Tuberculous Pericarditis—Own Experiences and Recent Recommendations
Abstract
:1. Introduction
- Originate from regions with a large prevalence of tuberculosis;
- Are receiving immunosuppressive treatment or treatment with biological drugs;
- Are infected with HIV;
- Are diagnosed with renal insufficiency, particularly in those receiving dialysis therapy;
- Are diagnosed with diabetes mellitus;
2. Clinical Vignette
3. Historical Group of Patients
- Positive result of a pericardial fluid culture for M. tuberculosis;
- Effusive pericarditis and positive culture for M. tuberculosis obtained from another site;
- Positive tuberculin skin test (TST), pericardial effusion diagnosed as lymphocytic exudate, and positive result of treatment with antituberculous drugs after the exclusion of any other cause of pericarditis [15].
4. Tuberculous Pericarditis—Current Diagnostic Approach
- Step 1—Confirmation of pericardial effusion in imaging tests;
- Step 2—Choice of further diagnostic approach;
- Step 3—Confirmation of tuberculous aetiology of pericarditis.
4.1. Step 1: Imaging Tests
4.1.1. Echocardiography
- Collapse of the free right ventricular wall in diastole;
- Dilatation of the inferior vena cava with absence or significant limitation of its respiratory motility;
- Finding of significant respiratory variability in the tricuspid (>50%) and mitral (>25%) flow.
4.1.2. Chest X-ray
4.1.3. Chest Computed Tomography
4.1.4. Magnetic Resonance Imaging
4.1.5. Fluorodeoxyglucose Positron Emission Tomography
4.2. Step 2: Choice of Further Diagnostic Approach
| Fever | 1 point |
| Night sweats | 1 point |
| Loss of body mass | 2 points |
| Globulin level > 40 g/L | 3 points |
| Peripheral leukocyte count < 10 × 109/L | 3 points |
4.3. Step 3: Confirmation of Tuberculous Aetiology of Pericarditis
- Direct Ziehl–Neelsen staining for mycobacteria [7]
- 2.
- Cultures for M. tuberculosis
- 3.
- 4.
- Indirect analyses regarding tuberculosis infection: concentration of interferon-gamma, activity of adenosine deaminase, or lysozyme in pericardial fluid [7]
- 5.
- Pericardial biopsy samples
- 6.
- Tests assessing latent tuberculosis (LTBI)
5. Principles of Recognition of Tuberculous Pericarditis (ESC 2015)
- Positive direct staining of pericardial fluid or pericardial biopsy specimens for mycobacteria and positive genetic test for M. tuberculosis of pericardial fluid;
- Positive result of pericardial fluid or pericardial biopsy culture for M. tuberculosis;
- Caseating granulomas in pericardial biopsy and positive genetic test for M. tuberculosis.
- Active tuberculosis of another organ, confirmed with positive culture and lymphocytic pericardial effusion with increased concentration of unstimulated interferon-gamma, ADA activity, or lysozyme activity;and/or
- Positive clinical response to antituberculous treatment in endemic regions.
6. Treatment
7. Prognosis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayosi, B.M.; Burgess, L.J.; Doubell, A.F. Tuberculous pericarditis. Circulation 2005, 112, 3608–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isiguzo, G.; Du Bruyn, E.; Howlett, P.; Ntsekhe, M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr. Cardiol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Picchi, C.; Mastrangelo, G.; Imazio, M.; Brucato, A. Recent advances in pericarditis. Eur. J. Intern. Med. 2021, 95, 24–31. [Google Scholar] [CrossRef]
- Lima, N.D.A.; Stancic, C.; Vos, D.; Insua, M.M.d.C.D.; Lima, C.C.D.V.; de Castro, R.L.; Maravelas, R.; Melgar, T.A. Hospital admissions for tuberculous pericarditis in the United States 2002–2014. Int. J. Mycobacteriol. 2019, 8, 347–350. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A.; The Neglected Tropical Diseases. Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Wiysonge, C.; Ntsekhe, M.; Gumedze, F.N.; Volmink, J.A.; Maartens, G.; Aje, A.; Thomas, B.M.; Thomas, K.M.; Awotedu, A.A.; et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S. Afr. Med. J. 2008, 98, 36–40. [Google Scholar]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Reuter, H.; Burgess, L.J.; Doubell, A.F. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol. Infect. 2005, 133, 393–399. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F. Diagnosis and treatment of pericarditis. Heart 2015, 101, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial Issues in the Management of Pericardial Diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef]
- Mayosi, B.M. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart 2007, 93, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Naicker, K.; Ntsekhe, M. Tuberculous pericardial disease: A focused update on diagnosis, therapy and prevention of complications. Cardiovasc. Diagn. Ther. 2020, 10, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Schwartzman, K.; Pai, M. Immune-based tests for tuberculosis. In Tuberculosis a Comprehensive Clinical Reference; Zumla, A.I., Ed.; Saunders: London, UK, 2009; pp. 179–197. [Google Scholar]
- Naia, L.; Rabadão, T.; Teixeira, M.; Ferreira, F.; Pinto, S.; Ferreira, R.; Eulálio, M. Heart failure as a first sign of disseminated tuberculosis. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 558–562. [Google Scholar] [CrossRef]
- Maisch, B.; Seferović, P.M.; Ristić, A.D.; Erbel, R.; Rienmüller, R.; Adler, Y.; Tomkowski, W.Z.; Thiene, G.; Yacoub, M.H.; Priori, S.G.; et al. Guidelines on the Diagnosis and Management of Pericardial Diseases Executive Summary the Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2004, 25, 587–610. [Google Scholar] [CrossRef]
- Kearns, M.J.; Walley, K.R. Tamponade: Hemodynamic and Echocardiographic Diagnosis. Chest 2018, 153, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B. Tuberculous pericarditis and myocarditis in adults and children. Tuberculosis 2009, 351–360. [Google Scholar] [CrossRef]
- Ling, L.H.; Oh, J.K.; Breen, J.F.; Schaff, H.V.; Danielson, G.K.; Mahoney, D.W.; Seward, J.B.; Tajik, A.J. Calcific constrictive pericarditis: Is it still with us? Ann. Intern. Med. 2000, 132, 444–450. [Google Scholar] [CrossRef]
- Verhaert, D.; Gabriel, R.S.; Johnston, D.; Lytle, B.W.; Desai, M.Y.; Klein, A.L. The Role of Multimodality Imaging in the Management of Pericardial Disease. Circ. Cardiovasc. Imaging 2010, 3, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, G.; Habashy, A.G.; Uthaman, B.; Cherian, J.M.; Salama, A.; Anim, J.T. Detection and follow-up of mediastinal lymph node enlargement in tuberculous pericardial effusions using computed tomography. Am. J. Med. 2003, 114, 319–322. [Google Scholar] [CrossRef]
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography Clinical Recommendations for Multimodality Cardiovascular Imaging of Patients with Pericardial Disease. J. Am. Soc. Echocardiogr. 2013, 26, 965–1012.e15. [Google Scholar] [CrossRef]
- Cosyns, B.; Plein, S.; Nihoyanopoulos, P.; Smiseth, O.; Achenbach, S.; Andrade, M.J.; Pepi, M.; Ristic, A.; Imazio, M.; Paelinck, B.; et al. European Association of Cardiovascular Imaging (EACVI) position paper: Multimodality imaging in pericardial disease. Eur. Heart J. Cardiovasc. Imaging 2014, 16, 12–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Kim, E.K.; Choi, J.Y.; Oh, J.K.; Chang, S.A. Clinical Utility of [18F]FDG-PET/CT in Pericardial Disease. Curr. Cardiol. Rep. 2019, 21, 107. [Google Scholar] [CrossRef]
- Xu, B.; Huang, S.S.; Jellis, C.; Flamm, S.D. Diagnosis of active pericarditis by positron emission tomography (PET)/cardiac magnetic resonance (CMR) imaging. Eur. Heart J. 2018, 39, 179. [Google Scholar] [CrossRef] [PubMed]
- Salomäki, S.P.; Hohenthal, U.; Kemppainen, J.; Pirilä, L.; Saraste, A. Visualization of pericarditis by fluorodeoxyglucose PET. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 291. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.; Dong, H.; Wang, Y.; Cheng, C.; Zuo, C.; Lu, J. 18F-FDG PET/CT in differentiating acute tuberculous from idiopathic pericarditis: Preliminary study. Clin. Nucl. Med. 2013, 38, 160–165. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ristic, A.D.; Maksimovic, R.; Tatić, V.; Ostojić, M.; Kanjuh, V. Diagnostic value of pericardial biopsy. Improvement with extensive sampling enabled by pericardioscopy. Circulation 2003, 107, 978–983. [Google Scholar] [CrossRef] [Green Version]
- Wiysonge, C.S.; Ntsekhe, M.; Thabane, L.; Volmink, J.; Majombozi, D.; Gumedze, F.; Pandie, S.; Mayosi, B.M. Interventions for treating tuberculous pericarditis. Cochrane Database Syst. Rev. 2017, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, H.; Burgess, L.; van Vuuren, W.; Doubell, A. Diagnosing tuberculous pericarditis. Q. J. Med. 2006, 99, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.G.; Ternouth, I.; Mushangi, E.; Siziya, S.; Robertson, V.; Malin, A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 2000, 84, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Dhana, A.V.; Howell, P.; Sanne, I.; Spencer, D. Identification of Mycobacterium tuberculosis from pericardial fluid using the new Xpert MTB/RIF assay. BMJ Case Rep. 2013, 2013, bcr2013200615. [Google Scholar] [CrossRef] [PubMed]
- Pandie, S.; Peter, J.G.; Kerbelker, Z.S.; Meldau, R.; Theron, G.; Govender, U.; Ntsekhe, M.; Dheda, K.; Mayosi, B.M. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-γ in a high burden setting: A prospective study. BMC Med. 2014, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianto, A.; Mertaniasih, N.M.; Gandi, P.; Al-Farabi, M.J.; Azmi, Y.; Jonatan, M.; Silahooij, S.I. Diagnostic test accuracy of Xpert MTB/RIF for tuberculous pericarditis: A systematic review and meta-analysis. F1000Research 2020, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update; World Health Organization: Geneva, Switzerland, 2013.
- Yu, G.; Zhong, F.; Shen, Y.; Zheng, H. Diagnostic accuracy of the Xpert MTB/RIF assay for tuberculous pericarditis: A systematic review and meta-analysis. PLoS ONE 2021, 10, e0257220. [Google Scholar] [CrossRef]
- Gori, A.; Bandera, A.; Marchetti, G.; Degli Esposti, A.; Catozzi, L.; Nardi, G.P.; Gazzola, L.; Ferrario, G.; van Embden, J.D.A.; van Soolingen, D.; et al. Spoligotyping and Mycobacterium tuberculosis. Emerg. Infect. Dis. 2005, 11, 1242–1248. [Google Scholar] [CrossRef]
- Augustynowicz-Kopeć, E.; Jagielski, T.; Kozińska, M.; Zabost, A.; Zwolska, Z. The significance of spoligotyping method in epidemiological investigations of tuberculosis. Pneumonol. Alergol. Pol. 2007, 75, 22–31. [Google Scholar]
- Gardy, J.L.; Johnston, J.C.; Ho Sui, S.J.; Cook, V.J.; Shah, L.; Brodkin, E.; Rempel, S.; Moore, R.; Zhao, Y.; Holt, R.; et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N. Engl. J. Med. 2011, 364, 730–739, Erratum in N. Engl. J. Med. 2011, 364, 2174. [Google Scholar] [CrossRef] [Green Version]
- Comas, I.; Homolka, S.; Niemann, S.; Gagneux, S. Genotyping of genetically monomorphic bacteria: DNA sequencing in Mycobacterium tuberculosis highlights the limitations of current methodologies. PLoS ONE 2009, 4, e7815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.C.; Bryant, J.M.; Einer-Jensen, K.; Holdstock, J.; Houniet, D.T.; Chan, J.Z.; Depledge, D.P.; Nikolayevskyy, V.; Broda, A.; Stone, M.J.; et al. Rapid Whole-Genome Sequencing of Mycobacterium tuberculosis Isolates Directly from Clinical Samples. J. Clin. Microbiol. 2015, 53, 2230–2237. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, C.W.; Lee, S.G.; Yang, H.S.; Hong, M.K.; Kim, J.J.; Park, S.W.; Chi, H.S.; Park, S.J. Comparison of polymerase chain reaction with adenosine deaminase activity in pericardial fluid for the diagnosis of tuberculous pericarditis. Am. J. Med. 2002, 113, 519–521. [Google Scholar] [CrossRef]
- Seo, H.T.; Kim, Y.S.; Ock, H.S.; Kang, L.H.; Byun, K.S.; Jeon, D.S.; Kim, S.J. Diagnostic performance of interferon-gamma release assay for diagnosis of tuberculous pericarditis: A meta-analysis. Int. J. Clin. Pract. 2020, 74, e13479. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xing, B.; Wang, W.; Yang, P.; Sun, Y.; Zheng, X.; Shang, Y.; Chen, F.; Liu, N.; Yang, L.; et al. Diagnostic values of Xpert MTB/RIF, T-SPOT.TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: A prospective observational study. Sci. Rep. 2020, 10, 16325. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ye, B.; Chen, D.; Zhong, F.; Chen, G.; Yang, J.; Xu, L.; Xu, X. Comparison between the diagnostic validities of Xpert MTB/RIF and interferon-γ release assays for tuberculous pericarditis using pericardial tissue. PLoS ONE 2017, 12, e0188704. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Ntsekhe, M.; Volmink, J.A.; Commerford, P.J. Interventions for treating tuberculous pericarditis. Cochrane Database Syst. Rev. 2002, 4, CD000526. [Google Scholar]
- Reuter, H.; Burgess, L.J.; Louw, V.J.; Doubell, A.F. The management of tuberculous pericardial effusion: Experience in 233 consecutive patients. Cardiovasc. J. S. Afr. 2007, 18, 20–25. [Google Scholar] [PubMed]
- Mayosi, B.M.; Ntsekhe, M.; Bosch, J.; Pandie, S.; Jung, H.; Gumedze, F.; Pogue, J.; Thabane, L.; Smieja, M.; Francis, V.; et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N. Engl. J. Med. 2014, 371, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Mayosi, B.M.; Ntsekhe, M.; Bosch, J.; Pogue, J.; Gumedze, F.; Badri, M.; Jung, H.; Pandie, S.; Smieja, M.; Thabane, L.; et al. Rationale and design of the Investigation of the Management of Pericarditis (IMPI) trial: A 2 × 2 factorial randomized double-blind multicenter trial of adjunctive prednisolone and Mycobacterium w immunotherapy in tuberculous pericarditis. Am. Heart J. 2013, 165, 109–115.e3. [Google Scholar] [CrossRef]
- Schutz, C.; Davis, A.G.; Sossen, B.; Lai, R.P.; Ntsekhe, M.; Harley, Y.X.; Wilkinson, R.J. Corticosteroids as an adjunct to tuberculosis therapy. Expert Rev. Respir. Med. 2018, 12, 881–891. [Google Scholar] [CrossRef]
- George, I.A.; Thomas, B.; Sadhu, J.S. Systematic review and meta-analysis of adjunctive corticosteroids in the treatment of tuberculous pericarditis. Int. J. Tuberc. Lung Dis. 2018, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Gillaspie, E.A.; Stulak, J.M.; Daly, R.C.; Greason, K.L.; Joyce, L.D.; Oh, J.; Schaff, H.V.; Dearani, J.A. A 20-year experience with isolated pericardiectomy: Analysis of indications and outcomes. J. Thorac. Cardiovasc. Surg. 2016, 152, 448–458. [Google Scholar] [CrossRef]
- Sagristà-Sauleda, J.; Angel, J.; Sanchez, A.; Permanyer-Miralda, G.; Soler-Soler, J. Effusive-constrictive pericarditis. N. Engl. J. Med. 2004, 350, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Wiyeh, A.B.; Ochodo, E.A.; Wiysonge, C.S.; Kakia, A.; Awotedu, A.A.; Ristic, A.; Mayosi, B.M. A systematic review of the efficacy and safety of intrapericardial fibrinolysis in patients with pericardial effusion. Int. J. Cardiol. 2018, 250, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
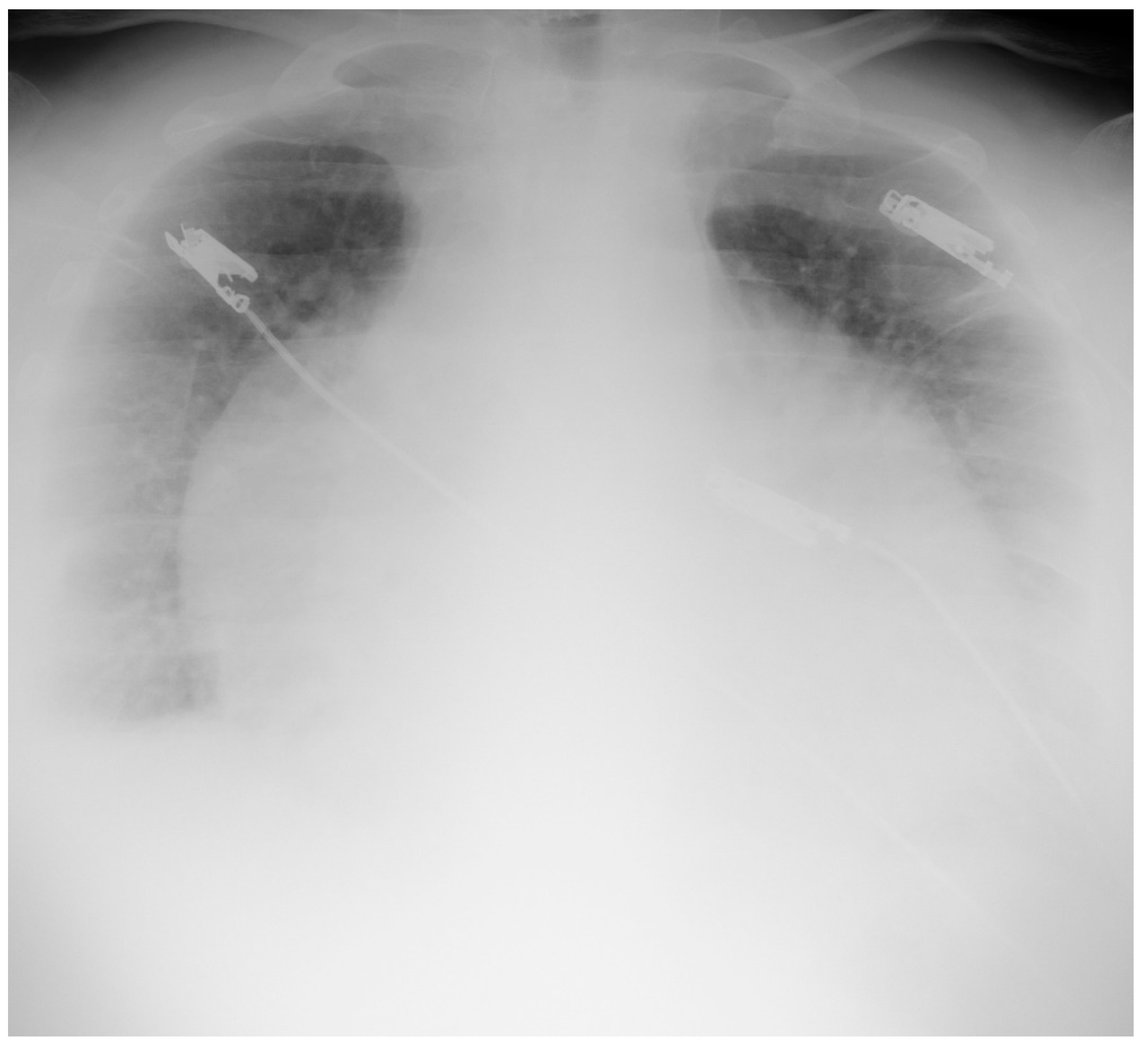
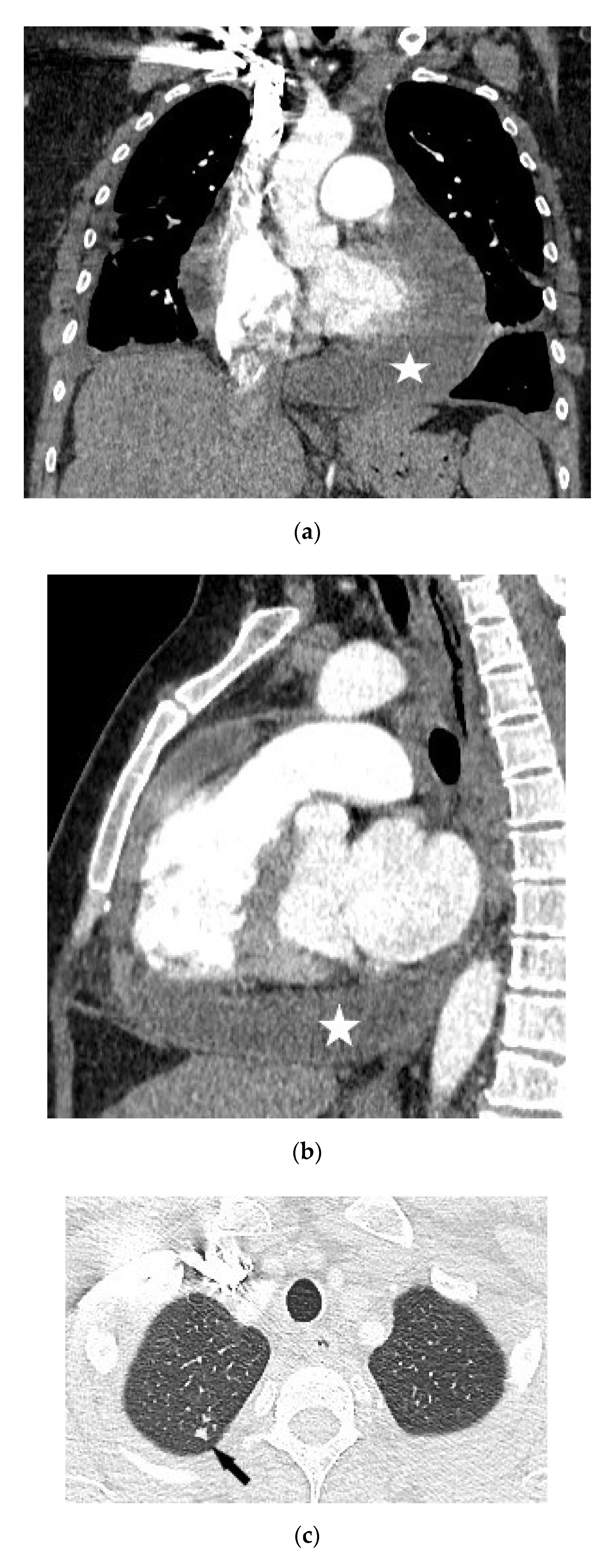
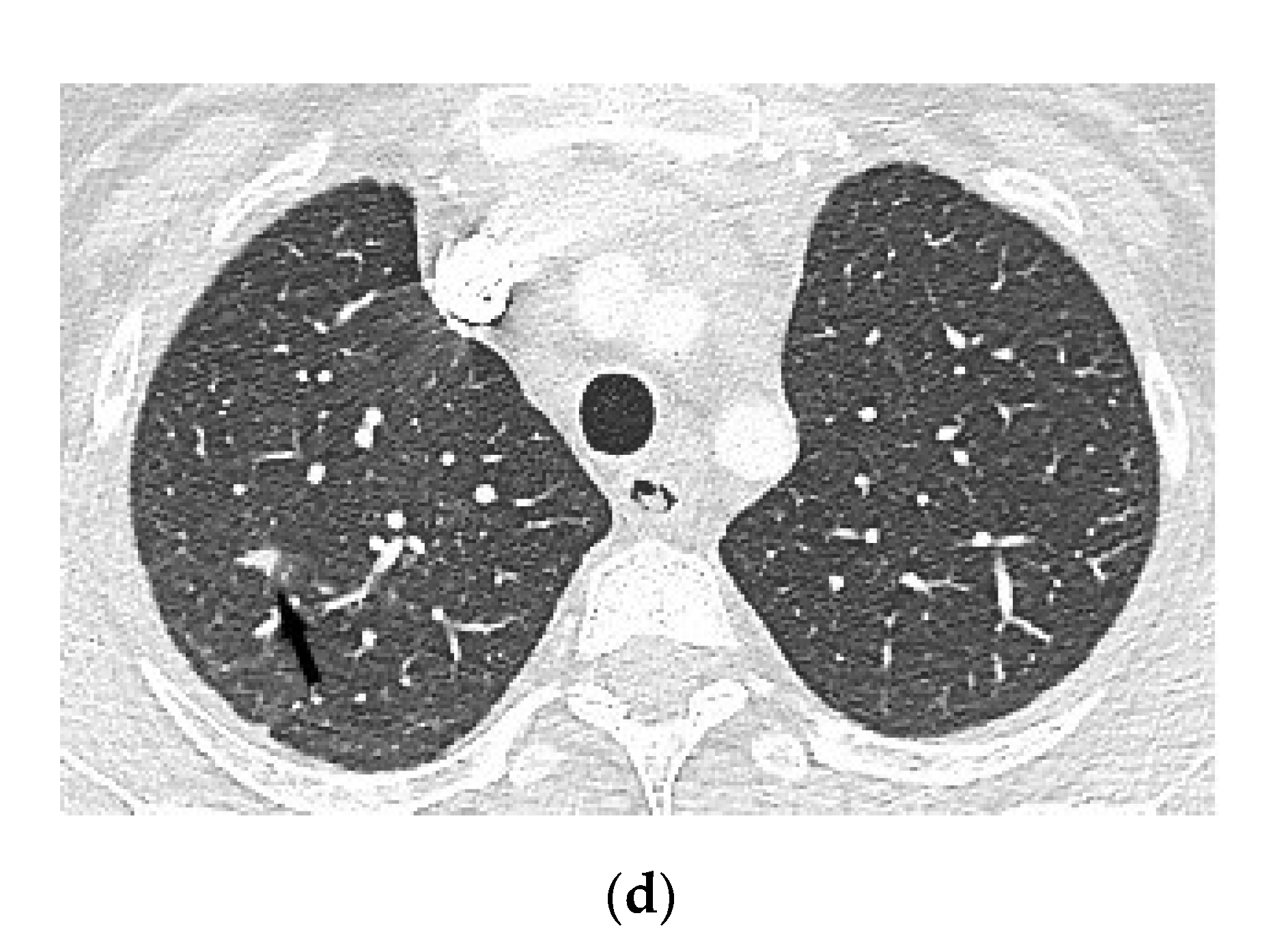
| No | Age (Years) | Gender | TST (mm) | Type of Pericarditis | PF Fluid Layer (mm) (CT or Echo) | Cardiac Tamponade (Echo) | Pericardial Constriction (Echo) |
|---|---|---|---|---|---|---|---|
| 1 | 72 | f | 17 | effusive | 23 | ||
| 2 | 52 | f | 22 | effusive | 34 | ||
| 3 | 61 | f | na | effusive-constrictive | na | + | + |
| 4 | 73 | m | 11 | constrictive | 3 | + | |
| 5 | 89 | f | 25 | effusive | 25 | ||
| 6 | 47 | m | 5 | effusive-constrictive | 40 | + | |
| 7 | 16 | m | 8 | effusive | 24 | ||
| 8 | 37 | m | na | effusive-constrictive | 15 | + | |
| 9 | 63 | f | 13 | effusive | 30 | + | |
| 10 | 58 | f | 0 | effusive | 30 | ||
| 11 | 77 | f | 0 | effusive | 17 |
| Type of Procedure | Amount of Drained Fluid (mL) | Macroscopic Appearance | PF Protein Concentration (mg/dL) | Lymphocyte (%) | Positive Culture for TB (Pericardial Fluid or Other Sites) | |
|---|---|---|---|---|---|---|
| 1 | pericardiotomy | 600 | serous | 4.9 | 62 | + |
| 2 | pericardioscopy | 800 | haemorrhagic | 6.0 | 100 | |
| 3 | pericardiocentesis | 120 | serous | 2.1 | 7 | + |
| 4 | - | - | - | - | - | |
| 5 | pericardiocentesis | 700 | serous | 5.0 | 100 | |
| 6 | pericardiocentesis | 1600 | na | na | na | |
| 7 | - | - | - | - | - | + |
| 8 | - | - | - | - | - | |
| 9 | pericardioscopy | 570 | serous | 6.0 | 30 | |
| 10 | pericardioscopy | 900 | haemorrhagic | 5.4 | 74 | |
| 11 | pericardiotomy | 100 | serous | 6.5 | 20 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybowska, M.; Błasińska, K.; Gątarek, J.; Klatt, M.; Augustynowicz-Kopeć, E.; Tomkowski, W.; Szturmowicz, M. Tuberculous Pericarditis—Own Experiences and Recent Recommendations. Diagnostics 2022, 12, 619. https://doi.org/10.3390/diagnostics12030619
Dybowska M, Błasińska K, Gątarek J, Klatt M, Augustynowicz-Kopeć E, Tomkowski W, Szturmowicz M. Tuberculous Pericarditis—Own Experiences and Recent Recommendations. Diagnostics. 2022; 12(3):619. https://doi.org/10.3390/diagnostics12030619
Chicago/Turabian StyleDybowska, Małgorzata, Katarzyna Błasińska, Juliusz Gątarek, Magdalena Klatt, Ewa Augustynowicz-Kopeć, Witold Tomkowski, and Monika Szturmowicz. 2022. "Tuberculous Pericarditis—Own Experiences and Recent Recommendations" Diagnostics 12, no. 3: 619. https://doi.org/10.3390/diagnostics12030619
APA StyleDybowska, M., Błasińska, K., Gątarek, J., Klatt, M., Augustynowicz-Kopeć, E., Tomkowski, W., & Szturmowicz, M. (2022). Tuberculous Pericarditis—Own Experiences and Recent Recommendations. Diagnostics, 12(3), 619. https://doi.org/10.3390/diagnostics12030619









