Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
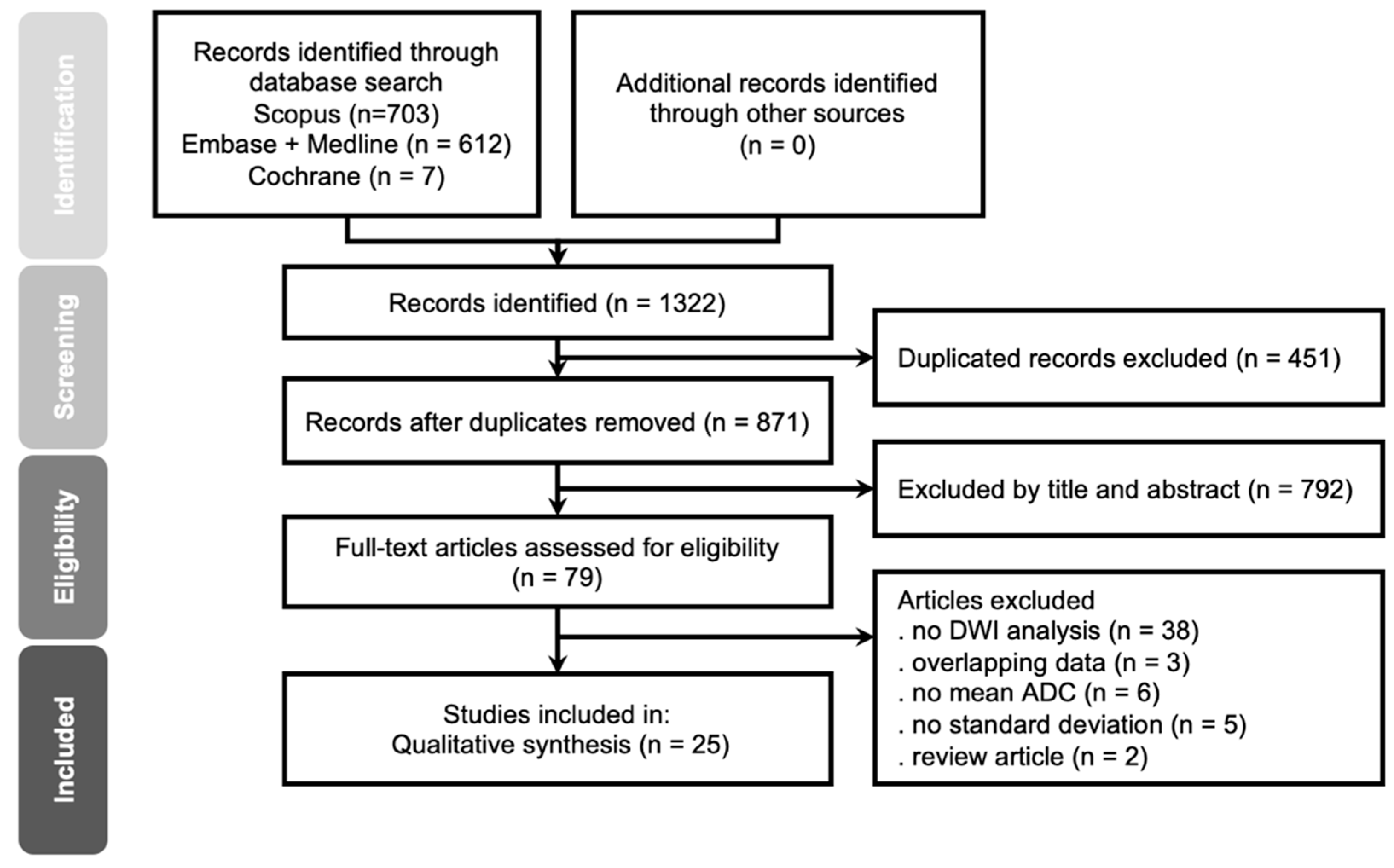
3.1. Literature Search and Study Characteristics
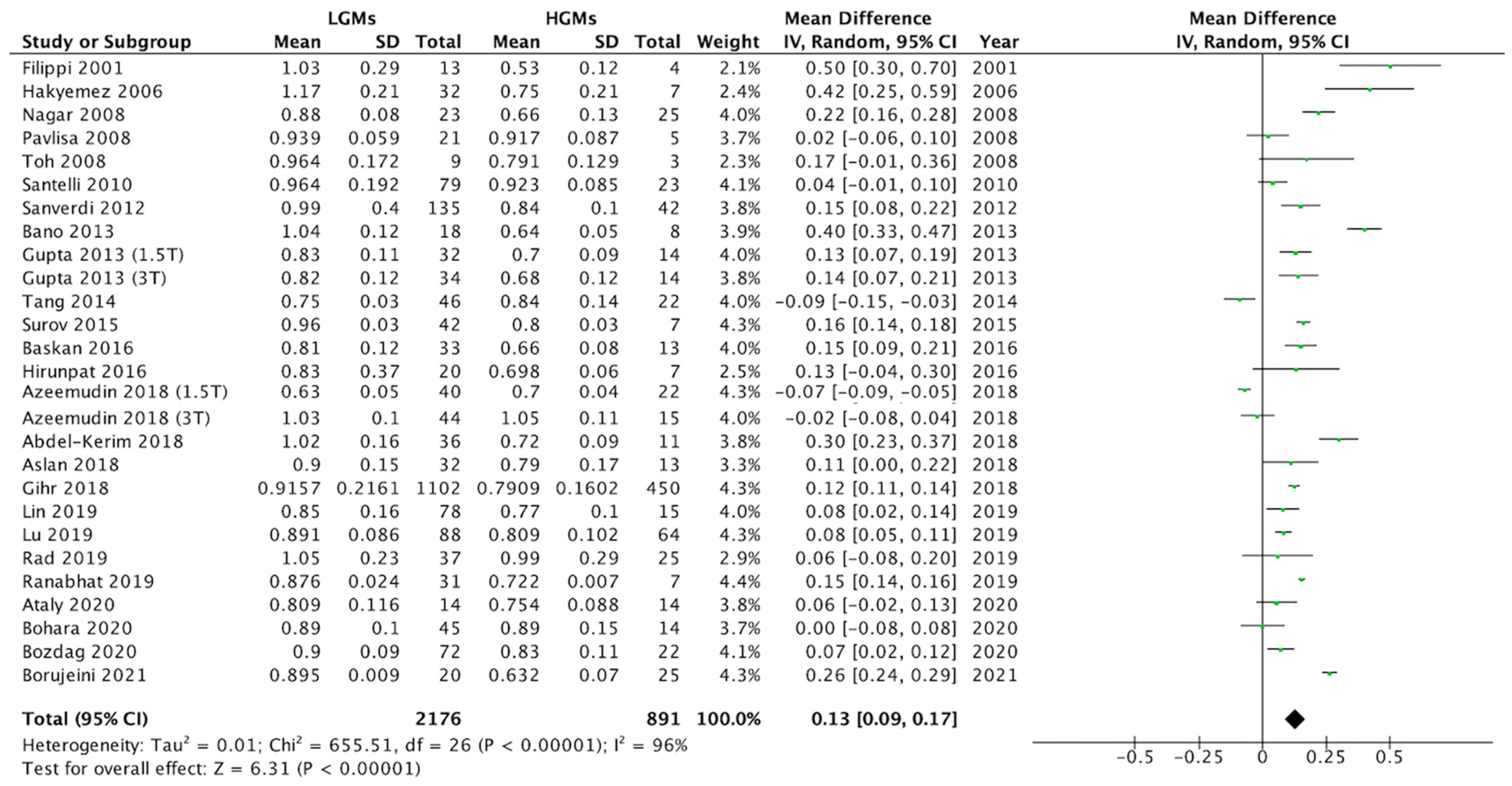
3.2. Differences in ADC Values between LGMs and HGMs
3.3. Subgroup Analysis for 1.5T and 3T MRI Scanners
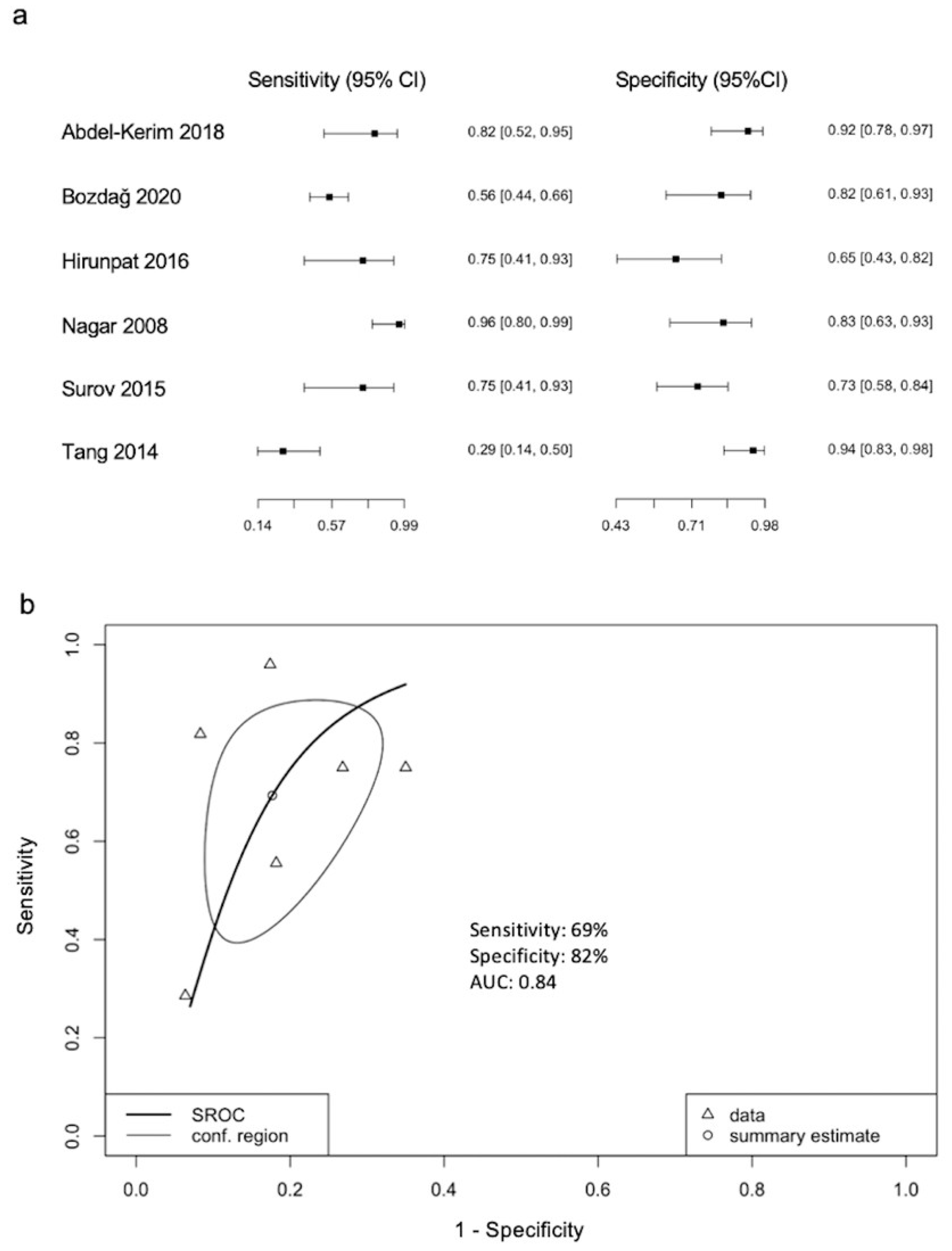
3.4. ADC Threshold Values for Differentiation between LGMs and HGMs
3.5. Correlation Coefficients (r) between Mean ADC and Ki-67
3.6. Quality Assessment and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamarides, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fevre-Montange, M.; Guyotat, J. WHO grade II and III meningiomas: A study of prognostic factors. J. Neurooncol. 2009, 95, 367–375. [Google Scholar] [CrossRef] [PubMed]
- van Alkemade, H.; de Leau, M.; Dieleman, E.M.T.; Kardaun, J.W.P.F.; van Os, R.; Vandertop, W.P.; van Furth, W.R.; Stalpers, L.J.A. Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 2012, 14, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef]
- Kaur, G.; Sayegh, E.T.; Larson, A.; Bloch, O.; Madden, M.; Sun, M.Z.; Barani, I.J.; James, C.D.; Parsa, A.T. Adjuvant radiotherapy for atypical and malignant meningiomas: A systematic review. Neuro-Oncology 2014, 16, 628–636. [Google Scholar] [CrossRef]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Le Bihan, D.; Iima, M. Diffusion Magnetic Resonance Imaging: What Water Tells Us about Biological Tissues. PLoS Biol. 2015, 13, e1002203. [Google Scholar] [CrossRef]
- Yamasaki, F.; Kurisu, K.; Satoh, K.; Arita, K.; Sugiyama, K.; Ohtaki, M.; Takaba, J.; Tominaga, A.; Hanaya, R.; Yoshioka, H.; et al. Apparent Diffusion Coefficient of Human Brain Tumors at MR Imaging. Radiology 2005, 235, 985–991. [Google Scholar] [CrossRef]
- Tang, Y.; Dundamadappa, S.K.; Thangasamy, S.; Flood, T.; Moser, R.; Smith, T.; Cauley, K.; Takhtani, D. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am. J. Roentgenol. 2014, 202, 1303–1308. [Google Scholar] [CrossRef]
- Filippi, C.G.; Edgar, M.A.; Uluǧ, A.M.; Prowda, J.C.; Heier, L.A.; Zimmerman, R.D. Appearance of Meningiomas on Diffusion-weighted Images: Correlating Diffusion Constants with Histopathologic Findings. AJNR Am. J. Neuroradiol. 2001, 22, 65–72. [Google Scholar] [PubMed]
- Surov, A.; Ginat, D.T.; Sanverdi, E.; Lim, C.C.T.; Hakyemez, B.; Yogi, A.; Cabada, T.; Wienke, A. Use of Diffusion Weighted Imaging in Differentiating Between Maligant and Benign Meningiomas. A Multicenter Analysis. World Neurosurg. 2016, 88, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Baskan, O.; Silav, G.; Bolukbasi, F.H.; Canoz, O.; Geyik, S.; Elmaci, I. Relation of apparent diffusion coefficient with Ki-67 proliferation index in meningiomas. Br. J. Radiol. 2016, 89, 20140842. [Google Scholar] [CrossRef] [PubMed]
- Ranabhat, K.; Bishokarma, S.; Agrawal, P.; Shrestha, P.; Panth, R.; Ghimire, R.K. Role of MR Morphology and Diffusion-Weighted Imaging in the Evaluation of Meningiomas: Radio-Pathologic Correlation. JNMA J. Nepal Med. Assoc. 2019, 57, 37–44. [Google Scholar] [CrossRef]
- Haghighi Borujeini, M.; Farsizaban, M.; Yazdi, S.R.; Tolulope Agbele, A.; Ataei, G.; Saber, K.; Hosseini, S.M.; Abedi-Firouzjah, R. Grading of meningioma tumors based on analyzing tumor volumetric histograms obtained from conventional MRI and apparent diffusion coefficient images. Egypt. J. Radiol. Nucl. Med. 2021, 52, 167. [Google Scholar] [CrossRef]
- Sanverdi, S.E.; Ozgen, B.; Oguz, K.K.; Mut, M.; Dolgun, A.; Soylemezoglu, F.; Cila, A. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur. J. Radiol. 2012, 81, 2389–2395. [Google Scholar] [CrossRef]
- Santelli, L.; Ramondo, G.; Della Puppa, A.; Ermani, M.; Scienza, R.; d’Avella, D.; Manara, R. Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir. 2010, 152, 1315–1319. [Google Scholar] [CrossRef]
- Yiping, L.; Kawai, S.; Jianbo, W.; Li, L.; Daoying, G.; Bo, Y. Evaluation parameters between intra-voxel incoherent motion and diffusion-weighted imaging in grading and differentiating histological subtypes of meningioma: A prospective pilot study. J. Neurol. Sci. 2017, 372, 60–69. [Google Scholar] [CrossRef]
- Rad, H.S.; Safari, M.; Kazerooni, A.F.; Moharamzad, Y.; Taheri, M. Apparent Diffusion Coefficient (ADC) and First-Order Histogram Statistics in Differentiating Malignant Versus Benign Meningioma in Adults. Iran. J. Radiol. 2018, 16, e74324. [Google Scholar]
- Meyer, H.J.; Wienke, A.; Surov, A. ADC values of benign and high grade meningiomas and associations with tumor cellularity and proliferation—A systematic review and meta-analysis. J. Neurol. Sci. 2020, 415, 116975. [Google Scholar] [CrossRef]
- Atalay, B.; Ediz, S.S.; Ozbay, N.O. Apparent Diffusion Coefficient in Predicting the Preoperative Grade of Meningiomas. J. Coll. Physicians Surg. Pak. 2020, 30, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Bohara, M.; Nakajo, M.; Kamimura, K.; Yoneyama, T.; Fukukura, Y.; Kiyao, Y.; Yonezawa, H.; Higa, N.; Kirishima, M.; Yoshiura, T. Histological Grade of Meningioma: Prediction by Intravoxel Incoherent Motion Histogram Parameters. Acad. Radiol. 2020, 27, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, M.; Er, A.; Ekmekci, S. Association of apparent diffusion coefficient with Ki-67 proliferation index, progesterone- receptor status and various histopathological parameters, and its utility in predicting the high grade in meningiomas. Acta Radiol. 2021, 62, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007, 8, 239–251. [Google Scholar] [CrossRef]
- Hakyemez, B.; Yildirim, N.; Gokalp, G.; Erdogan, C.; Parlak, M. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology 2006, 48, 513–520. [Google Scholar] [CrossRef]
- Nagar, V.A.; Ye, J.R.; Ng, W.H.; Chan, Y.H.; Hui, F.; Lee, C.K.; Lim, C.C. Diffusion-weighted MR imaging: Diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am. J. Neuroradiol. 2008, 29, 1147–1152. [Google Scholar] [CrossRef]
- Pavlisa, G.; Rados, M.; Pazanin, L.; Padovan, R.S.; Ozretic, D.; Pavlisa, G. Characteristics of typical and atypical meningiomas on ADC maps with respect to schwannomas. Clin. Imaging 2008, 32, 22–27. [Google Scholar] [CrossRef]
- Bano, S.; Waraich, M.M.; Khan, M.A.; Buzdar, S.A.; Manzur, S. Diagnostic value of apparent diffusion coefficient for the accurate assessment and differentiation of intracranial meningiomas. Acta Radiol. Short Rep. 2013, 2, 2047981613512484. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Mawrin, C.; Prell, J.; Spielmann, R.P.; Wienke, A.; Fiedler, E. Diffusion-Weighted Imaging in Meningioma: Prediction of Tumor Grade and Association with Histopathological Parameters. Transl. Oncol. 2015, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kerim, A.; Shehata, M.; El Sabaa, B.; Fadel, S.; Heikal, A.; Mazloum, Y. Differentiation between benign and atypical cranial Meningiomas. Can ADC measurement help? MRI findings with hystopathologial correlation. Egypt. J. Radiol. Nucl. Med. 2018, 49, 172–175. [Google Scholar] [CrossRef]
- Gihr, G.A.; Horvath-Rizea, D.; Garnov, N.; Kohlhof-Meinecke, P.; Ganslandt, O.; Henkes, H.; Meyer, H.J.; Hoffmann, K.T.; Surov, A.; Schob, S. Diffusion Profiling via a Histogram Approach Distinguishes Low-grade from High-grade Meningiomas, Can Reflect the Respective Proliferative Potential and Progesterone Receptor Status. Mol. Imaging Biol. 2018, 20, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Gunbey, H.P.; Tomak, L.; Incesu, L. The diagnostic value of using combined MR diffusion tensor imaging parameters to differentiate between low- and high-grade meningioma. Br. J. Radiol. 2018, 91, 20180088. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.H.; Castillo, M.; Wong, A.M.; Wei, K.C.; Wong, H.F.; Ng, S.H.; Wan, Y.L. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008, 29, 1630–1635. [Google Scholar] [CrossRef]
- Hirunpat, S.; Sanghan, N.; Watcharakul, C.; Kayasut, K.; Ina, N.; Pornrujee, H. Is Apparent Diffusion Coefficient Value Measured on Picture Archiving and Communication System Workstation Helpful in Prediction of High-grade Meningioma? Hong Kong J. Radiol. 2016, 19, 84–90. [Google Scholar] [CrossRef]
- Lin, L.; Xue, Y.; Duan, Q.; Chen, X.; Chen, H.; Jiang, R.; Zhong, T.; Xu, G.; Geng, D.; Zhang, J. Grading meningiomas using mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging. Clin. Radiol. 2019, 74, 651.e15–651.e23. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Luan, S.; Xiong, J.; Geng, D.; Yin, B. The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: An attempt using decision tree and decision forest. Eur. Radiol. 2019, 29, 1318–1328. [Google Scholar] [CrossRef]
- Gupta, A.; Prabhu, S.M.; Sureka, J.; Chacko, G. Role of diffusion weighted MRI in differentiating typical from atypical meningiomas at 1.5 and 3T MRI. Egypt. J. Radiol. Nucl. Med. 2013, 44, 635–640. [Google Scholar] [CrossRef][Green Version]
- Azeemuddin, M.; Nizamani, W.M.; Tariq, M.U.; Wasay, M. Role of ADC values and ratios of MRI scan in differentiating typical from atypical/anaplastic meningiomas. J. Pak. Med. Assoc. 2018, 68, 1403–1406. [Google Scholar] [PubMed]
- Weber, D.C.; Lovblad, K.O.; Rogers, L. New pathology classification, imagery techniques and prospective trials for meningiomas: The future looks bright. Curr. Opin. Neurol. 2010, 23, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Wani, M.A.; Kirmani, A.R.; Ramzan, A.U. Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas). J. Neurosci. Rural Pract. 2014, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Uppin, S.G.; Uppin, M.S.; Panigrahi, M.K.; Saradhi, V.; Bhattacharjee, S.; Sahu, B.P.; Purohit, A.K.; Challa, S. Meningiomas: Correlation of Ki67 with histological grade. Neurol. India 2011, 59, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Abry, E.; Thomassen, I.O.; Salvesen, O.O.; Torp, S.H. The significance of Ki-67/MIB-1 labeling index in human meningiomas: A literature study. Pathol. Res. Pract. 2010, 206, 810–815. [Google Scholar] [CrossRef]
- Pond, J.B.; Morgan, T.G.; Hatanpaa, K.J.; Yetkin, Z.F.; Mickey, B.E.; Mendelsohn, D.B. Chordoid Meningioma: Differentiating a Rare World Health Organization Grade II Tumor from Other Meningioma Histologic Subtypes Using MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1253–1258. [Google Scholar] [CrossRef]
- Cohen, A.D.; LaViolette, P.S.; Prah, M.; Connelly, J.; Malkin, M.G.; Rand, S.D.; Mueller, W.M.; Schmainda, K.M. Effects of perfusion on diffusion changes in human brain tumors. J. Magn. Reson. Imaging 2013, 38, 868–875. [Google Scholar] [CrossRef]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Ugga, L.; Perillo, T.; Cuocolo, R.; Stanzione, A.; Romeo, V.; Green, R.; Cantoni, V.; Brunetti, A. Meningioma MRI radiomics and machine learning: Systematic review, quality score assessment, and meta-analysis. Neuroradiology 2021, 63, 1293–1304. [Google Scholar] [CrossRef]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef]
- Stafford, S.L.; Pollock, B.E.; Foote, R.L.; Link, M.J.; Gorman, D.A.; Schomberg, P.J.; Leavitt, J.A. Meningioma radiosurgery: Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001, 49, 1029–1037. [Google Scholar] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Brastianos, P.K.; Loeffler, J.S. Radiation Treatment for WHO Grade II and III Meningiomas. Front. Oncol. 2013, 3, 227. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | MRI | ROI | b Value | LGMs | HGMs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tesla | (s/mm2) | Numbers | Mean ADC | SD | Numbers | Mean ADC | SD | |||
| (×10−3 mm2/s) | (×10−3 mm2/s) | |||||||||
| Filippi (2001) | Prospective | 1.5T | Single | 0,1000 | 13 | 1.03 | 0.29 | 4 | 0.53 | 0.12 |
| Hakyemez (2006) | Prospective | 1.5T | Single | 0,1000 | 32 | 1.17 | 0.21 | 7 | 0.75 | 0.21 |
| Nagar (2008) | Retrospective | 1.5T | Single | 0,1000 | 23 | 0.88 | 0.08 | 25 | 0.66 | 0.13 |
| Pavlisa (2008) | Prospective | 1.5T | Single | 0,500,1000 | 21 | 0.94 | 0.06 | 5 | 0.92 | 0.09 |
| Toh (2008) | Prospective | 3T | Single | 0,1000 | 9 | 0.96 | 0.17 | 3 | 0.79 | 0.13 |
| Santelli (2010) | Retrospective | 1T | Single | 0,800 | 79 | 0.96 | 0.19 | 23 | 0.92 | 0.09 |
| Sanverdi (2012) | Retrospective | 1.5T | Single | 0,500,1000 | 135 | 0.99 | 0.4 | 42 | 0.84 | 0.10 |
| Bano (2013) | Prospective | 1.5T | Single | 0,1000,2000 | 18 | 1.04 | 0.12 | 8 | 0.64 | 0.05 |
| Gupta (2013) | Retrospective | 1.5T | Single | 0,1000 | 32 | 0.83 | 0.11 | 14 | 0.70 | 0.09 |
| 3T | Single | 0,1000 | 34 | 0.82 | 0.12 | 14 | 0.68 | 0.12 | ||
| Tang (2014) | Retrospective | 1.5T | Single | 0,1000 | 46 | 0.75 | 0.03 | 22 | 0.84 | 0.14 |
| Surov (2015) | Retrospective | 1.5T | Whole | 0,1000 | 42 | 0.96 | 0.03 | 7 | 0.80 | 0.03 |
| Baskan (2016) | Retrospective | 3T | Single | 0,1000 | 33 | 0.81 | 0.12 | 13 | 0.66 | 0.08 |
| Hirunpat (2016) | Retrospective | 3T | Single | 0,1000 | 20 | 0.83 | 0.37 | 7 | 0.70 | 0.06 |
| Abdel-Kerim (2018) | Prospective | 1.5T | Single | 0,1000 | 36 | 1.02 | 0.16 | 11 | 0.72 | 0.09 |
| Aslan (2018) | Retrospective | 1.5T | Single | 0,1000 | 32 | 0.90 | 0.15 | 13 | 0.79 | 0.17 |
| Azeemudin (2018) | Retrospective | 1.5T | Single | 0,1000 | 40 | 0.63 | 0.05 | 22 | 0.70 | 0.04 |
| 3T | Single | 0,1000 | 44 | 1.03 | 0.10 | 15 | 1.05 | 0.11 | ||
| Gihr (2018) | Retrospective | 1.5T | Whole | 0,1000 | 28 | 0.99 | 0.14 | 9 | 0.78 | 0.07 |
| Lin (2019) | Prospective | 3T | Single | 0,1000 | 78 | 0.85 | 0.16 | 15 | 0.77 | 0.10 |
| Lu (2019) | Retrospective | 3T | Single | 0,1000 | 88 | 0.89 | 0.09 | 64 | 0.81 | 0.10 |
| Rad (2019) | Retrospective | 1.5T | Whole | 0,1000 | 37 | 1.05 | 0.23 | 25 | 0.99 | 0.29 |
| Ranabhat (2019) | Retrospective | 1.5T | Single | 0,90,1000 | 31 | 0.88 | 0.02 | 7 | 0.72 | 0.01 |
| Ataly (2020) | Retrospective | 1.5T | Single | 0,1000 | 14 | 0.81 | 0.12 | 14 | 0.75 | 0.09 |
| Bohara (2020) | Retrospective | 3T | Whole | 0,1000 | 45 | 0.89 | 0.10 | 14 | 0.89 | 0.15 |
| Bozdag (2020) | Retrospective | 1.5T | Single | 0,1000 | 72 | 0.90 | 0.09 | 22 | 0.83 | 0.11 |
| Borujeini (2021) | Retrospective | 1.5T | Whole | 0,500,1000 | 20 | 0.90 | 0.01 | 25 | 0.63 | 0.01 |
| 159 | ||||||||||
| Study | ADC Threshold Values (×10−3 mm2/s) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Nagar (2008) | 0.80 | 96 | 82 | 86 | 95 |
| Tang (2014) | 0.70 | 29 | 94 | 67 | 75 |
| Surov (2015) | 0.85 | 73 | 73 | 33 | 97 |
| Hirunpat (2016) | 0.80 | 75 | 65 | 46 | 87 |
| Abdel-Kerim (2018) | 0.79 | 81 | 92 | 75 | 94 |
| Bozdag (2020) | 0.89 | 56 | 82 | 91 | 36 |
| Study | r |
|---|---|
| Tang (2014) | −0.34 |
| Surov (2015) | −0.61 |
| Baskan (2016) | −0.33 |
| Gihr (2018) | −0.32 |
| Bozdag (2020) | −0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Hung, K.-C.; Shih, Y.-J.; Lim, S.-W.; Yang, C.-C.; Kuo, Y.-T.; Chen, J.-H.; Ko, C.-C. Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 630. https://doi.org/10.3390/diagnostics12030630
Tsai Y-T, Hung K-C, Shih Y-J, Lim S-W, Yang C-C, Kuo Y-T, Chen J-H, Ko C-C. Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(3):630. https://doi.org/10.3390/diagnostics12030630
Chicago/Turabian StyleTsai, Yueh-Ting, Kuo-Chuan Hung, Yun-Ju Shih, Sher-Wei Lim, Cheng-Chun Yang, Yu-Ting Kuo, Jeon-Hor Chen, and Ching-Chung Ko. 2022. "Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis" Diagnostics 12, no. 3: 630. https://doi.org/10.3390/diagnostics12030630
APA StyleTsai, Y.-T., Hung, K.-C., Shih, Y.-J., Lim, S.-W., Yang, C.-C., Kuo, Y.-T., Chen, J.-H., & Ko, C.-C. (2022). Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis. Diagnostics, 12(3), 630. https://doi.org/10.3390/diagnostics12030630






