast Cancer: 246 Invasive Secretory Carcinomas from the National Cancer Data Base. J. Surg. Oncol. 2016, 113, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Uguen, A. Spitz Tumors with NTRK1 Fusions: TRK-A and Pan-TRK Immunohistochemistry as Ancillary Diagnostic Tools. Am. J. Surg. Pathol. 2019, 43, 1438–1439. [Google Scholar] [CrossRef]
- Yeh, I.; Tee, M.K.; Botton, T.; Shain, A.H.; Sparatta, A.J.; Gagnon, A.; Vemula, S.S.; Garrido, M.C.; Nakamaru, K.; Isoyama, T.; et al. NTRK3 Kinase Fusions in Spitz Tumours. J. Pathol. 2016, 240, 282–290. [Google Scholar] [CrossRef]
- Goto, K.; Pissaloux, D.; Tirode, F.; de la Fouchardière, A. Spitz Nevus with a Novel TFG-NTRK2 Fusion: The First Case Report of NTRK2-Rearranged Spitz/Reed Nevus. J. Cutan. Pathol. 2021, 48, 1193–1196. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Dias-Santagata, D.; Farahani, A.A.; Boyraz, B.; Faquin, W.C.; Nosé, V.; Sadow, P.M. Clinicopathologic and Molecular Characterization of NTRK-Rearranged Thyroid Carcinoma (NRTC). Mod. Pathol. 2020, 33, 2186–2197. [Google Scholar] [CrossRef]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.; Chłopek, M.; Lamoureux, J.; Christiansen, J.; Kowalik, A.; Wasąg, B.; Felisiak-Gołąbek, A.; Agaimy, A.; Biernat, W.; Canzonieri, V.; et al. Colonic Adenocarcinomas Harboring NTRK Fusion Genes: A Clinicopathologic and Molecular Genetic Study of 16 Cases and Review of the Literature. Am. J. Surg. Pathol. 2020, 44, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Fraser, T.; Ahadi, M.; Fuchs, T.; Sioson, L.; Clarkson, A.; Sheen, A.; Singh, N.; Corless, C.L.; Gill, A.J. NTRK Gene Rearrangements Are Highly Enriched in MLH1/PMS2 Deficient, BRAF Wild-Type Colorectal Carcinomas-a Study of 4569 Cases. Mod. Pathol. 2020, 33, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO Classification: What’s New in Soft Tissue Tumor Pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Krebs, M.G.; Le Tourneau, C.; Sokol, E.S.; Maund, S.L.; Wilson, T.R.; Jin, D.X.; Newberg, J.Y.; Fabrizio, D.; Veronese, L.; et al. Genomic Context of NTRK1/2/3 Fusion-Positive Tumours from a Large Real-World Population. NPJ Precis. Oncol. 2021, 5, 69. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Capelletti, M.; Le, A.T.; Kako, S.; Butaney, M.; Ercan, D.; Mahale, S.; Davies, K.D.; Aisner, D.L.; Pilling, A.B.; et al. Oncogenic and Drug-Sensitive NTRK1 Rearrangements in Lung Cancer. Nat. Med. 2013, 19, 1469–1472. [Google Scholar] [CrossRef]
- Park, D.Y.; Choi, C.; Shin, E.; Lee, J.H.; Kwon, C.H.; Jo, H.-J.; Kim, H.-R.; Kim, H.S.; Oh, N.; Lee, J.S.; et al. NTRK1 Fusions for the Therapeutic Intervention of Korean Patients with Colon Cancer. Oncotarget 2016, 7, 8399–8412. [Google Scholar] [CrossRef][Green Version]
- Greco, A.; Miranda, C.; Pagliardini, S.; Fusetti, L.; Bongarzone, I.; Pierotti, M.A. Chromosome 1 Rearrangements Involving the Genes TPR and NTRK1 Produce Structurally Different Thyroid-Specific TRK Oncogenes. Genes Chromosomes Cancer 1997, 19, 112–123. [Google Scholar] [CrossRef]
- Carter-Febres, M.; Schneller, N.; Fair, D.; Solomon, D.; Perry, A.; Roy, A.; Linscott, L.; Alashari, M.; Kestle, J.R.; Bruggers, C.S. Adjuvant Maintenance Larotrectinib Therapy in 2 Children with NTRK Fusion-Positive High-Grade Cancers. J. Pediatr. Hematol./Oncol. 2020, 43, e987–e990. [Google Scholar] [CrossRef]
- Wu, L.W.; Pavlock, T.; Patterson, A.; Post, A.; Ambrose, C.; Rajaram, V.; Pavlick, D.C.; Cooke, M.; Miller, V.A.; Albacker, L.A.; et al. Durable Clinical Response to Larotrectinib in an Adolescent Patient with an Undifferentiated Sarcoma Harboring an STRN-NTRK2 Fusion. JCO Precis. Oncol. 2018, 2, PO.18.00101. [Google Scholar] [CrossRef]
- Zhao, R.; Yao, F.; Xiang, C.; Zhao, J.; Shang, Z.; Guo, L.; Ding, W.; Ma, S.; Yu, A.; Shao, J.; et al. Identification of NTRK Gene Fusions in Lung Adenocarcinomas in the Chinese Population. J. Pathol. Clin. Res. 2021, 7, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Church, A.J.; Calicchio, M.L.; Nardi, V.; Skalova, A.; Pinto, A.; Dillon, D.A.; Gomez-Fernandez, C.R.; Manoj, N.; Haimes, J.D.; Stahl, J.A.; et al. Recurrent EML4-NTRK3 Fusions in Infantile Fibrosarcoma and Congenital Mesoblastic Nephroma Suggest a Revised Testing Strategy. Mod. Pathol. 2018, 31, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Classe, M.; Malouf, G.G.; Su, X.; Yao, H.; Thompson, E.J.; Doss, D.J.; Grégoire, V.; Lenobin, J.; Fantoni, J.-C.; Sudour-Bonnange, H.; et al. Incidence, Clinicopathological Features and Fusion Transcript Landscape of Translocation Renal Cell Carcinomas. Histopathology 2017, 70, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.E.; Lipson, D.; Stephens, P.J.; Otto, G.; Lehmann, B.D.; Lyle, P.L.; Vnencak-Jones, C.L.; Ross, J.S.; Pietenpol, J.A.; Sosman, J.A.; et al. BRAF Fusions Define a Distinct Molecular Subset of Melanomas with Potential Sensitivity to MEK Inhibition. Clin. Cancer Res. 2013, 19, 6696–6702. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Bailey, M.; He, J.; Elvin, J.; Vergilio, J.-A.; Ramkissoon, S.; Suh, J.; Frampton, G.M.; Sun, J.X.; Morley, S.; et al. Genomic Profiling of a Large Set of Diverse Pediatric Cancers Identifies Known and Novel Mutations across Tumor Spectra. Cancer Res. 2017, 77, 509–519. [Google Scholar] [CrossRef]
- Sigal, D.S.; Bhangoo, M.S.; Hermel, J.A.; Pavlick, D.C.; Frampton, G.; Miller, V.A.; Ross, J.S.; Ali, S.M. Comprehensive Genomic Profiling Identifies Novel NTRK Fusions in Neuroendocrine Tumors. Oncotarget 2018, 9, 35809–35812. [Google Scholar] [CrossRef]
- Jiao, X.; Lokker, A.; Snider, J.; Castellanos, E.; Nanda, S.; Fisher, V.; Zong, J.; Keating, K.; Fellous, M. Co-Occurrence of NTRK Fusions with Other Genomic Biomarkers in Cancer Patients. Ann. Oncol. 2019, 30, v29–v30. [Google Scholar] [CrossRef]
- Schrock, A.B.; Zhu, V.W.; Hsieh, W.-S.; Madison, R.; Creelan, B.; Silberberg, J.; Costin, D.; Bharne, A.; Bonta, I.; Bosemani, T.; et al. Receptor Tyrosine Kinase Fusions and BRAF Kinase Fusions Are Rare but Actionable Resistance Mechanisms to EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1312–1323. [Google Scholar] [CrossRef]
- Van der Wekken, A.J.; Pelgrim, R.; ’t Hart, N.; Werner, N.; Mastik, M.F.; Hendriks, L.; van der Heijden, E.H.F.M.; Looijen-Salamon, M.; de Langen, A.J.; Staal-van den Brekel, J.; et al. Dichotomous ALK-IHC Is a Better Predictor for ALK Inhibition Outcome than Traditional ALK-FISH in Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 4251–4258. [Google Scholar] [CrossRef]
- Guo, R.; Berry, L.D.; Aisner, D.L.; Sheren, J.; Boyle, T.; Bunn, P.A.; Johnson, B.E.; Kwiatkowski, D.J.; Drilon, A.; Sholl, L.M.; et al. MET IHC Is a Poor Screen for MET Amplification or MET Exon 14 Mutations in Lung Adenocarcinomas: Data from a Tri-Institutional Cohort of the Lung Cancer Mutation Consortium. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1666–1671. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Butter, R.; ’t Hart, N.A.; Hooijer, G.K.J.; Monkhorst, K.; Speel, E.-J.; Theunissen, P.; Thunnissen, E.; von der Thüsen, J.H.; Timens, W.; van de Vijver, M.J. Multicentre Study on the Consistency of PD-L1 Immunohistochemistry as Predictive Test for Immunotherapy in Non-Small Cell Lung Cancer. J. Clin. Pathol. 2020, 73, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying Patients with NTRK Fusion Cancer. Ann. Oncol. 2019, 30, viii16–viii22. [Google Scholar] [CrossRef] [PubMed]
- Guibourg, B.; Cloarec, E.; Conan-Charlet, V.; Quintin-Roué, I.; Grippari, J.-L.; Le Flahec, G.; Marcorelles, P.; Uguen, A. EPR17341 and A7H6R Pan-TRK Immunohistochemistry Result in Highly Different Staining Patterns in a Series of Salivary Gland Tumors. Appl. Immunohistochem. Mol. Morphol. AIMM 2020, 28, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Roche Diagnostics VENTANA Pan-TRK (EPR17341) Assay. Available online: https://diagnostics.roche.com/us/en/landing-pages/ventana-pan-trk--epr17341--assay.html (accessed on 30 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).