Abstract
Fetal malformations occur in 2–3% of pregnancies. They require invasive procedures for cytogenetics and molecular testing. “Structural anomalies” include non-transient anatomic alterations. “Soft markers” are often transient minor ultrasound findings. Anomalies not fitting these definitions are categorized as “dynamic”. This meta-analysis aims to evaluate the diagnostic yield and the rates of variants of uncertain significance (VUSs) in fetuses undergoing molecular testing (chromosomal microarray (CMA), exome sequencing (ES), genome sequencing (WGS)) due to ultrasound findings. The CMA diagnostic yield was 2.15% in single soft markers (vs. 0.79% baseline risk), 3.44% in multiple soft markers, 3.66% in single structural anomalies and 8.57% in multiple structural anomalies. Rates for specific subcategories vary significantly. ES showed a diagnostic rate of 19.47%, reaching 27.47% in multiple structural anomalies. WGS data did not allow meta-analysis. In fetal structural anomalies, CMA is a first-tier test, but should be integrated with karyotype and parental segregations. In this class of fetuses, ES presents a very high incremental yield, with a significant VUSs burden, so we encourage its use in selected cases. Soft markers present heterogeneous CMA results from each other, some of them with risks comparable to structural anomalies, and would benefit from molecular analysis. The diagnostic rate of multiple soft markers poses a solid indication to CMA.
1. Introduction
Fetal malformations are diagnosed in 2–3% of pregnancies [1,2,3,4,5], representing a significant public health concern, requiring further morphological examinations, genetic counseling, invasive procedures for cytogenetics and molecular testing, and appropriate obstetric/perinatal management [6]. Some ultrasonographic (US) investigations are usually planned. The first-trimester US scan, commonly performed at 11–13 weeks of gestational age (wga), assesses nuchal translucency (NT) and nasal bone presence, and excludes tricuspid regurgitation and ductus venosus reversal flow. The mid-trimester US scan (18–22 wga) consists of a systematic sequential study of fetal organs. US scan efficiency for the detection of anomalies spans from 15% to 85%, depending on the wga, the sonographer’s expertise, the woman’s body mass index and the involved fetal organ [7]. Different kinds of anomalies can be identified. Structural anomalies include non-transient anatomic alterations of organs or limbs development. Nonspecific and often transient minor US findings that cannot be considered as structural are called “soft markers” (increased NT, short femur, echogenic intracardiac focus, mild ventriculomegaly, enlarged cisterna magna, choroid plexus cysts, echogenic bowel, mild hydronephrosis, absent/hypoplastic nasal bone and single umbilical artery) [8,9,10,11]. Several papers consider increased NT as either a soft marker or structural anomaly or a separate entity. We categorized as “dynamic” those anomalies that can regress or worsen during pregnancies, not fitting either the soft marker or structural anomaly definition. These include quantitative changes of amniotic fluid (anhydramnios, oligohydramnios and polyhydramnios), fetal growth restriction (FGR), effusions (hydrops, pleural or pericardial effusions, ascites, hydroderma) and cystic hygroma. Isolated soft markers are noted in up to 10% of fetuses [12]. They are considered mainly risk factors for chromosomopathies, and invasive diagnostic testing is usually not recommended after the detection of an isolated soft marker, following low-risk results of screening evaluations for aneuploidies (combined first-trimester screen; non-invasive prenatal screening (NIPS)). Diagnostic approaches for US anomalies are improving, and genetic testing provides valuable information about the prognosis of the fetus, being crucial to proper genetic counseling, and can be necessary to discuss future reproductive options. Cytogenetics and molecular testing can be requested. Standard karyotyping consists in the visualization of the entire mitotic structures, identifying chromosomal anomalies of number and structure with resolution limits of 5–10 Mb.
Molecular investigations are performed on fetal DNA extracted from invasive samples (chorionic villus, amniotic fluid and fetal blood) [13]. Array-based molecular cytogenetic analyses (chromosomal microarray analysis (CMA)) permit the detection of even smaller chromosomal imbalances (copy number variations (CNVs)), reaching a resolution of 50 Kb. The main approaches used are array-comparative genomic hybridization (a-CGH) and single nucleotide polymorphism array (SNP-array). SNP-array analyzes the sample through hybridization with allele-specific oligonucleotide probes, additionally identifying runs of homozygosity (ROHs), which can be caused by consanguinity or uniparental disomy, and mosaicism at a lower percentage than a-CGH [14]. Next generation sequencing (NGS) approaches revolutionized clinical practice in medical genetics, identifying point mutations within a limited timeframe and becoming a staple of prenatal diagnosis. NGS applications are represented by targeted panels, exome sequencing (ES), and whole genome sequencing (WGS). Diagnostic ES is used in selected fetuses with US anomalies and negative cytogenetics testing. WGS is an emerging tool whose diagnostic potential is currently being explored, mainly in research settings.
This meta-analysis aims to evaluate the diagnostic yield, the rates of results of uncertain significance (variants of uncertain significance (VUSs)) and incidental findings (IFs), pathogenic variants in genes unrelated to the clinical indication, in cohorts of fetuses undergoing non-targeted molecular testing due to structural and dynamic anomalies and soft markers. This review highlights characteristics, limitations, implications and challenges of the main molecular techniques adopted in prenatal diagnosis assessments, pointing out the importance of contextualizing molecular findings within a multidisciplinary team of experts in fetal medicine.
2. Materials and Methods
The research was conducted following PRISMA guidelines [15] (Figure 1). We searched the Pubmed database (https://pubmed.ncbi.nlm.nih.gov/, accessed on 12 November 2021) for “prenatal” AND/OR (specific string for the molecular technique), with a 10-year filter for publication date (1 January 2011–12 November 2021). All titles and abstracts were examined. Only papers with full text available in English language were retained. Case reports and papers not describing the prenatal diagnostic application of the specific molecular technique on invasive samples were excluded.
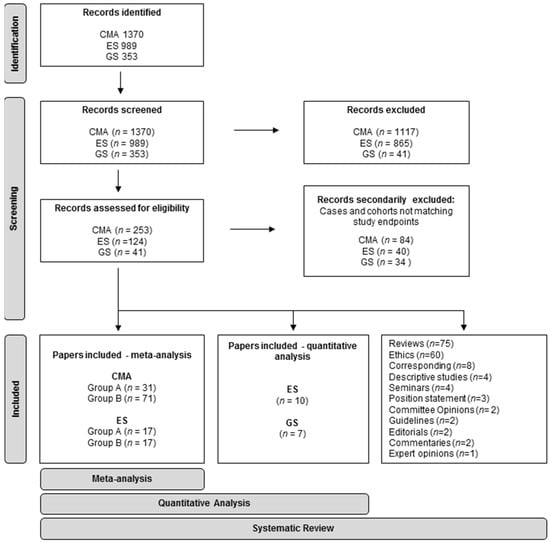
Figure 1.
PRISMA flowchart of the systematic review and meta-analysis.
Prenatal CMA and ES cohorts deemed eligible for quantitative analysis were divided into two groups for each approach: Group A, including cohorts with pooled different indications, and Group B, including fetuses enrolled for a specific single-district anomaly. Cases with postnatal diagnosis, fetuses enrolled for known chromosomal/molecular variants and familiarity/recurrence for genetic disorders, cases enrolled after fetal demise and twin pregnancies were excluded. Papers in which these categories were included but could not be separated from eligible cases were secondarily excluded.
Cases from eligible papers were classified into categories and subcategories. For each cohort and category, the findings were classified as either single, multiple or pooled, regardless of association (identified as “pooled” or “any”). The categories were: no indication of invasive testing; advanced maternal age; soft marker(s); structural anomalies, single, multiple, or pooled, regardless of association; and dynamic anomalies. Subcategories identifying specific districts for structural anomalies were: central nervous system, musculoskeletal, genitourinary, gastrointestinal, cardiovascular, thorax/respiratory/chest, craniofacial, abdominal and body wall. Subcategories for dynamic anomalies were: amniotic fluid quantity anomalies (polyhydramnios and oligohydramnios), FGR and hydrops. Subcategories for soft markers were: echogenic intracardiac focus, mild ventriculomegaly, enlarged cisterna magna, choroid plexus cysts, echogenic bowel, mild hydronephrosis, absent/hypoplastic nasal bone, short femur and single umbilical artery.
Due to the inconsistency in the classification of NT as a soft marker, a structural anomaly, or an autonomous condition, we regarded it as independent. For each paper, we annotated study design and sample types, when available. Pathogenic and likely pathogenic variants relevant to the phenotype were considered as diagnostic and annotated as P/LP. We also annotated VUSs and IFs. IFs, when declared, were excluded from the count of diagnostic rate. We scored diagnostic and VUS yields for each specific indication and approach for each paper. The diagnostic yield was scored as (n. cases with P/LP variant identified)/(eligible cases), while VUS rate was (n. cases with VUS identified)/(eligible cases).
For prenatal WGS cohorts, because of the reduced number of studies and the different sensitivity of the approaches, we performed quantitative analysis without distinguishing between Group A and Group B.
The meta-analysis was carried out for the CMA and ES cohorts. It was performed by scoring diagnostic and VUS rates, pooling cases collected within the same category and undergoing the same testing from eligible papers. Standard deviations and 95% confidence intervals were scored with the =STDEV.S and =CONFIDENCE functions on Microsoft Excel (Office 365).
Guidelines, expert opinion papers, commentaries, correspondences, studies exploring ethical concerns, reviews, systematic reviews and meta-analyses were examined.
2.1. Chromosomal Microarray Analysis
The search string was “(prenatal) AND ((chromosomal microarray) OR (array-CGH) OR (a-CGH) OR (CGH-array) OR (SNP-array))”. Papers which did not declare a proper ruling out of aneuploidies and gross chromosomal imbalances (>10 Mb) were secondarily excluded. The results were calculated as incremental yield over karyotyping. Diagnostics/VUSs rates were scored for the following:
- -
- Group A: advanced maternal age, no indication of any chromosomal analysis, soft markers (single, multiple, pooled), NT (isolated), structural anomalies (single, multiple, pooled), dynamic anomalies, specific isolated soft markers, isolated structural anomalies subcategories, and isolated dynamic anomalies.
- -
- Group B: soft markers (single, multiple, pooled), NT (pooled), structural anomalies (single, multiple, pooled), dynamic anomalies, specific isolated soft markers, isolated available structural anomalies subcategories, and isolated dynamic anomalies.
2.2. Exome Sequencing
We searched for (“WES” OR ‘Whole Exome Sequencing’ OR ‘Exome Sequencing’ OR “CES” OR “Clinical Exome Sequencing”) AND (‘Prenatal’). Papers not describing ES applications or describing ES performed after negative targeted panels were excluded. In all cases, karyotype and CMA were inconclusive. We collected the number of secondary and IFs in a unique category due to the ambiguous use of both terms in the literature.
Diagnostics/VUSs rates were scored for the following:
- -
- Group A: the overall pooled anomalies and available subcategories.
- -
- Group B: specific anomalies for which at least >2 papers were available. The analyzed categories included isolated and non-isolated cases of non-immune hydrops fetalis (NIHF), skeletal anomalies, congenital heart anomalies and isolated NT. When a single or only two papers were available, the diagnostic rates were annotated but not included in the meta-analysis.
2.3. Whole Genome Sequencing
We searched for ((WGS) OR ((whole genome sequencing)) OR (genome sequencing)) AND (prenatal). We excluded papers focusing on NIPS/NIPT. We divided the cohorts into two subgroups, distinguishing between whole genome sequencing (WGS) (≥20–30× depth of coverage) (Group 1) and low-coverage WGS (LC-WGS) (≤0.5× depth of coverage) (Group 2). Only prenatal cohorts with US anomalies were considered. When possible, we reclassified cases in two categories, multiple US anomalies and isolated US anomalies, recalculating diagnostic yield. In contrast, the overall diagnostic yield was considered. We reported or recalculated VUSs rate when data were available.
3. Results
3.1. Chromosomal Microarray
The literature research yielded 1370 results. A total of 253 papers were retained and analyzed, and 186 papers were deemed eligible for quantitative analysis. Among these, 71 described cohorts with pooled different indications (Group A), and 115 described cohorts enrolled for a specific single-district anomaly (Group B). In total, 7 articles were correspondences, 21 studies with ethics concerns, 4 guidelines, and 29 reviews.
Group A: A total of 40 were secondarily excluded from quantitative analysis [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Of these, 26 did not allow the scoring of the incremental diagnostic rate of CMA for the classes of fetuses selected for quantitative analysis [17,18,19,21,22,24,26,27,30,33,34,35,36,37,38,39,40,41,43,45,46,49,50,51,53,54,55], 6 did not allow proper exclusion of aneuploidies or gross chromosomal imbalances [23,32,42,47,48,52], 3 did not separate pathogenic results from VUSs [20,25,29], 3 used only targeted or low-resolution approaches [16,28,31], and 1 was a validation study on fetal samples with known diagnosis [44]. A total of 31 papers were eligible for quantitative analysis [11,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Of these, 20 were retrospective studies, 10 were prospective, and 1 did not declare pro- or retrospectivity. For six papers, the fetal sample was amniotic fluid. For one, the sample was fetal blood from cordocentesis. Twelve used amniotic fluid or chorionic villus, three used amniotic fluid or fetal blood, and nine used amniotic fluid, chorionic villus or fetal blood. Eleven of the selected papers explicitly reported IFs. It was not possible to perform statistical analysis on such data, as the definition of IFs varied from the identification of ROHs, from neurodevelopmental disorder susceptibility imbalances with reduced penetrance to pathogenic CNVs unrelated to the fetal phenotype. Diagnostic and VUSs rates for each paper are presented in Table S1. Results of the meta-analysis are shown in Table 1 and Table 2. The diagnostic rate for oligohydramnios, short femur, enlarged cisterna magna and mild pyelectasis and the VUS rate for hydrops, cystic hygroma, short femur and single umbilical artery were inferred from single sources. The average incremental diagnostic yield over karyotype for P/LP CNVs in advanced maternal age cases and in cases with no indication to chromosomal analysis was 0.84% (0.82–0.86) and 0.79% (0.74–0.83), respectively. The VUS rates were 1.75% (1.65–1.85) and 0.27% (0.24–0.30). These values should approximate the background risk for significant CNVs in low-risk pregnancies. There was a slight but significant increase in the diagnostic yield for soft markers. The yield was 2.15% (2.11–2.19) for isolated and 3.44% (3.24–3.64) for multiple soft markers. By pooling cohorts including both isolated and single soft markers, the yield was 2.45. (2.48–2.41).

Table 1.
CMA—Meta-analysis Group A and B. Diagnostic yield.

Table 2.
CMA—Meta-analysis Group A and B. VUS rate.
For isolated NT, the rate of P/LP VUS was 2.63% (2.80–2.46), higher than isolated soft markers but lower than structural anomalies, and the VUS rate was 1.95% (1.82–2.08). When evaluating the diagnostic rate for structural anomalies, the yield was 3.66% (3.60–3.72) for single anomalies, 8.57% (7.92–9.22) for multiple anomalies and 5.72% (5.65–5.78) from cohorts including both single and multiple anomalies cases. VUSs yields for the same groups were 5.15% (5.05–5.26), 6.26% (5.95–6.57), and 2.86% (2.79–2.93). Concerning dynamic anomalies, the diagnostic and VUS rates were 4.90% (6.13–3.68) and not inferable for isolated fetal hydrops, 3.76% (4.31–3.20) and 25.00% (9.60–40.40) for cystic hygroma, 3.61% (3.88-3.34) and 4.98% (4.67–5.29) for amniotic fluid quantity anomalies, and 3.34% (3.52–3.17) and 6.35% (4.27–8.42) for FGR.
Group B: A total of 44 were secondarily excluded from quantitative analysis. Of these, 34 dealt with specific single-district malformations [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119], 2 included postnatal cases [120,121], 3 dealt only with cases from fetuses’ demise or livebirths [122,123,124], 3 did not provide a proper distinction between US features [125,126,127], 1 dealt familial cases of heart disease [128], and 1 concerned twin pregnancies [129]. A total of 71 papers were eligible for quantitative analysis [6,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199]: 44 were retrospective studies, 12 were prospective, and 15 did not declare pro- or retrospectivity.
Diagnostic and VUS rates for each paper are presented in Supplementary Table S2. The results of the meta-analysis are shown in Table 1 and Table 2. Single soft markers and single structural anomalies were two of the most consistently represented among all the studies, the former showing 5.30% (4.50–6.10) yield and the latter showing 5.64% (5.44–5.84) yield. The diagnostic rates for isolated mild ventriculomegaly, 6.95% (6.76–7.13), and for isolated short femur, 5.88% (5.37–6.40), were significantly higher than for other isolated soft markers. Concerning pooled NT, the rate of P/LP and VUS was 3.58% (3.47–3.68) and 2.96%. (2.88–3.04). In dynamic anomalies, the diagnostic and VUS rates were 3.28% (3.13–3.43) and 3.31% (3.09–3.54) for isolated FGR.
The most common incidental CNVs in both groups were PMP22 (*6010979) deletions or duplications, leading to recurrent neuropathy with pressure palsies (MIM#162500) or Charcot–Marie–Tooth disease 1A (MIM#118220), respectively, and Xp22.31 deletions encompassing STS (MIM*300747), implied in X-linked ichthyosis (MIM#308100).
3.2. Exome Sequencing
The literature research yielded 989 results. Of these, 124 papers were retained, resulting in: 44 case studies, 36 reviews, 26 studies with ethics concerns, 4 seminars, 4 descriptive papers, 2 editorials, 2 comments, 3 correspondences, 2 position statements, and 1 committee opinion.
Group A: A total of 17 papers were included [13,75,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214] (Table S3). Group B: A total of 27 cohorts were enrolled [98,196,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237] (Table S4). For the quantitative analysis, 40 articles [121,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276] were secondarily excluded. Of these, three papers focused on fetal demises or stillbirths [256,257,258], three papers focused on information postmortem [242,250,254], three were case reports [238,246,252], five focused on a single specific phenotype [240,241,248,261,274], three presented inhomogeneity in inclusion criteria and chromosomal anomalies/CNV assessment [239,251,262], six included both fetuses and postnatal cases [244,249,253,259,260,269], three focused on candidate genes [243,247,263], three focused on recurrent phenotypes or previously described cohorts [245,255,272], five were excluded for the lack of inclusion or eligibility criteria [264,265,268,271,276], two were excluded for the higher a priori risk for consanguinity and recurrence [266,270], one because parents were tested for recessive disorders [267], two because they focused on gene panels [273,275], and one due to the postnatal diagnosis [121].
All 17 papers from Group A and 17 from Group B were eligible for meta-analysis: three focusing on NIHF [215,216,217], six on isolated and non-isolated skeletal anomalies [218,219,220,221,222,223], two on isolated NT [196,225], and five on isolated and not isolated cardiovascular anomalies [231,232,233,234]. In one paper [224], isolated and non-isolated NT categories retrieved from other studies [201,202] were possible to separate, and the two categories were counted separately in the analysis.
Group A: The pooled incremental diagnostic yield over CMA for P/LP variants was 19.47% (18.94–20.01%), with 27.47% (26.45–28.49%) of diagnostic yield in the category of multiple structural anomalies (≥2) [13,75,200,201,202,203,204,205,206,207] (Table 3). The overall VUS rate was 8.32% (7.94–8.69%) (Table 3).

Table 3.
ES meta-analysis. Any refers to cohorts pooling both isolated and associated anomalies. NIHF: non-immune hydrops fetalis; CNS: central nervous system.
Data for the meta-analysis of the specific organ anomalies was not available for all the papers included in Group A. When data were available, most papers included in the anomaly-specific subcategories both fetuses with isolated features and associated anomalies, without the possibility to discern the two categories. For some of them with more than one anomaly, the count was repeated in several subcategories with variable inclusion criteria.
The pooled diagnostic rate was 14.77% (13.23–16.32%) in the group of any cardiovascular anomaly, 34.11% (31.42–36.80%) in musculoskeletal anomalies, 21.23% (17.49–24.97%) in genitourinary anomalies, 34.88% (30.21–39.55%) in craniofacial anomalies, 24.70% (23.94–25.45%) in CNS anomalies and 10.53% (7.53–13.52%) in abdomen or body wall anomalies. The subcategory of isolated NT when data were retrievable (6/17 papers) showed a diagnostic rate of 13.46% (10.19–16.74%). The diagnostic rate in the group of any dynamic anomalies was 20.78% (18.71–22.84%) (Table 3).
Group B: Most cohorts selected for specific anomalies presented both isolated and non-isolated anomalies (Table S4). When the articles were >2 for a specific category, we performed a meta-analysis to score diagnostic and VUS rates (Table 3). In the group of any NIHF (three papers, isolated and non-isolated), the pooled diagnostic yield was 30.81% (28.56–33.07%) and the VUS rate was 10.47% (9.14–11.79%) [215,216,217]. The diagnostic rate for skeletal dysplasia (six papers, isolated and non-isolated) was 70.19% (68.80–71.57%), whereas the VUS rate was 4.44% (3.67–5.22%) [218,219,220,221,222,223]. The rate for increased isolated NT (three papers—in one data, were retrieved from [224]) was 4.33% (3.59–5.07%), and the rate of VUS was 1.44% (0.46–2.42%) [196,224,225] (Table 3). The unique cohort of fetuses presenting non-isolated NT [224] showed a diagnostic rate of 26% and a VUS rate of 9% (Table S4). The rate for isolated or non-isolated cardiovascular anomalies (five papers) was 11.02% (10.65%–11.39%), whereas the VUS rate was 7.62% (7.48–7.76%) [231,232,233,234] (Table 3). In single papers describing specific anomalies, we reviewed articles annotating diagnostic yields. Concerning CNS, in a cohort of fetuses presenting isolated anomalies, the diagnostic rate was 45% [236]; while in another cohort of isolated and non-isolated CNS anomalies, the diagnostic rate was 50% [230]; and in a third cohort of fetuses presenting with isolated and not isolated cerebellar vermis defects and Dandy–Walker malformation, the diagnostic rate was 42% [98]. In two cohorts of fetuses presenting corpus callosum anomalies [227,228], the diagnostic rate scored 40% and 19%, whereas the VUS rate of 10% was reported in only one paper [228]. In a cohort of fetuses presenting isolated and non-isolated genitourinary anomalies, the diagnostic rate was 12% [235], whereas in another paper, the diagnostic rate was 7% [237]. In one recent paper analyzing fetuses with isolated hypoplastic/absent nasal bone, the diagnostic rate was 17% [277] (Table S4). Concerning IFs, 6 of the selected papers in Group A and 14 papers in Group B explicitly reported IFs (Tables S3 and S4). We did not score a specific rate due to the overly heterogeneous definition of IFs.
3.3. Whole Genome Sequencing
We obtained 353 results, with 41 meeting the initial inclusion criteria. Among these, 17 articles included prenatal cohorts, tested with different approaches after invasive sampling: 12 with LC-WGS [278,279,280,281,282,283,284,285,286,287,288,289] (Group 2) and 5 with WGS [290,291,292,293,294] (Group 1). In addition, 1 correspondence, 10 reviews, and 13 studies about ethical concerns were also considered. Within Group 2, seven articles were secondarily excluded due to inadequate inclusion criteria [279,281], mixed class of high-risk pregnancies and abnormal US findings [285], specific disease studied [284], highly specific analysis performed [282], validation nature of the study [280] and sole inclusion of postnatal cases [287]. Among Group 1, three articles were secondarily excluded due to the highly specific genomic analysis performed [291], highly specific structural anomaly studied [293], and not clearly reported results [294]. Two articles were retained for Group 1 [290,292] and five for Group 2 [278,283,286,288,289]. Overall, five studies were prospective, and two were retrospective. In one study [290], we revised the diagnostic yield, excluding four twin couples and a fetus that did not show US anomalies. We also divided the cases into isolated (single soft marker/structural anomaly/dynamic anomaly) and multiple (≥2 structural anomalies/dynamic anomalies) to compare the diagnostic yield of these two classes. In one study of Group 1 [292] and three studies of Group 2 [278,288,289], CMA or karyotype were applied contextually with WGS, comparing yields. In one work of Group 1 [290], a two-step strategy consisting of CMA plus ES, other than WGS as first-tier test, was applied. Conversely, in two studies of Group 2 [283,286], CMA and/or other tests were applied only to confirm variants detected with WGS. Due to the paucity of the studies, the different approaches applied, and the heterogeneity of their classifications, we forwent quantitative analysis for these approaches. Nevertheless, to compare results, we recalculated/reported yields in each study for the cohort of interest, and VUS when available (Table S5).
4. Discussion
4.1. Before Molecular Testing
Karyotyping is a staple for detecting chromosomal anomalies in both prenatal and postnatal diagnosis. It is based on the analysis of mitotic structures, displayed as pairs of homologous chromosomes. Karyotype allows detection of inversions, translocations, triploidies, chromosomal markers and numeric or structural anomalies in mosaicism state. The main limitations are the time required to culture cells and the resolution [295]. Rearrangements of 5 Mb can be identified if the Giemsa-banding pattern is sufficiently detailed in the region of interest at 400–550-band definition [14]. Chromosomal anomalies are detected in up to 8–10% of fetuses with US anomalies [296].
4.2. Chromosomal Microarray Analysis
CMA was introduced in prenatal diagnostics due to the incremental 10–15% yield over karyotype in children showing malformations, intellectual disability and dysmorphisms [297]. Despite CMA not being able to diagnose balanced chromosomal anomalies and low-level mosaicism, it is widely accepted as a first-tier test in fetuses with malformations [48,298]. It has gradually replaced karyotyping in several countries for other indications too [82,299,300,301]. The detection of submicroscopic CNVs allows the identification of a wider range of causes of malformations [302,303,304,305,306]. It represents a more standardized technique than karyotyping, being less prone to human errors. It has a markedly higher resolution and faster turnaround times, without requiring cell culturing [301]. Both aCGH and SNP-array platforms are comparable in the detection rate of significant CNVs [307]. It is a controversial question whether to universally perform SNP-arrays in prenatal settings to systematically detect partial or whole chromosome uniparental disomy or to identify ROH [27]. Up to 55% of fetuses with ROH present with US anomalies, frequently represented by malformations and FGR [308]. In these circumstances, ES might be encouraged to rule out autosomal recessive conditions. CNVs correlated with neurodevelopmental disorders can present highly variable clinical expressivity or incomplete penetrance [309]. It is important to point out during post-test counseling with the couple that these findings are usually inherited from an asymptomatic parent [310], but the parental phenotype does not predict the features of the child. It would be desirable for geneticists to be joined by psychologists, ensuring that the consultants are making the best decision for them [311]. Other potential findings can be implied in non-actionable late-onset diseases (i.e., neurological conditions, susceptibility to cancer development) [310] potentially relevant to parental health. Parental consanguinity or misattributed paternity (through ROHs detection) can be suspected too.
In the current meta-analysis, we found a background rate for P/LP submicroscopic CNVs pregnancies without indications for chromosomal analysis of 0.79%, comparable with the advanced maternal age cohort (0.84%). These rates appear to be lower than those initially reported, which ranged up to 1.7% [82]. The rate of diagnostic findings appeared to increase along with the severity of the conditions. Single soft markers presented with an overall diagnostic yield (2.15%) higher than baseline risk. However, each soft marker behaves differently, and the rate varies significantly between Group A and B. Diagnostic rates for soft markers were consistently higher in Group B, possibly due to selection bias of the latter. For example, the detection rate for intracardiac echogenic focus is 0.56% in Group A and 2.01% in B. Mild ventriculomegaly showed a diagnostic rate significantly higher than other soft markers in both groups, comparable to that of structural anomalies (Group A: 4.41%; B: 6.12%). Multiple soft markers also had the noticeably higher yield of 3.44%, overlapping that of single structural anomalies. Isolated short femur displayed even higher yields, 12.50% for Group A (single paper, 34 cases) and 5.88% in B (three papers, 187 cases). If these results are confirmed, it might be necessary to classify these anomalies as separated from traditional soft markers. Concerning NT, we could only score rates for isolated NT in Group A, but we could only pool NT cohorts including both associated and isolated cases for Group B, obtaining slightly different yields (Group A: 2.63%; B: 3.48%). In both cases, the yield was higher than soft markers and lower than structural anomalies. Dynamic anomalies were not pooled, being classified from exclusion from the other categories. Isolated FGR yielded similar diagnostic rates between the two groups (3.34%; 3.79%). Isolated polyhydramnios in Group A presented with a 3.18% diagnostic rate, while in Group B it accounts for 2.42%. The main difference between the two groups concerned structural anomalies. For example, cardiovascular malformations yielded 3.18% in Group A and 6.49% in B, even if both groups consisted of thousands of cases. The average detection rate of submicroscopic CNVs in cohorts enrolled for any single structural anomaly and normal karyotype from Group A was 3.66%. The diagnostic rate for multiple structural anomalies in the same group was 8.57%. For Group A, there was no marked difference between the diagnostic yields for different categories. In Group B, cardiovascular anomalies had the highest diagnostic rate, 6.47% when isolated, while genitourinary anomalies yielded the lowest, 3.35%. As expected, the diagnostic rates in each subcategory of Group B were much higher when associated malformations were present along with the index anomaly. The comparison of VUS rates does not allow proper conclusions, as the classification and report policies, as well as the availability of parental samples for segregation analysis, vary greatly. A high VUS rate in both groups was noted for FGR (Group A: 6.35%; B: 9.35%) and for CNS anomalies (7.55%; 8.56%). In other categories, VUS rates do not appear to differ greatly from the diagnostic rates. Some groups with high diagnostic rates, like multiple structural anomalies, also display high VUSs rates, in this specific case 6.25%. Given the results in the advanced maternal age and no indication of chromosome analysis groups, 1% might be an educated guess of the background VUS risk. This is consistent with reports from previous studies [312]. Low-resolution CMA reduces reported VUSs by 32% [313] but also fails to detect some P/LP CNVs [314]. The possibility of performing targeted or low-resolution microarray testing to protect consultants from VUSs misinterpretation, rather than a genome-wide one, has been extensively discussed [315]. Nowadays, genome-wide platforms are almost universally used when there are clinical indications to CMA [307]. Ideally, prospective parents should be allowed to decide whether to opt for targeted or genome-wide approaches, but it requires a cultural substrate that permits the consultants to understand advantages and disadvantages of these methods, making a conscious choice.
Indications to perform CMA in fetuses detected with soft markers are not standardized. In this meta-analysis, we highlighted that soft markers present very different rates of P/LP submicroscopic CNVs. Some often transient anomalies usually included among soft makers, such as short femur (Group A: 12.5%; B: 5.88%) and mild ventriculomegaly (4.41%; 6.95%), showed yield rates comparable to those of structural anomalies. These observations lead us to consider these findings as distinct entities from other soft markers.
4.3. Exome Sequencing
ES enables the simultaneous examination of a large number of genes in a limited turnaround time, with acceptable costs [316], presenting advantages especially in prenatal settings [317], where time represents a significant limit. It is based on massive parallel DNA sequencing, examining protein coding regions, which make up to 2% of the genome but include more than 85% of all variants responsible for monogenic conditions [318]. ES is able to detect single nucleotide variations, indels and, with specific software analysis not widely validated in diagnostic practice, CNVs [319].
In this meta-analysis in Group A, ES showed an overall diagnostic rate of 19.47% (18.94–20.01%), with 27.47% (26.45–28.49%) yield in multiple structural anomalies. We stratified the diagnostic yields by single-district anomalies retrieved from Group A and B. However, the classification of the anomalies and their associations had little consistency among studies, confounding the ability to obtain specific diagnostic rates per organ system. Studies with homogeneous inclusion criteria are needed to determine the specific diagnostic value of ES in single-district anomalies [279].
Deep phenotyping is essential for proper interpretation of variants and incomplete phenotype information in prenatal settings makes interpretation of variants challenging [279]. Predefined terms, such as human phenotype ontology (HPO), are useful to make the ES indications as univocal as possible [320], and a list of prenatal HPOs will be available shortly [321].
In this meta-analysis, the VUS rate was 8.32% in Group A, whereas it ranged between 1.44% and 10.47% in Group B. Although it is often left to the autonomy of individual laboratories, American College of Medical Genetics and Genomics (ACMG) guidelines recommend reporting VUSs related to autosomal recessive diseases when they are congruent with phenotype and a P/LP variant has been identified in the other allele [322].
In the current review, the IF rate was not assessable, due to the large heterogeneity in analyzing and reporting choices in the prenatal setting. ACMG detailed pretest considerations of reporting, IFs (both fetal and parental) and secondary findings, explicitly separating the terms [322]. Concerning postnatal IFs, ACMG suggests reporting only P/LP variants in 73 genes associated with “medically actionable” diseases [323]. The approach to these findings in prenatal diagnosis is still not defined.
4.4. Whole Genome Sequencing
WGS allows the sequencing of an individual’s whole genome. Potentially identifying more genetic variations than any targeted approach, WGS raises deep questions about interpretation and reporting of the variants. The reviewed cohorts (Table S5) applied two distinct strategies to the fetus. The first one is the high-coverage WGS, which explores anomalies ranging from chromosomal to single nucleotide variants (SNVs) in a single analysis. One study [290] applied a trio-based WGS to a heterogeneous population of fetuses affected by structural and dynamic anomalies. Multiple US anomalies yielded a higher diagnostic rate than isolated anomalies, which do not change significatively if we consider only associated structural anomalies (10/32, 31.3%), because of the small number of multiple dynamic anomalies in this class. In contrast, the diagnostic yield of isolated anomalies is 2.4% higher if we consider only structural findings (6/47, 12.8%). This is probably due to the high number of undiagnosed isolated dynamic anomalies (16/18, 94.4%). One study [292], applied singleton WGS to a cohort with increased NT, showing higher diagnostic yield in fetuses with NT plus associated anomalies. The overall diagnostic rate is higher compared to the previous WGS study, possibly due to the smaller sample size and to the lower heterogeneity of this second cohort. The remaining five studies applied low-coverage genome sequencing (LC-GS) [278,283,286,288,289]. Strong evidence supports that this approach, identifying gross and submicroscopic CNVs and chromosomal anomalies, could be applied as an alternative to CMA in prenatal diagnosis [278,279], requiring less DNA (thus reducing the need for cell culture) and performing better in regions with lower CMA probes density. Among these cohorts, three showed heterogeneous US anomalies [278,286,288], one showed soft markers [283], and one was enrolled for cardiac anomalies, isolated or not [289] (Table S5). The diagnostic yield of LC-GS is lower than WGS, as expected due to the different sensitivity. Internal comparison between WGS and the respective CMA results highlights a significantly higher diagnostic yield of the first approach [290,292], which is consistent with the WGS ability to also detect SNVs. In contrast, LC-GS displays less marked increase in the diagnostic yield than the gold standard approach [278], with a more noticeable increment if compared to karyotype [289] (Table S5). Finally, the overall VUSs number is consistently higher in the WGS cohort that reported this result [292] than in the LC-GS studies [283,289] (38% vs. 1.4% and 3.8%), potentially imputable to the higher number of variants detected with the first approach.
4.5. Diagnostic Yield Comparison: CMA and ES
A comparison between the diagnostic rates of CMA and ES is hindered by differences in enrollment criteria among cohorts from the two groups. The yield is scored as incremental over lower-tier testing, so all fetuses from ES cohorts had previously undergone CMA, with negative results. Further inhomogeneity arises from the different enrollment criteria specificities, varying from the type of anomaly to its possible association. For these reasons, a quantitative comparison can only be performed for a limited set of conditions (Table 4). Nevertheless, such analysis is of great interest, and some general broader assumptions can be inferred from the remaining data. The incremental diagnostic yield for fetuses enrolled for any structural anomaly, regardless of possible associations and involved organs, was 5.72% for CMA Group A, and 6.84% for CMA Group B, while ES Group A yielded 19.47%. The results inferred from the ES meta-analysis are consistently higher than those from CMA studies, and it is evident that the diagnostic yield of the ES is particularly promising in those cases in which the CMA has not been conclusive. For example, the ES diagnostic rate is 24.7% (Group A) for anomalies of the nervous system, compared to the 5.74% incremental yield of CMA over karyotype (Group B). As expected, both methods resulted in high diagnostic rates in fetuses with multiple structural anomalies, being 8.57 and 15.58 in CMA Group A and B, respectively, and reaching the value of 27.47% in ES Group A. No assumptions can be made on soft markers, as most of them are excluded from the ES cohort. Increased NT, which is sometimes considered a soft marker for chromosomal anomalies, is known to be associated with an increased risk of pathogenic CNV and of some monogenic disorders. The diagnostic yield in isolated increased NT was 2.63% in CMA Group A, and ranged from 13.6% in ES Group A to 4.33% in ES Group B. This suggests that an analysis for monogenic conditions can be advantageous in increased NT as well as in structural anomalies cases.

Table 4.
CMA and ES comparison: detection rates and VUSs rates.
4.6. VUS Rate Comparison: CMA and ES
Further complications arise when comparing VUS rates, as the criteria for their classification and report varies greatly among studies. There are the same limitations described for the comparison between the diagnostic rates. A quantitative comparison is only available for pooled structural anomalies (2.86% and 3.34% in Group A and B via CMA versus 8.32% in Group B via ES), pooled cardiovascular anomalies (Group B: 5.56% via CMA versus 7.62% via ES) and isolated NT (1.95% in Group A via CMA versus 1.44% in Group B via ES) (Table 4). From these results, it is evident that in pooled structural anomalies, similarly to the greater diagnostic yield, ES presents a greater number of VUSs. As for cardiovascular anomalies and isolated NT, both techniques appear to reach comparable values.
4.7. Ethical Concerns
Each genetic test must be preceded and followed by non-directive counseling, aimed at educating consultants about the possible conditions related to the US picture and the array of available investigations, providing the basis for thoughtful decision making, according to psychological, socio-economic, cultural and religious backgrounds.
Interestingly, different communicative approaches have been reported depending on the type of society: where an individualistic culture prevails, both counselor and consultant are more assertive than more collectivistic countries [324]. Although genetic counseling must be non-directive, every geneticist is influenced by personal considerations on each single case, based on their experience or knowledge. It is essential that the geneticist shows empathy towards the couple without exposing himself excessively, not influencing the choices. Limited consultation time, different cultural backgrounds among patients and language barriers still represent great challenges [325]. The emotional state of the consultants, who often receive counseling immediately after the detection of the US anomaly, must also be considered. The resulting confusion and the disruption of the expectation of a healthy fetus can make it difficult to understand genetic implications and give true informed consent for testing. It is also essential to provide the basic communicative skills to the other health professionals, as fetal medicine is a multidisciplinary work, involving gynecologists, radiologists and pediatricians. Ideally, the communication should take place in a safe environment and in the presence of the whole team. Unfortunately, this clashes with practical concerns.
Reporting VUSs or IFs in prenatal settings is an extremely delicate question, and psychological support, often advocated but rarely proposed in actual clinical practice, should be considered [326]. VUSs are detected in about 5% of fetuses with structural anomalies via CMA and in around 8% of cases via ES (Table 2 and Table 3). These numbers inevitably increase when WGS is performed, due to the identification of VUSs also in the noncoding regions [290]. Consultants present different reactions after receiving information of unclear significance, which varies according to one’s own experiences, perceptions and family planning [326], and sometimes they feel reassured when inherited variants are found [327]. These findings can also negatively influence the doctor–patient relationship, if the potential VUSs and IFs identification has not been previously discussed [322,328].
These are among the most impacting findings in decision making, and prospective parents often do not have the time to evaluate what information they want to receive [329]. Indeed, there are no univocal protocols between the various countries, so there is great heterogeneity about what should be reported, who decides what to report [330], who must pay [331] and more.
Parental analysis is crucial for the interpretation of the results and for formulating recurrence risks. VUSs and IFs can present a clinical impact on both fetus and parents [332]. VUS carriers should be evaluated after birth [333] or if the couple is planning new pregnancies [334], even if no further phenotypic findings emerge. If the alteration is parentally inherited, instrumental analyses may be proposed for the carrier. This scenario opens up further ethical concerns. For example, it is sometimes impossible to undergo timely medical evaluations. The potential need for an out-of-pocket accession system for such assessments for a timely diagnosis introduces a different probability of reaching a correct interpretation of the variant based on the consultants’ economic status.
To complicate things, genetic findings are always reported under the mother’s name. Consequently, it can be impracticable to retrieve data during postnatal examinations [319,329]. As a final remark, negative results from genetic tests could be mistakenly interpreted by the consultants as necessarily reassuring findings [329], but it should be discussed that a negative analysis reduces the risk of a genetically determined condition without ever being able to exclude it.
4.8. Non-Invasive Prenatal Testing: From Screening to Diagnosis
After cell-free DNA (cfDNA) identification in maternal plasma, NIPT was developed to detect the most common fetal autosomal aneuploidies [335,336]. Later, sexual chromosomes aneuplodies analysis was developed, albeit with less reliable results. Today, NIPT is mainly performed with LC-GS (<0.5×), and recent development has focused on testing microdeletion/microduplication syndromes [337,338]. Nevertheless, more clinical validation studies are needed to test NIPT reliability on subchromosomal rearrangements before current use in clinical practice.
Some monogenic disorder assays have been considered for pregnancies at high risk for the specific condition as noninvasive prenatal diagnoses (NIPD), not requiring confirmation on fetal samples [339]. Paternally inherited or fetal de novo variants potentially causative for conditions with autosomal dominant inheritance are not present in the maternal genome. If the variant is detected in cfDNA, the fetus is predicted to be affected. Paternally inherited variants causing recessive disorders can be identified in the same way, if the partners carry different variants [340]. In this scenario, fetuses can present the paternal variant, being potentially affected or carrier, or not (unaffected). This approach can reduce the number of pregnant women undergoing invasive testing for known recessive conditions by a theoretical 50%. Relative mutation dosage is used when prospective parents carry the same recessive allele for maternally inherited dominant or X-linked pathogenic variants. It requires the exact quantification of the relative amount of reads. After linkage studies between the variant parental SNPs defining surrounding haplotypes, relative haplotype dosage [341,342] can be combined with relative mutation dosage, increasing the sensitivity.
4.9. RNA Sequencing
RNA-sequencing (RNA-seq) is an NGS-based technology that can sequence and quantitatively analyze RNA molecules in a biological sample. This approach can produce a plethora of valuable information on gene expression, novel transcript isoforms and allelic expression [343], which can help in characterizing cells, tissues and the human body. Due to the high amount of starting material needed and the restricted availability of fetal tissues, this approach has rarely been applied to prenatal fetuses, even for research purposes, causing an evident lack of data on prenatal tissues in the major gene expression atlases developed. Recently, the introduction of single-cell RNA-Seq, with a low amount of RNA required, has boosted the development of less invasive procedures which could in future be implemented in the molecular prenatal diagnosis and pregnancy monitoring, such as the detection of cell-free RNA in amniotic fluid [344], umbilical cord blood, placenta and maternal plasma [345].
4.10. Future Perspectives
The analysis of fetal DNA and RNA in maternal plasma, placenta and fetal fluids currently suffers from some issues, mainly due to the low quantity of fetal nucleic acids present, its mixed nature with maternal molecules, and technical and data analysis limitations. With further advances in WGS and RNA-seq techniques, the implementation of advanced sequencing platforms and bioinformatic analysis pipelines, as well as sequencing cost cutting, it can be foreseen that these less invasive approaches could be implemented in prenatal care and diagnosis, integrating additional valuable information on fetal health and complication of pregnancy [346]. In this view, the building of multidisciplinary teams of clinical and molecular geneticists and bioinformaticians could be essential to manage the interpretation of the enormous number of variants derived from the most advanced sequencing technologies in the time-efficient manner needed in prenatal diagnosis.
4.11. Diagnostic Workflow Suggestions
The definition of diagnostic workflows for prenatal diagnosis should not only consider diagnostic rate, but also turnaround time, VUSs management, economic factors and public health concerns. For fetuses with structural anomalies, CMA is universally considered a first-tier test, possibly after quantitative fluorescent polymerase chain reaction (for rapid detection of aneuploidies) negative results, to exclude aneuploidies [347]. This approach is undoubtedly valid, but we believe the information has to be integrated with structural chromosomal analysis and parental confirmations of the results. In this same class of fetuses, ES appears to have a very high incremental yield, at the cost of a significant VUSs burden. Given the economical, technical and interpretative limitations, a systematic application is not achievable yet. We encourage its use in cytogenetics-negative fetuses with multiple anomalies and in cases selected for familial history.
NT and fetal dynamic anomalies also benefit from the same approach as structural anomalies.
Regarding US minor findings, ES data are not available. We showed that soft markers present heterogeneous CMA results from each other. Short femur and mild ventriculomegaly present risks comparable to structural anomalies and should benefit from molecular analysis. For other isolated soft markers, the incremental yield is present but marginal, and a systematic recourse to CMA is far from unquestionable. The diagnostic rate of multiple soft markers poses a solid indication to CMA.
The moderate incremental yield over karyotyping of genome-wide CMA in low-risk pregnancies (advanced maternal age, parental anxiety) has unclear potential benefits due to the heterogeneity of possible results in fetuses without US anomalies, and a systematic application is not encouraged.
4.12. Limitations
Weaknesses of the literature search include the following:
- -
- Non-homogeneous/standardized nomenclature of distinct molecular approaches in the literature, potentially limiting the efficiency of the search string in retrieving articles.
Weaknesses of the current meta-analysis include the following:
- -
- Theoretically non-excludable heterogeneity in inclusion criteria of the cases chosen by the individual authors;
- -
- Cases in which an associated malformation was not specified were considered to be affected by a single malformation;
- -
- Diagnostic and VUS yields for specific soft markers were less reliable than US anomalies’ rates, due to smaller cohorts;
- -
- Discrepancy of results explained by the different numerosity of the samples;
- -
- Heterogeneity in molecular platforms.
5. Conclusions
Molecular approaches in the prenatal setting are critical tools for investigating the causes of fetal anomalies. From the current meta-analysis, it emerged that the overall diagnostic yield of mixed structural anomalies accounts for 5.72% for CMA and 19.47% for ES, with variability due to cohorts’ characteristics, the class of malformation and the numerosity of the samples. Among the most debated topics are the reporting and interpretation of VUSs in genes linked to the fetal phenotype and IFs. In this meta-analysis, VUSs reported for fetuses with structural anomalies were 2.86% for CMA and 8.32% for ES.
Interesting and original results emerged from the cohorts of fetuses with soft markers. We showed that soft markers present very different CMA diagnostic yields from each other, and some of them, including the short femur (Group A: 12.5%; B: 5.88%) and mild ventriculomegaly (Group A: 4.41%; B: 6.95%), have rates comparable to those of fetal structural anomalies. These observations lead us to consider some US findings as distinct entities from other soft markers. In our opinion, increased NT, short femur and mild ventriculomegaly should be considered like malformations, indicating the execution of CMA. Similarly, the detection of two or more soft markers should be managed in the same way as the detection of a structural anomaly. This information offers us new perspectives, and further studies are encouraged in order to definitively determine if all soft markers are truly “soft”.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12030575/s1, Table S1: CMA—Group A Systematic review. P/LP = Pathogenic and Likely Pathogenic Variants. VUS = Variants of Uncertain Significance. NT = Nuchal Translucency. Hyb = hybrid CGH/SNP technology. The term “ANY” refers to cohorts including both isolated and associated cases. The rates are scored on cases without aneuploidies and gross (>10 Mb) Copy Number Variations. Table S2: CMA—Systematic Review—Group B. Table S3: ES systematic review Group A Any refers to cohorts pooling both isolated and associated anomalies. DR Diagnostic Rate. IFs (Incidental Findings) refer to both incidental and secondary findings. NT: Nuchal Translucency. CNS: Central Nervous System. Table S4: ES systematic review Group B Any refers to cohorts pooling both isolated and associated anomalies. IFs (Incidental Findings) refer to both incidental and secondary findings. NIHF (Non-Immune Hydrops Fetalis). NT: Nuchal Translucency. CNS: Central Nervous System. Table S5: WGS systematic review. DR: diagnostic rate. NT: nuchal translucency. IUGR: intrauterine growth restriction.
Author Contributions
Study conceptualization, G.M.; methodology, D.G.; formal analysis, D.G., N.K.H., E.M., A.T.; data curation G.M., D.G., N.K.H., E.M., A.T., writing—original draft preparation G.M., D.G., N.K.H., E.M., A.T.; writing—review and editing G.M., D.G., N.K.H., E.M., A.T., A.P.; manuscript supervision, A.P.; project administration, G.M., A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the articles cited in the Reference Section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crane, J.P.; LeFevre, M.L.; Winborn, R.C.; Evans, J.K.; Ewigman, B.G.; Bain, R.P.; Frigoletto, F.D.; McNellis, D. A randomized trial of prenatal ultrasonographic screening: Impact on the detection, management, and outcome of anomalous fetuses. ACOG Curr. J. Rev. 1995, 8, 15–16. [Google Scholar] [CrossRef]
- Grandjean, H.; Larroque, D.; Levi, S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus Study. Am. J. Obstet. Gynecol. 1999, 181, 446–454. [Google Scholar] [CrossRef]
- Rydberg, C.; Tunón, K. Detection of fetal abnormalities by second-trimester ultrasound screening in a non-selected population. Acta Obstet. Gynecol. Scand. 2017, 96, 176–182. [Google Scholar] [CrossRef]
- Rayburn, W.F.; Jolley, J.A.; Simpson, L.L. Advances in ultrasound imaging for congenital malformations during early gestation. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, M.; Bricker, L.; Mullan, C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD007058. [Google Scholar] [CrossRef] [PubMed]
- Lostchuck, E.; Hui, L. Should second-trimester hypoplastic nasal bone be sole indication for diagnostic testing with chromosomal microarray analysis? Ultrasound Obstet. Gynecol. 2019, 53, 848–850. [Google Scholar] [CrossRef]
- Rao, R.; Platt, L.D. Ultrasound screening: Status of markers and efficacy of screening for structural abnormalities. Semin. Perinatol. 2016, 40, 67–78. [Google Scholar] [CrossRef]
- Nyberg, D.A.; Souter, V.L.; El-Bastawissi, A.; Young, S.; Luthhardt, F.; Luthy, D.A. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J. Ultrasound Med. 2001, 20, 1053–1063. [Google Scholar] [CrossRef]
- Van den Hof, M.C.; Wilson, R.D.; Bly, S.; Gagnon, R.; Lewthwaite, M.B.; Lim, K.; Morin, L.; Salem, S.; Allen, V.; Blight, C.; et al. Fetal Soft Markers in Obstetric Ultrasound. J. Obstet. Gynaecol. Can. 2005, 27, 592–612. [Google Scholar] [CrossRef]
- Agathokleous, M.; Chaveeva, P.; Poon, L.C.Y.; Kosinski, P.; Nicolaides, K.H. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet. Gynecol. 2013, 41, 247–261. [Google Scholar] [CrossRef]
- Hu, T.; Tian, T.; Zhang, Z.; Wang, J.; Hu, R.; Xiao, L.; Zhu, H.; Lai, Y.; Wang, H.; Liu, S. Prenatal chromosomal microarray analysis in 2466 fetuses with ultrasonographic soft markers: A prospective cohort study. Am. J. Obstet. Gynecol. 2021, 224, 516.e1–516.e16. [Google Scholar] [CrossRef]
- Dashe, J.S. Aneuploidy screening in pregnancy. Obstet. Gynecol. 2016, 128, 181–194. [Google Scholar] [CrossRef]
- Normand, E.A.; Braxton, A.; Nassef, S.; Ward, P.A.; Vetrini, F.; He, W.; Patel, V.; Qu, C.; Westerfield, L.E.; Stover, S.; et al. Clinical exome sequencing for fetuses with ultrasound abnormalities and a suspected Mendelian disorder. Genome Med. 2018, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.J.M.; Sutherland, G.R.; Shaffer, L.G. Chromosome Abnormalities and Genetic Counseling; Oxford University Press: Oxford, UK, 2011; ISBN 0195375335. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 1–9. [Google Scholar]
- Ahn, J.W.; Bint, S.; Irving, M.D.; Kyle, P.M.; Akolekar, R.; Mohammed, S.N.; Ogilvie, C.M. A new direction for prenatal chromosome microarray testing: Software-targeting for detection of clinically significant chromosome imbalance without equivocal findings. PeerJ 2014, 2014, e354. [Google Scholar] [CrossRef] [PubMed]
- Armengol, L.; Nevado, J.; Serra-Juhé, C.; Plaja, A.; Mediano, C.; García-Santiago, F.A.; García-Aragonés, M.; Villa, O.; Mansilla, E.; Preciado, C.; et al. Clinical utility of chromosomal microarray analysis in invasive prenatal diagnosis. Hum. Genet. 2012, 131, 513–523. [Google Scholar] [CrossRef]
- Brady, P.D.; Delle Chiaie, B.; Christenhusz, G.; Dierickx, K.; Van Den Bogaert, K.; Menten, B.; Janssens, S.; Defoort, P.; Roets, E.; Sleurs, E.; et al. A prospective study of the clinical utility of prenatal chromosomal microarray analysis in fetuses with ultrasound abnormalities and an exploration of a framework for reporting unclassified variants and risk factors. Genet. Med. 2014, 16, 469–476. [Google Scholar] [CrossRef]
- Breman, A.; Pursley, A.N.; Hixson, P.; Bi, W.; Ward, P.; Bacino, C.A.; Shaw, C.; Lupski, J.R.; Beaudet, A.; Patel, A.; et al. Prenatal chromosomal microarray analysis in a diagnostic laboratory; experience with >1000 cases and review of the literature. Prenat. Diagn. 2012, 32, 351–361. [Google Scholar] [CrossRef]
- Cai, M.; Lin, N.; Lin, Y.; Huang, H.; Xu, L. Evaluation of chromosomal abnormalities and copy number variations in late trimester pregnancy using cordocentesis. Aging 2020, 12, 15556–15565. [Google Scholar] [CrossRef]
- Chau, M.H.K.; Cao, Y.; Kwok, Y.K.Y.; Chan, S.; Chan, Y.M.; Wang, H.; Yang, Z.; Wong, H.K.; Leung, T.Y.; Choy, K.W. Characteristics and mode of inheritance of pathogenic copy number variants in prenatal diagnosis. Am. J. Obstet. Gynecol. 2019, 221, 493.e1–493.e11. [Google Scholar] [CrossRef]
- Cheng, S.S.W.; Chan, K.Y.K.; Leung, K.K.P.; Au, P.K.C.; Tam, W.K.; Li, S.K.M.; Luk, H.M.; Kan, A.S.Y.; Chung, B.H.Y.; Lo, I.F.M.; et al. Experience of chromosomal microarray applied in prenatal and postnatal settings in Hong Kong. Am. J. Med. Genet. Part C Semin. Med. Genet. 2019, 181, 196–207. [Google Scholar] [CrossRef]
- Chong, H.P.; Hamilton, S.; Mone, F.; Cheung, K.W.; Togneri, F.S.; Morris, R.K.; Quinlan-Jones, E.; Williams, D.; Allen, S.; McMullan, D.J.; et al. Prenatal chromosomal microarray testing of fetuses with ultrasound structural anomalies: A prospective cohort study of over 1000 consecutive cases. Prenat. Diagn. 2019, 39, 1064–1069. [Google Scholar] [CrossRef]
- D’Amours, G.; Kibar, Z.; Mathonnet, G.; Fetni, R.; Tihy, F.; Désilets, V.; Nizard, S.; Michaud, J.L.; Lemyre, E. Whole-genome array CGH identifies pathogenic copy number variations in fetuses with major malformations and a normal karyotype. Clin. Genet. 2012, 81, 128–141. [Google Scholar] [CrossRef]
- Donnelly, J.C.; Platt, L.D.; Rebarber, A.; Zachary, J.; Grobman, W.A.; Wapner, R.J. Association of Copy Number Variants With Specific Ultrasonographically Detected Fetal Anomalies. Obstet. Gynecol. 2014, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Faas, B.H.; Feenstra, I.; Eggink, A.J.; Kooper, A.J.; Pfundt, R.; Van Vugt, J.M.; De Leeuw, N. Non-targeted whole genome 250K SNP array analysis as replacement for karyotyping in fetuses with structural ultrasound anomalies: Evaluation of a one-year experience. Prenat. Diagn. 2012, 32, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Ganesamoorthy, D.; Bruno, D.L.; McGillivray, G.; Norris, F.; White, S.M.; Adroub, S.; Amor, D.J.; Yeung, A.; Oertel, R.; Pertile, M.D. Meeting the challenge of interpreting high-resolution single nucleotide polymorphism array data in prenatal diagnosis: Does increased diagnostic power outweigh the dilemma of rare variants? BJOG Int. J. Obstet. Gynaecol. 2013, 120, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Molina Gomes, D.; Ferreira, J.C.P.B.; Dupont, C.; Alesi, V.; Gouas, L.; Horelli-Kuitunen, N.; Choy, K.W.; García-Herrero, S.; de la Vega, A.G.; et al. Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat. Diagn. 2015, 35, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Y.; Zhang, M.; Lin, N.; An, G.; He, D.; Chen, M.; Chen, L.; Xu, L. Diagnostic accuracy and value of chromosomal microarray analysis for chromosomal abnormalities in prenatal detection. Medicine 2021, 100, e25999. [Google Scholar] [CrossRef]
- Kan, A.S.Y.; Lau, E.T.; Tang, W.F.; Chan, S.S.Y.; Ding, S.C.K.; Chan, K.Y.K.; Lee, C.P.; Hui, P.W.; Chung, B.H.Y.; Leung, K.Y.; et al. Whole-genome array CGH evaluation for replacing prenatal karyotyping in Hong Kong. PLoS ONE 2014, 9, e87988. [Google Scholar] [CrossRef]
- Klugman, S.; Suskin, B.; Spencer, B.L.; Dar, P.; Bajaj, K.; Powers, J.; Reichling, J.; Wasserman, D.; Dolan, S.M.; Merkatz, I.R. Clinical utility of chromosomal microarray analysis in prenatal diagnosis: Report of first 6 months in clinical practice. J. Matern. Neonatal Med. 2014, 27, 1333–1338. [Google Scholar] [CrossRef]
- Lallar, M.; Srivastava, P.; Rai, A.; Saxena, D.; Mandal, K.; Phadke, S.R. Cytogenetic microarray in structurally normal and abnormal foetuses: A five years experience elucidating increasing acceptance and clinical utility. J. Genet. 2019, 98, 1–9. [Google Scholar] [CrossRef]
- Lee, C.-N.; Lin, S.-Y.; Lin, C.-H.; Shih, J.-C.; Lin, T.-H.; Su, Y.-N. Clinical Utility of Array Comparative Genomic Hybridisation for Prenatal Diagnosis. Obstet. Gynecol. Surv. 2012, 67, 461–463. [Google Scholar] [CrossRef]
- Li, L.; He, Z.; Huang, X.; Lin, S.; Wu, J.; Huang, L.; Wan, Y.; Fang, Q. Chromosomal Abnormalities Detected by Karyotyping and Microarray Analysis in Twins With Structural Anomalies. Obstet. Gynecol. Surv. 2020, 75, 535–537. [Google Scholar] [CrossRef]
- Lovrecic, L.; Remec, Z.I.; Volk, M.; Rudolf, G.; Writzl, K.; Peterlin, B. Clinical utility of array comparative genomic hybridisation in prenatal setting. BMC Med. Genet. 2016, 17, 1–8. [Google Scholar] [CrossRef][Green Version]
- Oneda, B.; Baldinger, R.; Reissmann, R.; Reshetnikova, I.; Krejci, P.; Masood, R.; Ochsenbein-Kölble, N.; Bartholdi, D.; Steindl, K.; Morotti, D.; et al. High-resolution chromosomal microarrays in prenatal diagnosis significantly increase diagnostic power. Prenat. Diagn. 2014, 34, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Parchem, J.G.; Sparks, T.N.; Gosnell, K.; Norton, M.E. Utility of chromosomal microarray in anomalous fetuses. Prenat. Diagn. 2018, 38, 140–147. [Google Scholar] [CrossRef]
- Peng, H.H.; Lee, C.H.; Su, S.Y.; Chen, K.J.; Lee, Y.C.; You, S.H.; Lee, W.F.; Cheng, P.J. Prenatally diagnosed de novo segmental amplification or deletion by microarray-based comparative genomic hybridization: A retrospective study. Taiwan J. Obstet. Gynecol. 2019, 58, 662–666. [Google Scholar] [CrossRef]
- Pons, L.; Till, M.; Alix, E.; Abel, C.; Boggio, D.; Bordes, A.; Caloone, J.; Raskin, F.C.; Chatron, N.; Cordier, M.P.; et al. Prenatal microarray comparative genomic hybridization: Experience from the two first years of activity at the Lyon university-hospital. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 275–283. [Google Scholar] [CrossRef]
- Ridnõi, K.; Muru, K.; Keernik, M.; Pajusalu, S.; Ustav, E.L.; Tammur, P.; Mölter-Väär, T.; Kahre, T.; Šamarina, U.; Asser, K.; et al. A two-year prospective study assessing the performance of fetal chromosomal microarray analysis and next-generation sequencing in high-risk pregnancies. Mol. Genet. Genom. Med. 2021, 9, e1787. [Google Scholar] [CrossRef]
- Schmid, M.; Stary, S.; Springer, S.; Bettelheim, D.; Husslein, P.; Streubel, B. Prenatal microarray analysis as second-tier diagnostic test: Single-center prospective study. Ultrasound Obstet. Gynecol. 2013, 41, 267–273. [Google Scholar] [CrossRef]
- Shaffer, L.G.; Dabell, M.P.; Fisher, A.J.; Coppinger, J.; Bandholz, A.M.; Ellison, J.W.; Ravnan, J.B.; Torchia, B.S.; Ballif, B.C.; Rosenfeld, J.A. Experience With Microarray-Based Comparative Genomic Hybridization for Prenatal Diagnosis in Over 5000 Pregnancies. Obstet. Gynecol. Surv. 2013, 68, 93–95. [Google Scholar] [CrossRef][Green Version]
- Srebniak, M.; Boter, M.; Oudesluijs, G.; Joosten, M.; Govaerts, L.; Van Opstal, D.; Galjaard, R.J.H. Application of SNP array for rapid prenatal diagnosis: Implementation, genetic counselling and diagnostic flow. Eur. J. Hum. Genet. 2011, 19, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Srebniak, M.I.; Boter, M.; Oudesluijs, G.O.; Cohen-Overbeek, T.; Govaerts, L.C.P.; Diderich, K.E.M.; Oegema, R.; Knapen, M.F.C.M.; Van De Laar, I.M.B.H.; Joosten, M.; et al. Genomic SNP array as a gold standard for prenatal diagnosis of foetal ultrasound abnormalities. Mol. Cytogenet. 2012, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Srebniak, M.I.; Mout, L.; Van Opstal, D.; Galjaard, R.J.H. 0.5 Mb array as a first-line prenatal cytogenetic test in cases without ultrasound abnormalities and its implementation in clinical Practice. Hum. Mutat. 2013, 34, 1298–1303. [Google Scholar] [CrossRef]
- Srebniak, M.I.; Diderich, K.E.M.; Joosten, M.; Govaerts, L.C.P.; Knijnenburg, J.; De Vries, F.A.T.; Boter, M.; Lont, D.; Knapen, M.F.C.M.; De Wit, M.C.; et al. Prenatal SNP array testing in 1000 fetuses with ultrasound anomalies: Causative, unexpected and susceptibility CNVs. Eur. J. Hum. Genet. 2016, 24, 645–651. [Google Scholar] [CrossRef]
- Srebniak, M.I.; Knapen, M.F.C.M.; Polak, M.; Joosten, M.; Diderich, K.E.M.; Govaerts, L.C.P.; Boter, M.; Kromosoeto, J.N.R.; van Hassel, D.A.C.M.; Huijbregts, G.; et al. The influence of SNP-based chromosomal microarray and NIPT on the diagnostic yield in 10,000 fetuses with and without fetal ultrasound anomalies. Hum. Mutat. 2017, 38, 880–888. [Google Scholar] [CrossRef]
- Stern, S.; Hacohen, N.; Meiner, V.; Yagel, S.; Zenvirt, S.; Shkedi-Rafid, S.; Macarov, M.; Valsky, D.V.; Porat, S.; Yanai, N.; et al. Universal chromosomal microarray analysis reveals high proportion of copy-number variants in low-risk pregnancies. Ultrasound Obstet. Gynecol. 2021, 57, 813–820. [Google Scholar] [CrossRef]
- Vogel, I.; Petersen, O.B.; Christensen, R.; Hyett, J.; Lou, S.; Vestergaard, E.M. Chromosomal microarray as primary diagnostic genomic tool for pregnancies at increased risk within a population-based combined first-trimester screening program. Ultrasound Obstet. Gynecol. 2018, 51, 480–486. [Google Scholar] [CrossRef]
- Wright, D.; Carey, L.; Battersby, S.; Nguyen, T.; Clarke, M.; Nash, B.; Gulesserian, E.; Cross, J.; Darmanian, A. Validation of a Chromosomal Microarray for Prenatal Diagnosis Using a Prospective Cohort of Pregnancies with Increased Risk for Chromosome Abnormalities. Genet. Test. Mol. Biomark. 2016, 20, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Lin, N.; Xie, X.; Su, L.; Cai, M.; Lin, Y.; Wang, L.; Wang, M.; Xu, L.; et al. Chromosomal microarray analysis for pregnancies with abnormal maternal serum screening who undergo invasive prenatal testing. J. Cell. Mol. Med. 2021, 25, 6271–6279. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Ding, Y.; Song, X.; Mao, J.; Liu, M.; Liu, Y.; Huang, C.; Zhang, Q.; Wang, T. Clinical Utility of SNP Array Analysis in Prenatal Diagnosis: A Cohort Study of 5000 Pregnancies. Front. Genet. 2020, 11, 1401. [Google Scholar] [CrossRef]
- Yakut, S.; Cetin, Z.; Simsek, M.; Mendicioglu, I.I.; Toru, H.S.; Karauzum, S.B.; Luleci, G. Rare structural chromosomal abnormalities in prenatal diagnosis; clinical and cytogenetic findings on 10,125 prenatal cases. Turk. J. Pathol. 2014, 31, 36–44. [Google Scholar] [CrossRef][Green Version]
- Yatsenko, S.; Davis, S.; Hendrix, N.; Surti, U.; Emery, S.; Canavan, T.; Speer, P.; Hill, L.; Clemens, M.; Rajkovic, A. Application of chromosomal microarray in the evaluation of abnormal prenatal findings. Clin. Genet. 2013, 84, 47–54. [Google Scholar] [CrossRef]
- Žilina, O.; Teek, R.; Tammur, P.; Kuuse, K.; Yakoreva, M.; Vaidla, E.; Mölter-Väär, T.; Reimand, T.; Kurg, A.; Õunap, K. Chromosomal microarray analysis as a first-tier clinical diagnostic test: Estonian experience. Mol. Genet. Genom. Med. 2014, 2, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Bardin, R.; Hadar, E.; Haizler-Cohen, L.; Gabbay-Benziv, R.; Meizner, I.; Kahana, S.; Yeshaya, J.; Yacobson, S.; Cohen-Vig, L.; Agmon-Fishman, I.; et al. Cytogenetic analysis in fetuses with late onset abnormal sonographic findings. J. Perinat. Med. 2018, 46, 975–982. [Google Scholar] [CrossRef]
- Bornstein, E.; Berger, S.; Cheung, S.; Maliszewski, K.; Patel, A.; Pursley, A.; Lenchner, E.; Bacino, C.; Beaudet, A.; Divon, M. Universal Prenatal Chromosomal Microarray Analysis: Additive Value and Clinical Dilemmas in Fetuses with a Normal Karyotype. Am. J. Perinatol. 2016, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Brabbing-Goldstein, D.; Reches, A.; Svirsky, R.; Bar-Shira, A.; Yaron, Y. Dilemmas in genetic counseling for low-penetrance neuro-susceptibility loci detected on prenatal chromosomal microarray analysis. Am. J. Obstet. Gynecol. 2018, 218, 247.e1–247.e12. [Google Scholar] [CrossRef]
- Cai, M.; Lin, N.; Su, L.; Wu, X.; Xie, X.; Li, Y.; Lin, Y.; Xu, L.; Huang, H. Copy number variations in ultrasonically abnormal late pregnancy fetuses with normal karyotypes. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Cai, M.; Lin, N.; Chen, X.; Fu, M.; Guo, N.; Xu, L.; Huang, H. Evaluation of chromosomal abnormalities and copy number variations in fetuses with ultrasonic soft markers. BMC Med. Genom. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Charan, P.; Woodrow, N.; Walker, S.P.; Ganesamoorthy, D.; McGillivray, G.; Palma-Dias, R. High-resolution microarray in the assessment of fetal anomalies detected by ultrasound. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 46–52. [Google Scholar] [CrossRef]
- Fiorentino, F.; Napoletano, S.; Caiazzo, F.; Sessa, M.; Bono, S.; Spizzichino, L.; Gordon, A.; Nuccitelli, A.; Rizzo, G.; Baldi, M. Chromosomal microarray analysis as a first-line test in pregnancies with a priori low risk for the detection of submicroscopic chromosomal abnormalities. Eur. J. Hum. Genet. 2013, 21, 725–730. [Google Scholar] [CrossRef]
- Hillman, S.C.; McMullan, D.J.; Hall, G.; Togneri, F.S.; James, N.; Maher, E.J.; Meller, C.H.; Williams, D.; Wapner, R.J.; Maher, E.R.; et al. Use of prenatal chromosomal microarray: Prospective cohort study and systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.M.; Li, L.L.; Zhang, H.; Zhang, H.G.; Liu, R.Z.; Yu, Y. Clinical application of chromosomal microarray analysis in pregnant women with advanced maternal age and fetuses with ultrasonographic soft markers. Med. Sci. Monit. 2021, 27, e929074-1. [Google Scholar] [CrossRef]
- Hui, A.S.; Chau, M.H.K.; Chan, Y.M.; Cao, Y.; Kwan, A.H.; Zhu, X.; Kwok, Y.K.; Chen, Z.; Lao, T.T.; Choy, K.W.; et al. The role of chromosomal microarray analysis among fetuses with normal karyotype and single system anomaly or nonspecific sonographic findings. Acta Obstet. Gynecol. Scand. 2021, 100, 235–243. [Google Scholar] [CrossRef]
- Bajaj Lall, M.; Agarwal, S.; Paliwal, P.; Saviour, P.; Joshi, A.; Joshi, A.; Mahajan, S.; Bijarnia-Mahay, S.; Dua Puri, R.; Verma, I.C. Prenatal Diagnosis by Chromosome Microarray Analysis, An Indian Experience. J. Obstet. Gynecol. India 2021, 71, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Z.; Tang, H.-S. Chromosomal microarray analysis in pregnancies at risk for a molecular disorder. J. Matern. Neonatal Med. 2021, 34, 159–162. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Ye, M.; Chen, S.; Shen, Y.; Niu, J.; Wang, Y.; Xu, C. Should chromosomal microarray be offered to fetuses with ultrasonographic soft markers in second trimester: A prospective cohort study and meta-analysis. Prenat. Diagn. 2020, 40, 1569–1577. [Google Scholar] [CrossRef]
- Liao, C.; Fu, F.; Li, R.; Xie, G.E.; Zhang, Y.L.; Li, J.; Li, D.Z. Implementation of high-resolution SNP arrays in the investigation of fetuses with ultrasound malformations: 5 years of clinical experience. Clin. Genet. 2014, 86, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Jong, Y.J.; Huang, P.C.; Tsai, C. Detection of copy number variants with chromosomal microarray in 10,377 pregnancies at a single laboratory. Acta Obstet. Gynecol. Scand. 2020, 99, 775–782. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, H.; Wang, L.; Xiao, B.; Fan, Y.; Ye, H.; Ying, X.; Qiu, W.; Zhang, H.; Han, L.; et al. Chromosomal microarray analysis in fetuses with high-risk prenatal indications: A retrospective study in China. Taiwan. J. Obstet. Gynecol. 2021, 60, 299–304. [Google Scholar] [CrossRef]
- Moshonov, R.; Hod, K.; Azaria, B.; Abadi-Korek, I.; Berger, R.; Shohat, M. Benefit versus risk of chromosomal microarray analysis performed in pregnancies with normal and positive prenatal screening results: A retrospective study. PLoS ONE 2021, 16, e0250734. [Google Scholar] [CrossRef] [PubMed]
- Muys, J.; Blaumeiser, B.; Jacquemyn, Y.; Bandelier, C.; Brison, N.; Bulk, S.; Chiarappa, P.; Courtens, W.; De Leener, A.; De Rademaeker, M.; et al. The Belgian MicroArray Prenatal (BEMAPRE) database: A systematic nationwide repository of fetal genomic aberrations. Prenat. Diagn. 2018, 38, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Papoulidis, I.; Sotiriadis, A.; Siomou, E.; Papageorgiou, E.; Eleftheriades, M.; Papadopoulos, V.; Oikonomidou, E.; Orru, S.; Manolakos, E.; Athanasiadis, A. Routine use of array comparative genomic hybridization (aCGH) as standard approach for prenatal diagnosis of chromosomal abnormalities. Clinical experience of 1763 prenatal cases. Prenat. Diagn. 2015, 35, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Jiang, Y.; Zhou, X.; Meng, H.; Hao, N.; Chang, J.; Bai, J.; Wang, C.; Wang, M.; Guo, J.; et al. Simultaneous detection of cnvs and snvs improves the diagnostic yield of fetuses with ultrasound anomalies and normal karyotypes. Genes 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Revenga, L.; Madrigal, I.; Borrell, A.; Martinez, J.M.; Sabria, J.; Martin, L.; Jimenez, W.; Mira, A.; Badenas, C.; Milà, M. Chromosome microarray analysis should be offered to all invasive prenatal diagnostic testing following a normal rapid aneuploidy test result. Clin. Genet. 2020, 98, 379–383. [Google Scholar] [CrossRef]
- Rooryck, C.; Toutain, J.Ô.; Cailley, D.; Bouron, J.; Horovitz, J.; Lacombe, D.; Arveiler, B.; Saura, R. Prenatal diagnosis using array-CGH: A French experience. Eur. J. Med. Genet. 2013, 56, 341–345. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Maya, I.; Reches, A.; Frumkin, A.; Grinshpun-Cohen, J.; Segel, R.; Manor, E.; Khayat, M.; Tenne, T.; Banne, E. Chromosomal microarray analysis results from pregnancies with various ultrasonographic anomalies. Obstet. Gynecol. 2018, 132, 1368–1375. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Vig, L.C.; Kahana, S.; Yacobson, S.; Tenne, T.; Agmon-Fishman, I.; Klein, C.; Matar, R.; Basel-Salmon, L.; Maya, I. Chromosomal Microarray vs. NIPS: Analysis of 5541 Low-Risk Pregnancies. Obstet. Gynecol. Surv. 2020, 75, 222–224. [Google Scholar] [CrossRef]
- Shaffer, L.G.; Rosenfeld, J.A.; Dabell, M.P.; Coppinger, J.; Bandholz, A.M.; Ellison, J.W.; Ravnan, J.B.; Torchia, B.S.; Ballif, B.C.; Fisher, A.J. Detection rates of clinically significant genomic alterations by microarray analysis for specific anomalies detected by ultrasound. Prenat. Diagn. 2012, 32, 986–995. [Google Scholar] [CrossRef]
- Van Opstal, D.; de Vries, F.; Govaerts, L.; Boter, M.; Lont, D.; van Veen, S.; Joosten, M.; Diderich, K.; Galjaard, R.J.; Srebniak, M.I. Benefits and burdens of using a SNP Array in pregnancies at increased risk for the common aneuploidies. Hum. Mutat. 2015, 36, 319–326. [Google Scholar] [CrossRef]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal Microarray Versus Karyotyping for Prenatal Diagnosis. Obstet. Gynecol. Surv. 2013, 68, 276–278. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; An, G.; Xie, X.; Su, L.; Cai, M.; Chen, X.; Li, Y.; Lin, N.; He, D.; Wang, M.; et al. Chromosomal microarray analysis for pregnancies with or without ultrasound abnormalities in women of advanced maternal age. J. Clin. Lab. Anal. 2020, 34, e23117. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Tao, J.; Han, X.; Zhao, X.; Liu, C.; Gao, L.; Cheng, W. The clinical use of chromosomal microarray analysis in detection of fetal chromosomal rearrangements: A study from China Mainland. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Yang, X.; Fu, J.; Teng, Z.; Lv, Y.; Yu, L. Application of chromosome microarray analysis in prenatal diagnosis. BMC Pregnancy Childbirth 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.D.; DeKoninck, P.; Fryns, J.P.; Devriendt, K.; Deprest, J.A.; Vermeesch, J.R. Identification of dosage-sensitive genes in fetuses referred with severe isolated congenital diaphragmatic hernia. Prenat. Diagn. 2013, 33, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Z.; Rosenfeld, J.A.; Pursley, A.N.; Patel, A.; Huang, J.; Wang, H.; Chen, M.; Sun, X.; Leung, T.Y.; et al. Contribution of genomic copy-number variations in prenatal oral clefts: A multicenter cohort study. Genet. Med. 2016, 18, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Huang, L.; Liu, J.; Fang, F.; Liu, Z.; Zhang, Y.; Li, F.; Liao, C. Prenatal diagnosis of submicroscopic chromosomal aberrations in fetuses with congenital cystic adenomatoid malformation by chromosomal microarray analysis. J. Matern. Neonatal Med. 2021, 34, 2623–2629. [Google Scholar] [CrossRef]
- Du, L.; Xie, H.N.; Huang, L.H.; Xie, Y.J.; Wu, L.H. Prenatal diagnosis of submicroscopic chromosomal aberrations in fetuses with ventricular septal defects by chromosomal microarray-based analysis. Prenat. Diagn. 2016, 36, 1178–1184. [Google Scholar] [CrossRef]
- Fu, F.; Chen, F.; Li, R.; Zhang, Y.; Pan, M.; Li, D.; Liao, C. Prenatal diagnosis of fetal multicystic dysplastic kidney via high-resolution whole-genome array. Nephrol. Dial. Transplant. 2016, 31, 1693–1698. [Google Scholar] [CrossRef]
- Fu, F.; Deng, Q.; Lei, T.Y.; Li, R.; Jing, X.Y.; Yang, X.; Liao, C. Clinical application of SNP array analysis in fetuses with ventricular septal defects and normal karyotypes. Arch. Gynecol. Obstet. 2017, 296, 929–940. [Google Scholar] [CrossRef]
- Jin, H.; Yingqiu, C.; Zequn, L.; Yanjun, H.; Yunyan, Z.; Shufan, Z.; Yiyang, C.; Ru, L.; Li, Z.; Yongling, Z.; et al. Chromosomal microarray analysis in the prenatal diagnosis of orofacial clefts: Experience from a single medical center in mainland China. Medicine 2018, 97, e12057. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Won, H.S.; Han, Y.J.; Ryu, H.M.; Lee, D.E.; Jeong, B. Da Clinical value of chromosomal microarray analysis in prenatally diagnosed dextro-transposition of the great arteries. J. Matern. Neonatal Med. 2020, 33, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.C.; Boekhorst, F.; Mancini, G.M.; Smit, L.S.; Groenenberg, I.A.L.; Dudink, J.; de Vries, F.A.T.; Go, A.T.J.I.; Galjaard, R.J.H. Advanced genomic testing may aid in counseling of isolated agenesis of the corpus callosum on prenatal ultrasound. Prenat. Diagn. 2017, 37, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Huang, H.; Su, L.; Lin, N.; Wu, X.; Xie, X.; An, G.; Li, Y.; Lin, Y.; Xu, L.; et al. Chromosomal abnormalities and copy number variations in fetal ventricular septal defects. Mol. Cytogenet. 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Feng, J.L.; Xie, Y.J.; Xie, H.N.; Zheng, J.; Lin, M.F. Chromosomal aneuploidies and copy number variations in posterior fossa abnormalities diagnosed by prenatal ultrasonography. Prenat. Diagn. 2017, 37, 1160–1168. [Google Scholar] [CrossRef]
- Lemire, G.T.; Beauregard-Lacroix, É.; Campeau, P.M.; Parent, S.; Roy-Beaudry, M.; Soglio, D.D.; Grignon, A.; Rypens, F.; Wavrant, S.; Laberge, A.M.; et al. Retrospective analysis of fetal vertebral defects: Associated anomalies, etiologies, and outcome. Am. J. Med. Genet. Part A 2020, 182, 664–672. [Google Scholar] [CrossRef]
- Li, L.; Fu, F.; Li, R.; Xiao, W.; Yu, Q.; Wang, D.; Jing, X.; Zhang, Y.; Yang, X.; Pan, M.; et al. Genetic tests aid in counseling of fetuses with cerebellar vermis defects. Prenat. Diagn. 2020, 40, 1228–1238. [Google Scholar] [CrossRef]
- Maya, I.; Singer, A.; Yonath, H.; Reches, A.; Rienstein, S.; Zeligson, S.; Ben Shachar, S.; Sagi-Dain, L. What have we learned from 691 prenatal chromosomal microarrays for ventricular septal defects? Acta Obstet. Gynecol. Scand. 2020, 99, 757–764. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, J.; Peng, R.; Du, L.; Zheng, Q.; Lei, T.; Xie, H. Prenatal diagnosis of chromosomal aberrations in fetuses with conotruncal heart defects by genome-wide high-resolution SNP array. J. Matern. Neonatal Med. 2020, 33, 1211–1217. [Google Scholar] [CrossRef]
- Maya, I.; Singer, A.; Baris, H.N.; Goldberg, Y.; Shalata, A.; Khayat, M.; Ben-Shachar, S.; Sagi-Dain, L. Prenatal microarray analysis in right aortic arch—A retrospective cohort study and review of the literature. J. Perinatol. 2018, 38, 468–473. [Google Scholar] [CrossRef]
- O’Mahony, E.F.; Hutchinson, D.P.; McGillivray, G.; Nisbet, D.L.; Palma-Dias, R. Right-sided aortic arch in the age of microarray. Prenat. Diagn. 2017, 37, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zheng, J.; Xie, H.N.; He, M.; Lin, M.F. Genetic anomalies in fetuses with tetralogy of Fallot by using high-definition chromosomal microarray analysis. Cardiovasc. Ultrasound 2019, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Xie, H.N.; Zheng, J.; Zhou, Y.; Lin, M.F. Fetal right aortic arch: Associated anomalies, genetic anomalies with chromosomal microarray analysis, and postnatal outcome. Prenat. Diagn. 2017, 37, 329–335. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Singer, A.; Frumkin, A.; Shalata, A.; Koifman, A.; Segel, R.; Benyamini, L.; Rienstein, S.; Kahyat, M.; Sharony, R.; et al. Chromosomal microarray findings in pregnancies with an isolated pelvic kidney. J. Perinat. Med. 2019, 47, 30–34. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Singer, A.; Josefsberg, S.; Peleg, A.; Lev, D.; Samra, N.N.; Bar-Shira, A.; Zeligson, S.; Maya, I.; Ben-Shachar, S. Microarray analysis has no additional value in fetal aberrant right subclavian artery: Description of 268 pregnancies and systematic literature review. Ultrasound Obstet. Gynecol. 2019, 53, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Maya, I.; Falik-Zaccai, T.; Feingold-Zadok, M.; Lev, D.; Yonath, H.; Kaliner, E.; Frumkin, A.; Ben Shachar, S.; Singer, A. Isolated fetal horseshoe kidney does not seem to increase the risk for abnormal chromosomal microarray results. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 80–83. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Maya, I.; Peleg, A.; Reches, A.; Banne, E.; Baris, H.N.; Tenne, T.; Singer, A.; Ben-Shachar, S. Microarray analysis in pregnancies with isolated unilateral kidney agenesis. Pediatr. Res. 2018, 83, 825–828. [Google Scholar] [CrossRef]
- Singer, A.; Maya, I.; Frumkin, A.; Zeligson, S.; Josefsberg, S.B.Y.; Berger, R.; Ben Shachar, S.; Sagi-Dain, L. Is fetal isolated double renal collecting system an indication for chromosomal microarray? J. Matern. Neonatal Med. 2021, 34, 696–700. [Google Scholar] [CrossRef]
- Maya, I.; Kahana, S.; Yeshaya, J.; Tenne, T.; Yacobson, S.; Agmon-Fishman, I.; Cohen-Vig, L.; Levi, A.; Reinstein, E.; Basel-Vanagaite, L.; et al. Chromosomal microarray analysis in fetuses with aberrant right subclavian artery. Ultrasound Obstet. Gynecol. 2017, 49, 337–341. [Google Scholar] [CrossRef]
- Vedel, C.; Rode, L.; Jørgensen, F.S.; Petersen, O.B.; Sundberg, K.; Tabor, A.; Ekelund, C.K. Prenatally detected isolated ventricular septum defects and the association with chromosomal aberrations—A nationwide register-based study from Denmark. Prenat. Diagn. 2021, 41, 347–353. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Su, L.; Xie, X.; Cai, M.; Lin, N.; Huang, H.; Lin, Y.; Xu, L. Chromosomal Microarray Analysis for the Fetuses with Aortic Arch Abnormalities and Normal Karyotype. Mol. Diagn. Ther. 2020, 24, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Zhu, X.; Wang, Y.; Ru, T.; Dai, C.; Wang, Z.; Li, J.; Hu, Y. Copy number variations in multicystic dysplastic kidney: Update for prenatal diagnosis and genetic counseling. Prenat. Diagn. 2016, 36, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, T.; Fu, F.; Deng, Q.; Li, R.; Wang, D.; Yang, X.; Li, D.; Liao, C. Microarray analysis in fetuses with duodenal obstruction: It is not just trisomy 21. Prenat. Diagn. 2021, 41, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Huang, L.; Lin, S.; He, Z.; Zhu, H.; Zhang, Y.; Fang, Q.; Luo, Y. Prenatal diagnosis of posterior fossa anomalies: Additional value of chromosomal microarray analysis in fetuses with cerebellar hypoplasia. Prenat. Diagn. 2018, 38, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tang, H.; Lu, J.; Yang, X.; Ding, H.; Wu, J. Prenatal genetic diagnosis of omphalocele by karyotyping, chromosomal microarray analysis and exome sequencing. Ann. Med. 2021, 53, 1285–1291. [Google Scholar] [CrossRef]
- Svirsky, R.; Brabbing-Goldstein, D.; Rozovski, U.; Kapusta, L.; Reches, A.; Yaron, Y. The genetic and clinical outcome of isolated fetal muscular ventricular septal defect (VSD). J. Matern. Neonatal Med. 2019, 32, 2837–2841. [Google Scholar] [CrossRef]
- Svirsky, R.; Reches, A.; Brabbing-Goldstein, D.; Bar-Shira, A.; Yaron, Y. Association of aberrant right subclavian artery with abnormal karyotype and microarray results. Prenat. Diagn. 2017, 37, 808–811. [Google Scholar] [CrossRef]
- Singer, A.; Maya, I.; Banne, E.; Baris Feldman, H.; Vinkler, C.; Ben Shachar, S.; Sagi-Dain, L. Prenatal clubfoot increases the risk for clinically significant chromosomal microarray results—Analysis of 269 singleton pregnancies. Early Hum. Dev. 2020, 145, 105047. [Google Scholar] [CrossRef]
- des Portes, V.; Rolland, A.; Velazquez-Dominguez, J.; Peyric, E.; Cordier, M.P.; Gaucherand, P.; Massardier, J.; Massoud, M.; Curie, A.; Pellot, A.S.; et al. Outcome of isolated agenesis of the corpus callosum: A population-based prospective study. Eur. J. Paediatr. Neurol. 2018, 22, 82–92. [Google Scholar] [CrossRef]
- van Nisselrooij, A.E.L.; Lugthart, M.A.; Clur, S.A.; Linskens, I.H.; Pajkrt, E.; Rammeloo, L.A.; Rozendaal, L.; Blom, N.A.; van Lith, J.M.M.; Knegt, A.C.; et al. The prevalence of genetic diagnoses in fetuses with severe congenital heart defects. Genet. Med. 2020, 22, 1206–1214. [Google Scholar] [CrossRef]
- Huang, J.; Poon, L.C.; Akolekar, R.; Choy, K.W.; Leung, T.Y.; Nicolaides, K.H. Is high fetal nuchal translucency associated with submicroscopic chromosomal abnormalities on array CGH? Ultrasound Obstet. Gynecol. 2014, 43, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Krutzke, S.K.; Engels, H.; Hofmann, A.; Schumann, M.M.; Cremer, K.; Zink, A.M.; Hilger, A.; Ludwig, M.; Gembruch, U.; Reutter, H.; et al. Array-based molecular karyotyping in fetal brain malformations: Identification of novel candidate genes and chromosomal regions. Birth Defects Res. Part A Clin. Mol. Teratol. 2016, 106, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, H.; Tang, J.; Riaz Khan, M.; Wang, B.; Bukhari, I. Identification of minor chromosomal defects causing abnormal foetus and spontaneous abortions. Br. J. Biomed. Sci. 2016, 73, 67–73. [Google Scholar] [CrossRef]
- Cicatiello, R.; Pignataro, P.; Izzo, A.; Mollo, N.; Pezone, L.; Maruotti, G.M.; Sarno, L.; Sglavo, G.; Conti, A.; Genesio, R.; et al. Chromosomal Microarray Analysis versus Karyotyping in Fetuses with Increased Nuchal Translucency. Med. Sci. 2019, 7, 40. [Google Scholar] [CrossRef]
- Yi, J.L.; Zhang, W.; Meng, D.H.; Ren, L.J.; Yu, J.; Wei, Y.L. Epidemiology of fetal cerebral ventriculomegaly and evaluation of chromosomal microarray analysis versus karyotyping for prenatal diagnosis in a Chinese hospital. J. Int. Med. Res. 2019, 47, 5508–5517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, T.; Wang, J.; Li, Q.; Wang, H.; Liu, S. Prenatal Diagnostic Value of Chromosomal Microarray in Fetuses with Nuchal Translucency Greater than 2.5 mm. BioMed Res. Int. 2019, 2019, 6504159. [Google Scholar] [CrossRef]
- Sukenik-Halevy, R.; Sukenik, S.; Koifman, A.; Alpert, Y.; Hershkovitz, R.; Levi, A.; Biron-Shental, T. Clinical aspects of prenatally detected congenital heart malformations and the yield of chromosomal microarray analysis. Prenat. Diagn. 2016, 36, 1185–1191. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Huang, X.; He, Z.; Lin, S.; Wang, Y.; Li, L.; Luo, Y.; Fang, Q. Chromosomal aberrations and CNVs in twin fetuses with cardiovascular anomalies: Comparison between monochorionic diamniotic and dichorionic diamniotic twins. Prenat. Diagn. 2018, 38, 318–327. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, S.; Huang, L.; He, Z.; Huang, X.; Zhou, Y.; Fang, Q.; Luo, Y. Application of chromosomal microarray analysis in prenatal diagnosis of fetal growth restriction. Prenat. Diagn. 2016, 36, 686–692. [Google Scholar] [CrossRef]
- An, G.; Lin, Y.; Xu, L.P.; Huang, H.L.; Liu, S.P.; Yu, Y.H.; Yang, F. Application of chromosomal microarray to investigate genetic causes of isolated fetal growth restriction. Mol. Cytogenet. 2018, 11, 33. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Y.; Hu, H.; Yao, H.; Li, Y.; Tang, S.; Zheng, L.; Xu, Y.; Liang, Z. Karyotypic and Molecular Genetic Changes Associated With Fetal Cardiovascular Abnormalities: Results of a Retrospective 4-Year Ultrasonic Diagnosis Study. Int. J. Biol. Sci. 2013, 9, 463–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borrell, A.; Grande, M.; Meler, E.; Sabrià, J.; Mazarico, E.; Muñoz, A.; Rodriguez-Revenga, L.; Badenas, C.; Figueras, F. Genomic Microarray in Fetuses With Early Growth Restriction: A Multicenter Study. Obstet. Gynecol. Surv. 2018, 73, 73–74. [Google Scholar] [CrossRef]
- Brun, S.; Pennamen, P.; Mattuizzi, A.; Coatleven, F.; Vuillaume, M.L.; Lacombe, D.; Arveiler, B.; Toutain, J.; Rooryck, C. Interest of chromosomal microarray analysis in the prenatal diagnosis of fetal intrauterine growth restriction. Prenat. Diagn. 2018, 38, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Huang, H.; Su, L.; Lin, N.; Wu, X.; Xie, X.; An, G.; Li, Y.; Lin, Y.; Xu, L. Fetal congenital heart disease: Associated anomalies, identification of genetic anomalies by single-nucleotide polymorphism array analysis, and postnatal outcome. Medicine 2018, 97. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lin, N.; Su, L.; Wu, X.; Xie, X.; Li, Y.; Chen, X.; Lin, Y.; Huang, H.; Xu, L. Copy number variations associated with fetal congenital kidney malformations. Mol. Cytogenet. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Lin, N.; Su, L.; Wu, X.; Xie, X.; Li, Y.; Chen, X.; Dai, Y.; Lin, Y.; Huang, H.; et al. Detection of copy number disorders associated with congenital anomalies of the kidney and urinary tract in fetuses via single nucleotide polymorphism arrays. J. Clin. Lab. Anal. 2020, 34, e23025. [Google Scholar] [CrossRef]
- Cai, M.; Huang, H.; Su, L.; Wu, X.; Xie, X.; Xu, L.; Lin, N. Choroid Plexus Cysts: Single Nucleotide Polymorphism Array Analysis of Associated Genetic Anomalies and Resulting Obstetrical Outcomes. Risk Manag. Healthc. Policy 2021, 14, 2491–2497. [Google Scholar] [CrossRef]
- Cai, M.; Huang, H.; Xu, L.; Lin, N. Clinical Utility and the Yield of Single Nucleotide Polymorphism Array in Prenatal Diagnosis of Fetal Central Nervous System Abnormalities. Front. Mol. Biosci. 2021, 8, 452. [Google Scholar] [CrossRef]
- Chang, Q.; Yang, Y.; Peng, Y.; Liu, S.; Li, L.; Deng, X.; Yang, M.; Lan, Y. Prenatal detection of chromosomal abnormalities and copy number variants in fetuses with ventriculomegaly. Eur. J. Paediatr. Neurol. 2020, 25, 106–112. [Google Scholar] [CrossRef]
- de Wit, M.C.; Srebniak, M.I.; Joosten, M.; Govaerts, L.C.P.; Kornelisse, R.F.; Papatsonis, D.N.M.; de Graaff, K.; Knapen, M.F.C.M.; Bruggenwirth, H.T.; de Vries, F.A.T.; et al. Prenatal and postnatal findings in small-for-gestational-age fetuses without structural ultrasound anomalies at 18–24 weeks. Ultrasound Obstet. Gynecol. 2017, 49, 342–348. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Jiang, Y.; Luo, Q.; Shi, L.; Zeng, S.; Zhuang, J.; Lyu, G. The Genetic Etiology Diagnosis of Fetal Growth Restriction Using Single-Nucleotide Polymorphism-Based Chromosomal Microarray Analysis. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.-L.; Zhu, X.-Y.; Zhu, Y.-J.; Wu, X.; Zhao, G.-F.; Wang, W.-J.; Li, J. The application of chromosomal microarray analysis to the prenatal diagnosis of isolated mild ventriculomegaly. Taiwan. J. Obstet. Gynecol. 2019, 58, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Egloff, M.; Hervé, B.; Quibel, T.; Jaillard, S.; Le Bouar, G.; Uguen, K.; Saliou, A.H.; Valduga, M.; Perdriolle, E.; Coutton, C.; et al. Diagnostic yield of chromosomal microarray analysis in fetuses with isolated increased nuchal translucency: A French multicenter study. Ultrasound Obstet. Gynecol. 2018, 52, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Huang, H.; Lin, X.; Xue, H.; Cai, M.; Lin, N.; Xu, L. Performance of chromosomal microarray analysis for detection of copy number variations in fetal echogenic bowel. Risk Manag. Healthc. Policy 2021, 14, 1431–1438. [Google Scholar] [CrossRef]
- Fantasia, I.; Stampalija, T.; Sirchia, F.; Della Pietà, I.; Ottaviani Giammarco, C.; Guidolin, F.; Quadrifoglio, M.; Barresi, V.; Travan, L.; Faletra, F. First-trimester absent nasal bone: Is it a predictive factor for pathogenic CNVs in the low-risk population? Prenat. Diagn. 2020, 40, 1563–1568. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Nisbet, D.L.; Reidy, K.L.; Palma-Dias, R. Hypoplastic nasal bone: A potential marker for facial dysmorphism associated with pathogenic copy number variants on microarray. Prenat. Diagn. 2019, 39, 116–123. [Google Scholar] [CrossRef]
- He, M.; Zhang, Z.; Hu, T.; Liu, S. Chromosomal microarray analysis for the detection of chromosome abnormalities in fetuses with echogenic intracardiac focus in women without high-risk factors. Medicine 2020, 99, e19014. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Y.; Sun, R.; Cao, L.; Chen, X.; Liu, C.; Luo, C.; Ma, D.; Wang, W.; Fu, X.; et al. Copy Number Variations with Isolated Fetal Ventriculomegaly. Curr. Mol. Med. 2017, 17, 133–139. [Google Scholar] [CrossRef]
- Huang, H.; Cai, M.; Wang, Y.; Liang, B.; Lin, N.; Xu, L. Snp array as a tool for prenatal diagnosis of congenital heart disease screened by echocardiography: Implications for precision assessment of fetal prognosis. Risk Manag. Healthc. Policy 2021, 14, 345–355. [Google Scholar] [CrossRef]
- Huang, H.; Cai, M.; Ma, W.; Lin, N.; Xu, L. Chromosomal microarray analysis for the prenatal diagnosis in fetuses with nasal bone hypoplasia: A retrospective cohort study. Risk Manag. Healthc. Policy 2021, 14, 1533–1540. [Google Scholar] [CrossRef]
- Huang, R.N.; Chen, J.Y.; Pan, H.; Liu, Q.Q. Correlation between mild fetal ventriculomegaly, chromosomal abnormalities, and copy number variations. J. Matern. Neonatal Med. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cai, M.; Liu, L.; Xu, L.; Lin, N. Effectiveness of chromosomal microarray analysis for prenatal diagnosis of fetal echogenic intracardiac focus: A single-center experience. Int. J. Gen. Med. 2021, 14, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Hureaux, M.; Guterman, S.; Hervé, B.; Till, M.; Jaillard, S.; Redon, S.; Valduga, M.; Coutton, C.; Missirian, C.; Prieur, F.; et al. Chromosomal microarray analysis in fetuses with an isolated congenital heart defect: A retrospective, nationwide, multicenter study in France. Prenat. Diagn. 2019, 39, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Lazier, J.; Fruitman, D.; Lauzon, J.; Bernier, F.; Argiropoulos, B.; Chernos, J.; Caluseriu, O.; Simrose, R.; Thomas, M.A. Prenatal Array Comparative Genomic Hybridization in Fetuses With Structural Cardiac Anomalies. J. Obstet. Gynaecol. Can. 2016, 38, 619–626. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Zhang, G.; Jiao, H.; Zhu, J.; Li, Z.; Chen, C.; Zhang, X.P.; Huang, H.; Wang, J.Y. A Chinese multicenter retrospective study of isolated increased nuchal translucency associated chromosome anomaly and prenatal diagnostic suggestions. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.Y.; Au Yeung, K.C.; Leung, W.C.; Leung, K.Y.; Lo, T.K.; To, W.W.K.; Lau, W.L.; Chan, L.W.; Sahota, D.S.; Choy, R.K.W. Prenatal diagnosis of pathogenic genomic imbalance in fetuses with increased nuchal translucency but normal karyotyping using chromosomal microarray. Hong Kong Med. J. 2019, 25, 30–32. [Google Scholar]
- Li, S.; Han, X.; Wang, Y.; Chen, S.; Niu, J.; Qian, Z.; Li, P.; Jin, L.; Xu, C. Chromosomal microarray analysis in fetuses with congenital anomalies of the kidney and urinary tract: A prospective cohort study and meta-analysis. Prenat. Diagn. 2019, 39, 165–174. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Wang, J.; Zhang, H.; Zhu, H.; Lai, Y.; Liu, S.; Wang, H.; Hu, T. Prenatal diagnosis of genetic aberrations in fetuses with short femur detected by ultrasound: A prospective cohort study. Prenat. Diagn. 2021, 41, 1153–1163. [Google Scholar] [CrossRef]
- Liao, C.; Li, R.; Fu, F.; Xie, G.; Zhang, Y.; Pan, M.; Li, J.; Li, D. Prenatal diagnosis of congenital heart defect by genome-wide high-resolution SNP array. Prenat. Diagn. 2014, 34, 858–863. [Google Scholar] [CrossRef]
- Lin, S.; Shi, S.; Huang, L.; Lei, T.; Cai, D.; Hu, W.; Zhou, Y.; Luo, Y. Is an analysis of copy number variants necessary for various types of kidney ultrasound anomalies in fetuses? Mol. Cytogenet. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; He, Z.; Lin, S.; Wang, Y.; Luo, Y. Clinical value of genetic analysis in prenatal diagnosis of short femur. Mol. Genet. Genom. Med. 2019, 7, e978. [Google Scholar] [CrossRef]
- Lund, I.C.B.; Christensen, R.; Petersen, O.B.; Vogel, I.; Vestergaard, E.M. Chromosomal microarray in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 2015, 45, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Meng, D.; Li, Q.; Hu, X.; Chen, Y.; He, C.; Xie, B.; She, S.; Li, Y.; Fu, C. Genetic testing and pregnancy outcome analysis of 362 fetuses with congenital heart disease identified by prenatal ultrasound. Arq. Bras. Cardiol. 2018, 111, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Mademont-Soler, I.; Morales, C.; Soler, A.; Martínez-Crespo, J.M.; Shen, Y.; Margarit, E.; Clusellas, N.; Obon, M.; Wu, B.; Sanchez, A. Prenatal diagnosis of chromosomal abnormalities in fetuses with abnormal cardiac ultrasound findings: Evaluation of chromosomal microarray-based analysis. Ultrasound Obstet. Gynecol. 2013, 41, 375–382. [Google Scholar] [CrossRef]
- Maya, I.; Yacobson, S.; Kahana, S.; Yeshaya, J.; Tenne, T.; Agmon-Fishman, I.; Cohen-Vig, L.; Shohat, M.; Basel-Vanagaite, L.; Sharony, R. Cut-off value of nuchal translucency as indication for chromosomal microarray analysis. Ultrasound Obstet. Gynecol. 2017, 50, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Monier, I.; Receveur, A.; Houfflin-Debarge, V.; Goua, V.; Castaigne, V.; Jouannic, J.M.; Mousty, E.; Saliou, A.H.; Bouchghoul, H.; Rousseau, T.; et al. Should prenatal chromosomal microarray analysis be offered for isolated fetal growth restriction? A French multicenter study. Am. J. Obstet. Gynecol. 2021, 225, 676.e1–676.e15. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Jacobs, K.M.; Tessier, K.M.; Narasimhan, S.L.; Tofte, A.N.; McCarter, A.R.; Cross, S.N. Chromosomal microarray analysis in the investigation of prenatally diagnosed congenital heart disease. Am. J. Obstet. Gynecol. MFM 2020, 2, 100078. [Google Scholar] [CrossRef]
- Pan, M.; Han, J.; Zhen, L.; Yang, X.; Li, R.; Liao, C.; Li, D.Z. Prenatal diagnosis of fetuses with increased nuchal translucency using an approach based on quantitative fluorescent polymerase chain reaction and genomic microarray. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhou, Y.; Xie, H.N.; Lin, M.F.; Zheng, J. Chromosomal and subchromosomal anomalies associated to small for gestational age fetuses with no additional structural anomalies. Prenat. Diagn. 2017, 37, 1219–1224. [Google Scholar] [CrossRef]
- Petersen, O.B.; Smith, E.; Van Opstal, D.; Polak, M.; Knapen, M.F.C.M.; Diderich, K.E.M.; Bilardo, C.M.; Arends, L.R.; Vogel, I.; Srebniak, M.I. Nuchal translucency of 3.0-3.4 mm an indication for NIPT or microarray? Cohort analysis and literature review. Acta Obstet. Gynecol. Scand. 2020, 99, 765–774. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, Y.; Zhang, C.; Zhou, R.; Wu, Y.; Wang, C.; Meng, L.; Mao, P.; Cheng, Q.; Luo, C.; et al. Comprehensive evaluation of genetic variants using chromosomal microarray analysis and exome sequencing in fetuses with congenital heart defect. Ultrasound Obstet. Gynecol. 2021, 58, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Singer, A.; Falik-Zaccai, T.; Peleg, A.; Bar-Shira, A.; Feingold-Zadok, M.; Ben Shachar, S.; Maya, I. The effect of polyhydramnios degree on chromosomal microarray results: A retrospective cohort analysis of 742 singleton pregnancies. Arch. Gynecol. Obstet. 2021, 304, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Singer, A.; Ben Shachar, S.; Josefsberg Ben Yehoshua, S.; Feingold-Zadok, M.; Greenbaum, L.; Maya, I. Risk of Clinically Significant Chromosomal Microarray Analysis Findings in Fetuses With Nuchal Translucency From 3.0 mm Through 3.4 mm. Obstet. Gynecol. 2021, 137, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Singer, A.; Segel, R.; Berger, R.; Kanengisser-Pines, B.; Maya, I. The yield of chromosomal microarray in pregnancies with congenital cardiac defects and normal noninvasive prenatal screening. Am. J. Obstet. Gynecol. 2021, 225, 333.e1–333.e14. [Google Scholar] [CrossRef]
- Schmid, M.; Stary, S.; Blaicher, W.; Gollinger, M.; Husslein, P.; Streubel, B. Prenatal genetic diagnosis using microarray analysis in fetuses with congenital heart defects. Prenat. Diagn. 2012, 32, 376–382. [Google Scholar] [CrossRef]
- Sinajon, P.; Chitayat, D.; Roifman, M.; Wasim, S.; Carmona, S.; Ryan, G.; Noor, A.; Kolomietz, E.; Chong, K. Microarray and RASopathy-disorder testing in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 2020, 55, 383–390. [Google Scholar] [CrossRef]
- Singer, A.; Maya, I.; Koifman, A.; Samra, N.N.; Baris, H.N.; Falik-Zaccai, T.; Ben Shachar, S.; Sagi-Dain, L. Microarray analysis in pregnancies with isolated echogenic bowel. Early Hum. Dev. 2018, 119, 25–28. [Google Scholar] [CrossRef]
- Singer, A.; Maya, I.; Sukenik-Halevy, R.; Tenne, T.; Lev, D.; Ben Shachar, S.; Sagi-Dain, L. Microarray findings in pregnancies with oligohydramnios—A retrospective cohort study and literature review. J. Perinat. Med. 2019, 48, 53–58. [Google Scholar] [CrossRef]
- Song, T.; Wan, S.; Li, Y.; Xu, Y.; Dang, Y.; Zheng, Y.; Li, C.; Zheng, J.; Chen, B.; Zhang, J. Detection of copy number variants using chromosomal microarray analysis for the prenatal diagnosis of congenital heart defects with normal karyotype. J. Clin. Lab. Anal. 2019, 33, e22630. [Google Scholar] [CrossRef]
- Song, T.; Xu, Y.; Li, Y.; Jia, L.; Zheng, J.; Dang, Y.; Wan, S.; Zheng, Y.; Zhang, J.; Yang, H. Detection of submicroscopic chromosomal aberrations by chromosomal microarray analysis for the prenatal diagnosis of central nervous system abnormalities. J. Clin. Lab. Anal. 2020, 34, e23434. [Google Scholar] [CrossRef]
- Stuurman, K.E.; van der Mespel-Brouwer, M.H.; Engels, M.A.J.; Elting, M.W.; Bhola, S.L.; Meijers-Heijboer, H. Isolated Increased Nuchal Translucency in First Trimester Ultrasound Scan: Diagnostic Yield of Prenatal Microarray and Outcome of Pregnancy. Front. Med. 2021, 8, 1784. [Google Scholar] [CrossRef]
- Su, L.; Huang, H.; An, G.; Cai, M.; Wu, X.; Li, Y.; Xie, X.; Lin, Y.; Wang, M.; Xu, L. Clinical application of chromosomal microarray analysis in fetuses with increased nuchal translucency and normal karyotype. Mol. Genet. Genom. Med. 2019, 7, e811. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Qin, Z.; Fu, H.; Luo, J.; Huang, Y.; Huang, P.; Zhang, S.; Liu, T.; Lu, W.; Li, W.; et al. The correlations of prenatal renal ultrasound abnormalities with pathogenic CNVs in a large Chinese cohort. Ultrasound Obstet. Gynecol. 2021, 59, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, Q.; Jiang, S.W.; Yan, Y.; Wang, X.; Zhang, J.; Liu, Y.; Yao, L.; Ma, Y.; Wang, L. Prenatal diagnosis of central nervous system anomalies by high-resolution chromosomal microarray analysis. BioMed Res. Int. 2015, 2015, 426379. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lv, J.; Chen, X.; Bai, L.; Li, H.; Chen, C.; Wang, P.; Xu, X.; Lu, J. Prenatal Diagnosis of DNA Copy Number Variations by Genomic Single-Nucleotide Polymorphism Array in Fetuses with Congenital Heart Defects. Fetal Diagn. Ther. 2016, 39, 64–73. [Google Scholar] [CrossRef]
- Toren, A.; Alpern, S.; Berkenstadt, M.; Bar-Yosef, O.; Pras, E.; Katorza, E. Chromosomal microarray evaluation of fetal ventriculomegaly. Isr. Med. Assoc. J. 2020, 22, 573–578. [Google Scholar]
- Turan, S.; Asoglu, M.R.; Benziv, R.G.; Doyle, L.; Harman, C.; Turan, O.M. Yield rate of chromosomal microarray analysis in fetuses with congenital heart defects. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 172–176. [Google Scholar] [CrossRef]
- Tzadikevitch Geffen, K.; Singer, A.; Maya, I.; Sagi-Dain, L.; Khayat, M.; Ben-Shachar, S.; Daum, H.; Michaelson-Cohen, R.; Feingold-Zadok, M.; Sukenik Halevy, R. Chromosomal microarray should be performed for cases of fetal short long bones detected prenatally. Arch. Gynecol. Obstet. 2021, 303, 85–92. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Li, Q.; Zhu, H.; Lai, Y.; Luo, W.; Liu, S.; Wang, H.; Hu, T. Prenatal diagnosis of chromosomal aberrations by chromosomal microarray analysis in foetuses with ventriculomegaly. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Fu, F.; Zhang, Y.; Li, D.; Liao, C. Submicroscopic chromosomal abnormalities in fetuses with increased nuchal translucency and normal karyotype. J. Matern. Neonatal Med. 2017, 30, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Liang, D.; Meng, L.; Wu, Y.; Qiao, F.; Ji, X.; Luo, C.; Zhang, J.; Xu, T.; et al. Prenatal chromosomal microarray analysis in fetuses with congenital heart disease: A prospective cohort study. Am. J. Obstet. Gynecol. 2018, 218, 244.e1–244.e17. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Y.; Huang, S.; Wu, Y.; Li, P.; Zhuang, J. Clinical application of chromosomal microarray analysis for the prenatal diagnosis of chromosomal abnormalities and copy number variations in fetuses with congenital heart disease. Prenat. Diagn. 2018, 38, 406–413. [Google Scholar] [CrossRef]
- Xie, X.; Wu, X.; Su, L.; Cai, M.; Li, Y.; Huang, H.; Xu, L. Application of single nucleotide polymorphism microarray in prenatal diagnosis of fetuses with central nervous system abnormalities. Int. J. Gen. Med. 2021, 14, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xiang, Y.; Xu, X.; Zhou, L.; Li, H.; Dong, X.; Tang, S. Clinical application of chromosomal microarray analysis for fetuses with craniofacial malformations. Mol. Cytogenet. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Yan, H.; Chen, J.; Li, N.; Wang, J.; Liu, Y.; Zhang, H.; Li, S.; Zhang, W.; Chen, D.; et al. Genetic Examination for Fetuses with Increased Fetal Nuchal Translucency by Genomic Technology. Cytogenet. Genome Res. 2020, 160, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yu, A.; Lin, N.; Chen, X.; Lin, M.; Wang, Y.; Huang, H.; Xu, L. Detection of copy number variation associated with ventriculomegaly in fetuses using single nucleotide polymorphism arrays. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhao, X.R.; Gao, L.; Wu, Y.; Wang, Y.L. Application of chromosomal microarray in fetuses with increased nuchal translucency. J. Matern. Neonatal Med. 2020, 33, 1749–1754. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Ru, T.; Wang, Y.; Xu, Y.; Yang, Y.; Wu, X.; Cram, D.S.; Hu, Y. Identification of copy number variations associated with congenital heart disease by chromosomal microarray analysis and next-generation sequencing. Prenat. Diagn. 2016, 36, 321–327. [Google Scholar] [CrossRef]
- He, M.; Du, L.; Xie, H.; Zhang, L.; Gu, Y.; Lei, T.; Zheng, J.; Chen, D. The Added Value of Whole-Exome Sequencing for Anomalous Fetuses With Detailed Prenatal Ultrasound and Postnatal Phenotype. Front. Genet. 2021, 12, 627204. [Google Scholar] [CrossRef]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K. Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Fu, F.; Li, R.; Li, Y.; Nie, Z.Q.; Lei, T.; Wang, D.; Yang, X.; Han, J.; Pan, M.; Zhen, L.; et al. Whole exome sequencing as a diagnostic adjunct to clinical testing in fetuses with structural abnormalities. Ultrasound Obstet. Gynecol. 2018, 51, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, J.; Wang, C.; Chen, F.; Xie, Y.; Li, Y.; Li, N.; Wang, J.; Zhang, V.W.; Chen, D. Clinical application of medical exome sequencing for prenatal diagnosis of fetal structural anomalies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhou, L.; Xiong, J. jiao Whole-exome sequencing increases the diagnostic rate for prenatal fetal structural anomalies. Eur. J. Med. Genet. 2021, 64, 104288. [Google Scholar] [CrossRef] [PubMed]
- Pauta, M.; Campos, B.; Segura-Puimedon, M.; Arca, G.; Nadal, A.; Tubau, A.; Perez, S.P.; Marimon, E.; Martín, L.; López-Quesada, E.; et al. Next-Generation Sequencing Gene Panels and “Solo” Clinical Exome Sequencing Applied in Structurally Abnormal Fetuses. Fetal Diagn. Ther. 2021, 48, 746–756. [Google Scholar] [CrossRef]
- Pangalos, C.; Hagnefelt, B.; Lilakos, K.; Konialis, C. First applications of a targeted exome sequencing approach in fetuses with ultrasound abnormalities reveals an important fraction of cases with associated gene defects. PeerJ 2016, 2016, e1955. [Google Scholar] [CrossRef]
- Vora, N.L.; Powell, B.; Brandt, A.; Strande, N.; Hardisty, E.; Gilmore, K.; Foreman, A.K.M.; Wilhelmsen, K.; Bizon, C.; Reilly, J.; et al. Prenatal exome sequencing in anomalous fetuses: New opportunities and challenges. Genet. Med. 2017, 19, 1207–1216. [Google Scholar] [CrossRef]
- Boissel, S.; Fallet-Bianco, C.; Chitayat, D.; Kremer, V.; Nassif, C.; Rypens, F.; Delrue, M.-A.; Soglio, D.D.; Oligny, L.L.; Patey, N.; et al. Genomic Study of Severe Fetal Anomalies and Discovery of GREB1L Mutations in Renal Agenesis. Obstet. Gynecol. Surv. 2018, 73, 677–679. [Google Scholar] [CrossRef]
- Leung, G.K.C.; Mak, C.C.Y.; Fung, J.L.F.; Wong, W.H.S.; Tsang, M.H.Y.; Yu, M.H.C.; Pei, S.L.C.; Yeung, K.S.; Mok, G.T.K.; Lee, C.P.; et al. Identifying the genetic causes for prenatally diagnosed structural congenital anomalies (SCAs) by whole-exome sequencing (WES) 06 Biological Sciences 0604 Genetics 11 Medical and Health Sciences 1114 Paediatrics and Reproductive Medicine. BMC Med. Genom. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Daum, H.; Meiner, V.; Elpeleg, O.; Harel, T.; Bar-Or, L.; Eilat, A.; Fahham, D.; Gur, M.; Hacohen, N.; Kimchi, A.; et al. Fetal exome sequencing: Yield and limitations in a tertiary referral center. Ultrasound Obstet. Gynecol. 2019, 53, 80–86. [Google Scholar] [CrossRef]
- de Koning, M.A.; Haak, M.C.; Adama van Scheltema, P.N.; Peeters-Scholte, C.M.P.C.D.; Koopmann, T.T.; Nibbeling, E.A.R.; Aten, E.; den Hollander, N.S.; Ruivenkamp, C.A.L.; Hoffer, M.J.V.; et al. From diagnostic yield to clinical impact: A pilot study on the implementation of prenatal exome sequencing in routine care. Genet. Med. 2019, 21, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Becher, N.; Andreasen, L.; Sandager, P.; Lou, S.; Petersen, O.B.; Christensen, R.; Vogel, I. Implementation of exome sequencing in fetal diagnostics—Data and experiences from a tertiary center in Denmark. Acta Obstet. Gynecol. Scand. 2020, 99, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Haworth, A.; Ive, L.; Dubis, R.; Savage, H.; Serra, E.; Kenny, J.; Elmslie, F.; Greco, E.; Thilaganathan, B.; et al. A report on the impact of rapid prenatal exome sequencing on the clinical management of 52 ongoing pregnancies: A retrospective review. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1012–1019. [Google Scholar] [CrossRef]
- Al-Kouatly, H.B.; Makhamreh, M.M.; Rice, S.M.; Smith, K.; Harman, C.; Quinn, A.; Valcarcel, B.N.; Firman, B.; Liu, R.; Hegde, M.; et al. High diagnosis rate for nonimmune hydrops fetalis with prenatal clinical exome from the Hydrops-Yielding Diagnostic Results of Prenatal Sequencing (HYDROPS) Study. Genet. Med. 2021, 23, 1325–1333. [Google Scholar] [CrossRef]
- Mone, F.; Eberhardt, R.Y.; Hurles, M.E.; Mcmullan, D.J.; Maher, E.R.; Lord, J.; Chitty, L.S.; Dempsey, E.; Homfray, T.; Giordano, J.L.; et al. Fetal hydrops and the Incremental yield of Next-generation sequencing over standard prenatal Diagnostic testing (FIND) study: Prospective cohort study and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 58, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.N.; Lianoglou, B.R.; Adami, R.R.; Pluym, I.D.; Holliman, K.; Duffy, J.; Downum, S.L.; Patel, S.; Faubel, A.; Boe, N.M.; et al. Exome Sequencing for Prenatal Diagnosis in Nonimmune Hydrops Fetalis. Obstet. Gynecol. Surv. 2021, 76, 139–141. [Google Scholar] [CrossRef]
- Kucińska-Chahwan, A.; Roszkowski, T.; Nowakowska, B.; Geremek, M.; Paczkowska, M.; Bijok, J.; Massalska, D. Genetic causes of the skeletal system abnormalities diagnosed by prenatal sonography with the use of exome sequencing: Single institution experience. Ultrasound Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, S.; Huang, X.; Pang, J.; Liu, J.; Hu, J.; Shen, X.; Tang, C.; Wang, H. Whole Exome Sequencing Analysis in Fetal Skeletal Dysplasia Detected by Ultrasonography: An Analysis of 38 Cases. Front. Genet. 2021, 12, 728544. [Google Scholar] [CrossRef]
- Yang, K.; Shen, M.; Yan, Y.; Tan, Y.; Zhang, J.; Wu, J.; Yang, G.; Li, S.; Wang, J.; Ren, Z.; et al. Genetic Analysis in Fetal Skeletal Dysplasias by Trio Whole-Exome Sequencing. BioMed Res. Int. 2019, 2019, 2492590. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Teng, Y.; Liang, D.; Li, Z.; Wu, L. Molecular diagnosis for 55 fetuses with skeletal dysplasias by whole-exome sequencing: A retrospective cohort study. Clin. Genet. 2021, 100, 219–226. [Google Scholar] [CrossRef]
- Han, J.; Yang, Y.D.; He, Y.; Liu, W.J.; Zhen, L.; Pan, M.; Yang, X.; Zhang, V.W.; Liao, C.; Li, D.Z. Rapid prenatal diagnosis of skeletal dysplasia using medical trio exome sequencing: Benefit for prenatal counseling and pregnancy management. Prenat. Diagn. 2020, 40, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, C.; Shi, H.; Mo, Y.; Tan, W.; Sun, T.; Zhu, J.; Li, Q.; Li, H.; Li, Y. Prenatal diagnosis of skeletal dysplasias using whole exome sequencing in China. Clin. Chim. Acta 2020, 507, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mellis, R.; Eberhardt, R.Y.; Hamilton, S.J.; McMullan, D.J.; Kilby, M.D.; Maher, E.R.; Hurles, M.E.; Giordano, J.L.; Aggarwal, V.; Goldstein, D.B.; et al. Fetal exome sequencing for isolated increased nuchal translucency: Should we be doing it? BJOG Int. J. Obstet. Gynaecol. 2021, 129, 52–61. [Google Scholar] [CrossRef]
- Yang, X.; Huang, L.Y.; Pan, M.; Xu, L.L.; Zhen, L.; Han, J.; Li, D.Z. Exome sequencing improves genetic diagnosis of fetal increased nuchal translucency. Prenat. Diagn. 2020, 40, 1426–1431. [Google Scholar] [CrossRef]
- Schwab, M.E.; Dong, S.; Lianoglou, B.R.; Aguilar Lucero, A.F.; Schwartz, G.B.; Norton, M.E.; MacKenzie, T.C.; Sanders, S.J. Exome sequencing of fetuses with congenital diaphragmatic hernia supports a causal role for NR2F2, PTPN11, and WT1 variants. Am. J. Surg. 2021, 223, 182–186. [Google Scholar] [CrossRef] [PubMed]
- She, Q.; Tang, E.; Peng, C.; Wang, L.; Wang, D.; Tan, W. Prenatal genetic testing in 19 fetuses with corpus callosum abnormality. J. Clin. Lab. Anal. 2021, 35, e23971. [Google Scholar] [CrossRef] [PubMed]
- Heide, S.; Spentchian, M.; Valence, S.; Buratti, J.; Mach, C.; Lejeune, E.; Olin, V.; Massimello, M.; Lehalle, D.; Mouthon, L.; et al. Prenatal exome sequencing in 65 fetuses with abnormality of the corpus callosum: Contribution to further diagnostic delineation. Genet. Med. 2020, 22, 1887–1891. [Google Scholar] [CrossRef]
- Zhen, L.; Yang, Y.-D.; Xu, L.-L.; Cao, Q.; Li, D.-Z. Fetal micrognathia in the first trimester: An ominous finding even after a normal array. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Weitensteiner, V.; Zhang, R.; Bungenberg, J.; Marks, M.; Gehlen, J.; Ralser, D.J.; Hilger, A.C.; Sharma, A.; Schumacher, J.; Gembruch, U.; et al. Exome sequencing in syndromic brain malformations identifies novel mutations in ACTB, and SLC9A6, and suggests BAZ1A as a new candidate gene. Birth Defects Res. 2018, 110, 587–597. [Google Scholar] [CrossRef]
- Westphal, D.S.; Leszinski, G.S.; Rieger-Fackeldey, E.; Graf, E.; Weirich, G.; Meitinger, T.; Ostermayer, E.; Oberhoffer, R.; Wagner, M. Lessons from exome sequencing in prenatally diagnosed heart defects: A basis for prenatal testing. Clin. Genet. 2019, 95, 582–589. [Google Scholar] [CrossRef]
- Li, R.; Fu, F.; Yu, Q.; Wang, D.; Jing, X.; Zhang, Y.; Li, F.; Li, F.; Han, J.; Pan, M.; et al. Prenatal exome sequencing in fetuses with congenital heart defects. Clin. Genet. 2020, 98, 215–230. [Google Scholar] [CrossRef]
- Mone, F.; Eberhardt, R.Y.; Morris, R.K.; Hurles, M.E.; McMullan, D.J.; Maher, E.R.; Lord, J.; Chitty, L.S.; Giordano, J.L.; Wapner, R.J.; et al. COngenital heart disease and the Diagnostic yield with Exome sequencing (CODE) study: Prospective cohort study and systematic review. Ultrasound Obstet. Gynecol. 2021, 57, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yi, T.; Hao, X.; Yan, H.; Wang, J.; Li, Q.; Gu, X.; Zhou, X.; Wang, S.; Wang, X.; et al. Contribution of single-gene defects to congenital cardiac left-sided lesions in the prenatal setting. Ultrasound Obstet. Gynecol. 2020, 56, 225–232. [Google Scholar] [CrossRef]
- Lei, T.Y.; Fu, F.; Li, R.; Yu, Q.X.; Du, K.; Zhang, W.W.; Deng, Q.; Li, L.S.; Wang, D.; Yang, X.; et al. Whole-exome sequencing in the evaluation of fetal congenital anomalies of the kidney and urinary tract detected by ultrasonography. Prenat. Diagn. 2020, 40, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Xie, Y.; Chen, F.; Chen, M.; Yu, L.; Chen, D.; Chen, J. Novel and recurrent variants identified in fetuses with central nervous system abnormalities by trios-medical exome sequencing. Clin. Chim. Acta 2020, 510, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Shao, B.; Wang, C.; Hu, P.; Qiao, F.; Xu, Z. Molecular diagnostic in fetuses with isolated congenital anomalies of the kidney and urinary tract by whole-exome sequencing. J. Clin. Lab. Anal. 2020, 34, e23480. [Google Scholar] [CrossRef]
- Akalın, A.; Taskiran, E.Z.; Şimşek-Kiper, P.Ö.; Utine, E.; Alanay, Y.; Özçelik, U.; Boduroğlu, K. Spondyloepimetaphyseal dysplasia EXTL3-deficient type: Long-term follow-up and review of the literature. Am. J. Med. Genet. Part A 2021, 185, 3104–3110. [Google Scholar] [CrossRef]
- Aoi, H.; Mizuguchi, T.; Suzuki, T.; Makino, S.; Yamamoto, Y.; Takeda, J.; Maruyama, Y.; Seyama, R.; Takeuchi, S.; Uchiyama, Y. Whole exome sequencing of fetal structural anomalies detected by ultrasonography. J. Hum. Genet. 2021, 66, 499–507. [Google Scholar] [CrossRef]
- Biard, J.M.; Payrat, S.; Clapuyt, P.; Barrea, C.; Benoit, V.; Baldin, P.; Bernard, P.; Van Grambezen, B.; Sznajer, Y. Antenatal diagnosis of CHARGE syndrome: Prenatal ultrasound findings and crucial role of fetal dysmorphic signs. About a series of 10 cases and review of literature. Eur. J. Med. Genet. 2021, 64, 104189. [Google Scholar] [CrossRef]
- Cao, Q.; Yang, Y.; Pan, M.; Han, J.; Yang, X.; Li, D.Z. Fetal akinesia: The application of clinical exome sequencing in cases with decreased fetal movement. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 59–63. [Google Scholar] [CrossRef]
- Lefebvre, M.; Bruel, A.L.; Tisserant, E.; Bourgon, N.; Duffourd, Y.; Collardeau-Frachon, S.; Attie-Bitach, T.; Kuentz, P.; Assoum, M.; Schaefer, E.; et al. Genotype-first in a cohort of 95 fetuses with multiple congenital abnormalities: When exome sequencing reveals unexpected fetal phenotype-genotype correlations. J. Med. Genet. 2021, 58, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Lerman-Sagie, T.; Pogledic, I.; Leibovitz, Z.; Malinger, G. A practical approach to prenatal diagnosis of malformations of cortical development. Eur. J. Paediatr. Neurol. 2021, 34, 50–61. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, J.; Xi, L.; Lin, X.; Wang, C.; Yue, H.; Gu, J.; Hu, W.; Fu, W.; Wei, Z.; et al. Genetics Evaluation of Targeted Exome Sequencing in 223 Chinese Probands With Genetic Skeletal Dysplasias. Front. Cell Dev. Biol. 2021, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.E.; Van Ziffle, J.; Lianoglou, B.R.; Hodoglugil, U.; Devine, W.P.; Sparks, T.N. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2021, 226, 128.e1–128.e11. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Gaudino, G.; Torella, A.; Piluso, G.; Perrotta, S.; Miraglia del Giudice, E.; Nigro, V.; Grandone, A. Intermittent macrothrombocytopenia in a novel patient with Takenouchi-Kosaki syndrome and review of literature. Eur. J. Med. Genet. 2021, 64, 104358. [Google Scholar] [CrossRef]
- Shi, L.; Li, M.; Qi, H.; Zhu, J.; Yang, J.; Tang, J.; Wang, L. Whole-exome sequencing analysis to identify novel potential pathogenetic mutations in fetuses with abnormal brain structure. Ann. Transl. Med. 2021, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- So, P.L.; Luk, H.M.; Cheung, K.W.; Hui, W.; Chung, M.Y.; Mak, A.S.L.; Lok, W.Y.; Yu, K.P.T.; Cheng, S.S.W.; Hau, E.W.L.; et al. Prenatal phenotype of Kabuki syndrome: A case series and literature review. Prenat. Diagn. 2021, 41, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Stutterd, C.A.; Brock, S.; Stouffs, K.; Fanjul-Fernandez, M.; Lockhart, P.J.; McGillivray, G.; Mandelstam, S.; Pope, K.; Delatycki, M.B.; Jansen, A.; et al. Genetic heterogeneity of polymicrogyria: Study of 123 patients using deep sequencing. Brain Commun. 2021, 3, fcaa221. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hao, X.; Wang, X.; Zhou, X.; Zhang, Y.; Liu, X.; Han, J.; Gu, X.; Sun, L.; Zhao, Y.; et al. Genetics and Clinical Features of Noncompaction Cardiomyopathy in the Fetal Population. Front. Cardiovasc. Med. 2021, 7, 397. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Q.; Xiang, J.; Yin, L.; Wang, J.; Wang, T. Whole Exome Sequencing Aids the Diagnosis of Fetal Skeletal Dysplasia. Front. Genet. 2021, 12, 178. [Google Scholar] [CrossRef]
- Wu, W.J.; Ma, G.C.; Chang, T.Y.; Lee, M.H.; Chen, Y.N.; Chen, M. Hydrops in first trimester as unreported prenatal finding of dyssegmental dysplasia confirmed by exome sequencing. Ultrasound Obstet. Gynecol. 2021, 58, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhang, Z.; Shi, P.; Martin, D.M.; Kong, X. Incorporation of exome-based CNV analysis makes trio-WES a more powerful tool for clinical diagnosis in neurodevelopmental disorders: A retrospective study. Hum. Mutat. 2021, 42, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, Y.; Song, R.; Wang, L.; Xu, H.; Xie, X.; Zhou, H.; Sun, P.; Zhang, M.; Zhao, Q.; et al. Combined exome sequencing and deep phenotyping in highly selected fetuses with skeletal dysplasia during the first and second trimesters improves diagnostic yield. Prenat. Diagn. 2021, 41, 1401–1413. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Wei, X.; Yao, R.; Yang, Y.; Deng, L.; Zou, G.; Wang, X.; Yang, Y.; Duan, T.; et al. Value of Exome Sequencing in Diagnosis and Management of Recurrent Non-immune Hydrops Fetalis: A Retrospective Analysis. Front. Genet. 2021, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.L.; Monaghan, K.G.; Copenheaver, D.; Retterer, K.; Scuffins, J.; Kucera, C.R.; Friedman, B.; Richard, G.; Juusola, J. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: Expanding our knowledge of genetic disease during fetal development. Genet. Med. 2017, 19, 1171–1178. [Google Scholar] [CrossRef]
- Quinlan-Jones, E.; Lord, J.; Williams, D.; Hamilton, S.; Marton, T.; Eberhardt, R.Y.; Rinck, G.; Prigmore, E.; Keelagher, R.; McMullan, D.J.; et al. Molecular autopsy by trio exome sequencing (ES) and postmortem examination in fetuses and neonates with prenatally identified structural anomalies. Genet. Med. 2019, 21, 1065–1073. [Google Scholar] [CrossRef]
- Meier, N.; Bruder, E.; Lapaire, O.; Hoesli, I.; Kang, A.; Hench, J.; Hoeller, S.; De Geyter, J.; Miny, P.; Heinimann, K.; et al. Exome sequencing of fetal anomaly syndromes: Novel phenotype-genotype discoveries. Eur. J. Hum. Genet. 2019, 27, 730–737. [Google Scholar] [CrossRef]
- Carss, K.J.; Hillman, S.C.; Parthiban, V.; McMullan, D.J.; Maher, E.R.; Kilby, M.D.; Hurles, M.E. Exome sequencing improves genetic diagnosis of structural fetal abnormalities revealed by ultrasound. Hum. Mol. Genet. 2014, 23, 3269–3277. [Google Scholar] [CrossRef]
- Mackie, F.; Carss, K.; Hillman, S.; Hurles, M.; Kilby, M. Exome Sequencing in Fetuses with Structural Malformations. J. Clin. Med. 2014, 3, 747–762. [Google Scholar] [CrossRef]
- Yauy, K.; Mau-Them, F.T.; Willems, M.; Coubes, C.; Blanchet, P.; Herlin, C.; Arrada, I.T.; Sanchez, E.; Faure, J.-M.; Le Gac, M.-P. B3GAT3-related disorder with craniosynostosis and bone fragility due to a unique mutation. Genet. Med. 2018, 20, 269–274. [Google Scholar] [CrossRef]
- Quaio, C.R.D.C.; Moreira, C.M.; Novo-Filho, G.M.; Sacramento-Bobotis, P.R.; Groenner Penna, M.; Perazzio, S.F.; Dutra, A.P.; da Silva, R.A.; Santos, M.N.P.; de Arruda, V.Y.N. Diagnostic power and clinical impact of exome sequencing in a cohort of 500 patients with rare diseases. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 955–964. [Google Scholar] [CrossRef] [PubMed]
- De Tomasi, L.; David, P.; Humbert, C.; Silbermann, F.; Arrondel, C.; Tores, F.; Fouquet, S.; Desgrange, A.; Niel, O.; Bole-Feysot, C. Mutations in GREB1L cause bilateral kidney agenesis in humans and mice. Am. J. Hum. Genet. 2017, 101, 803–814. [Google Scholar] [CrossRef]
- Westerfield, L.E.; Stover, S.R.; Mathur, V.S.; Nassef, S.A.; Carter, T.G.; Yang, Y.; Eng, C.M.; Van den Veyver, I.B. Reproductive genetic counseling challenges associated with diagnostic exome sequencing in a large academic private reproductive genetic counseling practice. Prenat. Diagn. 2015, 35, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, C.L.; Powis, Z.; Farwell, K.; Shahmirzadi, L.; Weltmer, E.C.; Turocy, J.; Lowe, T.; Kobelka, C.; Chen, E.; Basel, D.; et al. Exome sequencing positively identified relevant alterations in more than half of cases with an indication of prenatal ultrasound anomalies. Prenat. Diagn. 2015, 35, 1073–1078. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Kurdi, W.; Almusafri, F.; Alnemer, M.; Alkaff, A.; Babay, Z.; Alhashem, A.; Tulbah, M.; Alsahan, N.; Khan, R.; et al. Molecular autopsy in maternal-fetal medicine. Genet. Med. 2018, 20, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Stals, K.L.; Wakeling, M.; Baptista, J.; Caswell, R.; Parrish, A.; Rankin, J.; Tysoe, C.; Jones, G.; Gunning, A.C.; Lango Allen, H.; et al. Diagnosis of lethal or prenatal-onset autosomal recessive disorders by parental exome sequencing. Prenat. Diagn. 2018, 38, 33–43. [Google Scholar] [CrossRef]
- Harris, S.; Gilmore, K.; Hardisty, E.; Lyerly, A.D.; Vora, N.L. Ethical and counseling challenges in prenatal exome sequencing. Prenat. Diagn. 2018, 38, 897–903. [Google Scholar] [CrossRef]
- Aarabi, M.; Sniezek, O.; Jiang, H.; Saller, D.N.; Bellissimo, D.; Yatsenko, S.A.; Rajkovic, A. Importance of complete phenotyping in prenatal whole exome sequencing. Hum. Genet. 2018, 137, 175–181. [Google Scholar] [CrossRef]
- Greenbaum, L.; Pode-Shakked, B.; Eisenberg-Barzilai, S.; Dicastro-Keidar, M.; Bar-Ziv, A.; Goldstein, N.; Reznik-Wolf, H.; Poran, H.; Rigbi, A.; Barel, O. Evaluation of diagnostic yield in fetal whole-exome sequencing: A report on 45 consecutive families. Front. Genet. 2019, 10, 425. [Google Scholar] [CrossRef]
- Vora, N.L.; Gilmore, K.; Brandt, A.; Gustafson, C.; Strande, N.; Ramkissoon, L.; Hardisty, E.; Foreman, A.K.M.; Wilhelmsen, K.; Owen, P. An approach to integrating exome sequencing for fetal structural anomalies into clinical practice. Genet. Med. 2020, 22, 954–961. [Google Scholar] [CrossRef]
- Lei, T.; Fu, F.; Li, R.; Wang, D.; Wang, R.; Jing, X.; Deng, Q.; Li, Z.; Liu, Z.; Yang, X. Whole-exome sequencing for prenatal diagnosis of fetuses with congenital anomalies of the kidney and urinary tract. Nephrol. Dial. Transplant. 2017, 32, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Sunde, L.; Nielsen, M.L.; Ramsing, M.; Petersen, A.; Hjortshøj, T.D.; Olsen, T.E.; Tabor, A.; Hertz, J.M.; Johnsen, I. Targeted gene sequencing and whole-exome sequencing in autopsied fetuses with prenatally diagnosed kidney anomalies. Clin. Genet. 2018, 93, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Mary, L.; Chennen, K.; Stoetzel, C.; Antin, M.; Leuvrey, A.; Nourisson, E.; Alanio-Detton, E.; Antal, M.C.; Attié-Bitach, T.; Bouvagnet, P. Bardet-Biedl syndrome: Antenatal presentation of forty-five fetuses with biallelic pathogenic variants in known Bardet-Biedl syndrome genes. Clin. Genet. 2019, 95, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Chandler, N.; Best, S.; Hayward, J.; Faravelli, F.; Mansour, S.; Kivuva, E.; Tapon, D.; Male, A.; DeVile, C.; Chitty, L.S. Rapid prenatal diagnosis using targeted exome sequencing: A cohort study to assess feasibility and potential impact on prenatal counseling and pregnancy management. Genet. Med. 2018, 20, 1430–1437. [Google Scholar] [CrossRef]
- Reches, A.; Hiersch, L.; Simchoni, S.; Barel, D.; Greenberg, R.; Ben Sira, L.; Malinger, G.; Yaron, Y. Whole-exome sequencing in fetuses with central nervous system abnormalities. J. Perinatol. 2018, 38, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Long, W.; Zhou, Q.; Wang, J.; Shi, Y.; Liu, J.; Wang, Q. Is Prenatal Diagnosis Necessary for Fetal Isolated Nasal Bone Absence or Hypoplasia? Int. J. Gen. Med. 2021, 14, 4435. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Z.; Zhang, R.; Chau, M.H.K.; Yang, Z.; Tsang, K.Y.C.; Wong, H.K.; Gui, B.; Meng, Z.; Xiao, K.; et al. Low-pass genome sequencing versus chromosomal microarray analysis: Implementation in prenatal diagnosis. Genet. Med. 2020, 22, 500–510. [Google Scholar] [CrossRef]
- Chau, M.H.K.; Wang, H.; Lai, Y.; Zhang, Y.; Xu, F.; Tang, Y.; Wang, Y.; Chen, Z.; Leung, T.Y.; Chung, J.P.W.; et al. Low-pass genome sequencing: A validated method in clinical cytogenetics. Hum. Genet. 2020, 139, 1403–1415. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Jiang, Y.; Qi, Q.; Hao, N.; Liu, C.; Xu, M.; Cram, D.S.; Liu, J. A rapid PCR-free next-generation sequencing method for the detection of copy number variations in prenatal samples. Life 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Dan, S.; Chen, F.; Choy, K.W.; Jiang, F.; Lin, J.; Xuan, Z.; Wang, W.; Chen, S.; Li, X.; Jiang, H.; et al. Prenatal detection of aneuploidy and imbalanced chromosomal arrangements by massively parallel sequencing. PLoS ONE 2012, 7, e27835. [Google Scholar] [CrossRef]
- Shi, P.; Xia, Y.; Li, Q.; Kong, X. Usefulness of copy number variant detection following monogenic disease exclusion in prenatal diagnosis. J. Obstet. Gynaecol. Res. 2021, 47, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Zhou, C.; Wang, L.; Xie, H.; Xiao, Y.; Zhu, H.; Hu, T.; Zhang, Z.; Zhu, Q.; et al. Prospective chromosome analysis of 3429 amniocentesis samples in China using copy number variation sequencing. Am. J. Obstet. Gynecol. 2018, 219, 287.e1–287.e18. [Google Scholar] [CrossRef]
- Zhao, R.; Ruan, Y.; Wang, X. Whole-exome sequencing and whole genome re-sequencing for prenatal diagnosis of achondroplasia. Int. J. Clin. Exp. Med. 2015, 8, 19241–19249. [Google Scholar] [PubMed]
- Dong, Z.; Zhang, J.; Hu, P.; Chen, H.; Xu, J.; Tian, Q.; Meng, L.; Ye, Y.; Wang, J.; Zhang, M.; et al. Low-pass whole-genome sequencing in clinical cytogenetics: A validated approach. Genet. Med. 2016, 18, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.H.K.; Qian, J.; Chen, Z.; Li, Y.; Zheng, Y.; Tse, W.T.; Kwok, Y.K.; Leung, T.Y.; Dong, Z.; Choy, K.W. Trio-Based Low-Pass Genome Sequencing Reveals Characteristics and Significance of Rare Copy Number Variants in Prenatal Diagnosis. Front. Genet. 2021, 12, 742325. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Ji, X.; Hu, H.; Zhang, J.; Meng, L.; Lin, Y.; Ma, D.; Jiang, T.; Jiang, H.; et al. Clinical application of whole-genome low-coverage next-generation sequencing to detect and characterize balanced chromosomal translocations. Clin. Genet. 2017, 91, 605–610. [Google Scholar] [CrossRef]
- Walker, L.; Watson, C.M.; Hewitt, S.; Crinnion, L.A.; Bonthron, D.T.; Cohen, K.E. An alternative to array-based diagnostics: A prospectively recruited cohort, comparing arrayCGH to next-generation sequencing to evaluate foetal structural abnormalities. J. Obstet. Gynaecol. 2019, 39, 328–334. [Google Scholar] [CrossRef]
- Huang, J.; Deng, X.; Wang, Y.; Tang, N.; Zeng, D. Analysis of Copy Number Variations by Low-Depth Whole-Genome Sequencing in Fetuses with Congenital Cardiovascular Malformations. Cytogenet. Genome Res. 2021, 160, 643–649. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Z.; Sun, J.; Liu, L.; Zhou, X.; Liu, F.; Xing, Y.; Cui, S.; Xiong, S.; Liu, X.; et al. Whole genome sequencing in the evaluation of fetal structural anomalies: A parallel test with chromosomal microarray plus whole exome sequencing. Genes 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Yu, M.H.C.; Chau, J.F.T.; Au, S.L.K.; Lo, H.M.; Yeung, K.S.; Fung, J.L.F.; Mak, C.C.Y.; Chung, C.C.Y.; Chan, K.Y.K.; Chung, B.H.Y.; et al. Evaluating the Clinical Utility of Genome Sequencing for Cytogenetically Balanced Chromosomal Abnormalities in Prenatal Diagnosis. Front. Genet. 2021, 11, 620162. [Google Scholar] [CrossRef]
- Choy, K.W.; Wang, H.; Shi, M.; Chen, J.; Yang, Z.; Zhang, R.; Yan, H.; Wang, Y.; Chen, S.; Chau, M.H.K.; et al. Prenatal Diagnosis of Fetuses With Increased Nuchal Translucency by Genome Sequencing Analysis. Front. Genet. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yang, Y.; Wen, H.; Wang, B.; Zhang, T.; Li, S. Abnormal Sylvian fissure at 20–30 weeks as an indicator of malformations of cortical development: Role for prenatal whole-genome sequencing. Ultrasound Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Yang, Y.K.; Liang, Y.; Zhang, T.J.; Liang, N.; Yang, L.M.; Li, S.J.; Shan, D.; Wu, Q.Q. Prenatal diagnosis of fetal skeletal dysplasia using targeted next-generation sequencing: An analysis of 30 cases. Diagn. Pathol. 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.D.; Vermeesch, J.R. Genomic microarrays: A technology overview. Prenat. Diagn. 2012, 32, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Jelin, A.C.; Vora, N. Whole Exome Sequencing: Applications in Prenatal Genetics. Obstet. Gynecol. Clin. N. Am. 2018, 45, 69–81. [Google Scholar] [CrossRef]
- Crolla, J.A.; Wapner, R.; Van Lith, J.M.M. Controversies in prenatal diagnosis 3: Should everyone undergoing invasive testing have a microarray? Prenat. Diagn. 2014, 34, 18–22. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine. Committee Opinion No.682: Microarrays and Next-Generation Sequencing Technology: The Use of Advanced Genetic Diagnostic Tools in Obstetrics and Gynecology. Obstet. Gynecol. 2016, 128, e262–e268. [Google Scholar] [CrossRef]
- Callaway, J.L.A.; Shaffer, L.G.; Chitty, L.S.; Rosenfeld, J.A.; Crolla, J.A. The clinical utility of microarray technologies applied to prenatal cytogenetics in the presence of a normal conventional karyotype: A review of the literature. Prenat. Diagn. 2013, 33, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, C.R.; Scharer, G.H.; Shaikh, T.H. Clinical impact of copy number variation analysis using high-resolution microarray technologies: Advantages, limitations and concerns. Genome Med. 2012, 4, 1–12. [Google Scholar] [CrossRef]
- American College of Obstetricians. Committee Opinion No. 581: The use of chromosomal microarray analysis in prenatal diagnosis. Obstet. Gynecol. 2013, 122, 1374–1377. [Google Scholar] [CrossRef]
- Evangelidou, P.; Alexandrou, A.; Moutafi, M.; Ioannides, M.; Antoniou, P.; Koumbaris, G.; Kallikas, I.; Velissariou, V.; Sismani, C.; Patsalis, P.C. Implementation of high resolution whole genome array cgh in the prenatal clinical setting: Advantages, challenges, and review of the literature. BioMed Res. Int. 2013, 2013, 346762. [Google Scholar] [CrossRef] [PubMed]
- Jelin, A.C.; Sagaser, K.G.; Wilkins-Haug, L. Prenatal Genetic Testing Options. Pediatr. Clin. N. Am. 2019, 66, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.U.; Koo, S.H. Clinical implementation of chromosomal microarray technology in prenatal diagnosis. Mol. Med. Rep. 2012, 6, 1219–1222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leavitt, K.; Goldwaser, T.; Bhat, G.; Kalia, I.; Klugman, S.D.; Dolan, S.M. Chromosomal microarray in prenatal diagnosis: Case studies and clinical challenges. Per. Med. 2016, 13, 249–255. [Google Scholar] [CrossRef]
- Hillman, S.C.; McMullan, D.J.; Williams, D.; Maher, E.R.; Kilby, M.D. Microarray comparative genomic hybridization in prenatal diagnosis: A review. Ultrasound Obstet. Gynecol. 2012, 40, 385–391. [Google Scholar] [CrossRef]
- Levy, B.; Wapner, R. Prenatal diagnosis by chromosomal microarray analysis. Fertil. Steril. 2018, 109, 201–212. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Lin, S.; Wang, Y.; Huang, L.; Huang, X.; Luo, Y. Absence of heterozygosity detected by single-nucleotide polymorphism array in prenatal diagnosis. Ultrasound Obstet. Gynecol. 2021, 57, 314–323. [Google Scholar] [CrossRef]
- Baker, J.; Shuman, C.; Chitayat, D.; Wasim, S.; Okun, N.; Keunen, J.; Hofstedter, R.; Silver, R. Informed Decision-Making in the Context of Prenatal Chromosomal Microarray. J. Genet. Couns. 2018, 27, 1130–1147. [Google Scholar] [CrossRef]
- Mcgillivray, G.; Rosenfeld, J.A.; Mckinlay Gardner, R.J.; Gillam, L.H. Genetic counselling and ethical issues with chromosome microarray analysis in prenatal testing. Prenat. Diagn. 2012, 32, 389–395. [Google Scholar] [CrossRef]
- Millo, T.; Douiev, L.; Popper, D.; Shkedi-Rafid, S. Personalized prenatal genomic testing: Couples’ experience with choice regarding uncertain and adult-onset findings from chromosomal-microarray-analysis. Prenat. Diagn. 2021, 41, 376–383. [Google Scholar] [CrossRef]
- De Jong, A.; Dondorp, W.J.; Macville, M.V.E.; De Die-Smulders, C.E.M.; Van Lith, J.M.M.; De Wert, G.M.W.R. Microarrays as a diagnostic tool in prenatal screening strategies: Ethical reflection. Hum. Genet. 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Shaffer, L.G.; Dabell, M.P.; Rosenfeld, J.A.; Neill, N.J.; Ballif, B.C.; Coppinger, J.; Diwan, N.R.; Chong, K.; Shohat, M.; Chitayat, D. Referral patterns for microarray testing in prenatal diagnosis. Prenat. Diagn. 2012, 32, 611. [Google Scholar] [CrossRef] [PubMed]
- Stark, Z.; Gillam, L.; Walker, S.P.; McGillivray, G. Ethical controversies in prenatal microarray. Curr. Opin. Obstet. Gynecol. 2013, 25, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Bejjani, B.A.; Shaffer, L.G. Application of Array-Based Comparative Genomic Hybridization to Clinical Diagnostics. J. Mol. Diagn. 2006, 8, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ruivenkamp, C.A.L.; Hoffer, M.J.V.; Vrijenhoek, T.; Kriek, M.; van Asperen, C.J.; den Dunnen, J.T.; Santen, G.W.E. Next-generation diagnostics: Gene panel, exome, or whole genome? Hum. Mutat. 2015, 36, 648–655. [Google Scholar] [CrossRef]
- Wou, K.; DeBie, I.; Carroll, J.; Brock, J.A.; Douglas Wilson, R. Fetal Exome Sequencing on the Horizon. J. Obstet. Gynaecol. Can. 2019, 41, 64–67. [Google Scholar] [CrossRef]
- Castleman, J.S.; Wall, E.; Allen, S.; Williams, D.; Doyle, S.; Kilby, M.D. The prenatal exome—A door to prenatal diagnostics? Expert Rev. Mol. Diagn. 2021, 21, 465–474. [Google Scholar] [CrossRef]
- Abou Tayoun, A.N.; Spinner, N.B.; Rehm, H.L.; Green, R.C.; Bianchi, D.W. Prenatal DNA Sequencing: Clinical, Counseling, and Diagnostic Laboratory Considerations. Prenat. Diagn. 2018, 38, 26–32. [Google Scholar] [CrossRef]
- Wise, A.L.; Manolio, T.A.; Mensah, G.A.; Peterson, J.F.; Roden, D.M.; Tamburro, C.; Williams, M.S.; Green, E.D. Genomic medicine for undiagnosed diseases. Lancet 2019, 394, 533–540. [Google Scholar] [CrossRef]
- Köhler, S.; Gargano, M.; Matentzoglu, N.; Carmody, L.C.; Lewis-Smith, D.; Vasilevsky, N.A.; Danis, D.; Balagura, G.; Baynam, G.; Brower, A.M. The human phenotype ontology in 2021. Nucleic Acids Res. 2021, 49, D1207–D1217. [Google Scholar] [CrossRef]
- Monaghan, K.G.; Leach, N.T.; Pekarek, D.; Prasad, P.; Rose, N.C. The use of fetal exome sequencing in prenatal diagnosis: A points to consider document of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2020, 22, 675–680. [Google Scholar] [CrossRef]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J. ACMG SF v3. 0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Muys, J.; Blaumeiser, B.; Janssens, K.; Loobuyck, P.; Jacquemyn, Y. Chromosomal microarray analysis in prenatal diagnosis: Ethical considerations of the Belgian approach. J. Med. Ethics 2020, 46, 104–109. [Google Scholar] [CrossRef]
- Naqvi, M.; Goldfarb, I.T.; Hanmer, K.J.; Bryant, A. Chromosomal microarray use among women undergoing invasive prenatal diagnosis. Prenat. Diagn. 2016, 36, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; Ormond, K.E. Ethical considerations in prenatal testing: Genomic testing and medical uncertainty. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2018; Volume 23, pp. 1–6. [Google Scholar]
- Lou, S.; Lomborg, K.; Lewis, C.; Riedijk, S.; Petersen, O.B.; Vogel, I. It’s probably nothing, but… couples’ experiences of pregnancy following a prenatally diagnosed and uncertain copy number variant. Acta Obs. Gynecol. Scand. 2020, 99, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Quinlan-Jones, E.; Kilby, M.D.; Greenfield, S.; Parker, M.; McMullan, D.; Hurles, M.E.; Hillman, S.C. Prenatal whole exome sequencing: The views of clinicians, scientists, genetic counsellors and patient representatives. Prenat. Diagn. 2016, 36, 935–941. [Google Scholar] [CrossRef]
- Guadagnolo, D.; Mastromoro, G.; Di Palma, F.; Pizzuti, A.; Marchionni, E. Prenatal Exome Sequencing: Background, Current Practice and Future Perspectives—A Systematic Review. Diagnostics 2021, 11, 224. [Google Scholar] [CrossRef]
- Shkedi-Rafid, S.; Fenwick, A.; Dheensa, S.; Wellesley, D.; Lucassen, A.M. What results to disclose, when, and who decides? Healthcare professionals’ views on prenatal chromosomal microarray analysis. Prenat. Diagn. 2016, 36, 252–259. [Google Scholar] [CrossRef]
- Singletary, C.N.; Krstic, N.C.; Czerwinski, J.L.; Choates, M.G.; Wagner, C. Prenatal chromosomal microarray uptake with invasive prenatal diagnosis: How many patients take the leap? Prenat. Diagn. 2018, 38, 748–754. [Google Scholar] [CrossRef]
- Westerfield, L.; Darilek, S.; Van Den Veyver, I.B. Counseling challenges with variants of uncertain significance and incidental findings in prenatal genetic screening and diagnosis. J. Clin. Med. 2014, 3, 1018–1032. [Google Scholar] [CrossRef]
- Klapwijk, J.E.; Srebniak, M.I.; Go, A.T.J.I.; Govaerts, L.C.P.; Lewis, C.; Hammond, J.; Hill, M.; Lou, S.; Vogel, I.; Ormond, K.E.; et al. How to deal with uncertainty in prenatal genomics: A systematic review of guidelines and policies. Clin. Genet. 2021, 100, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Mellis, R.; Chandler, N.; Chitty, L.S. Next-generation sequencing and the impact on prenatal diagnosis. Expert Rev. Mol. Diagn. 2018, 18, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Blumenfeld, Y.J.; Chitkara, U.; Hudgins, L.; Quake, S.R. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc. Natl. Acad. Sci. USA 2008, 105, 16266–16271. [Google Scholar] [CrossRef] [PubMed]
- Thung, D.T.; Beulen, L.; Hehir-Kwa, J.; Faas, B.H. Implementation of whole genome massively parallel sequencing for noninvasive prenatal testing in laboratories. Expert Rev. Mol. Diagn. 2015, 15, 111–124. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wu, J.; Zhou, P.; Fu, J.; Sun, J.; Cai, W.; Liu, H.; Yang, Y. Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/microduplications in a cohort of 8141 single pregnancies. Hum. Genom. 2019, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Mao, X.; Lei, W.; He, M.; Lu, W. Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/microduplications in a cohort of 42,910 single pregnancies with different clinical features. Hum. Genom. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Drury, S.; Mason, S.; McKay, F.; Lo, K.; Boustred, C.; Jenkins, L.; Chitty, L.S. Implementing non-invasive prenatal diagnosis (Nipd) in a national health service laboratory; From dominant to recessive disorders. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 924, pp. 71–75. [Google Scholar]
- Milunsky, A.; Milunsky, J.M. Genetic Disorders and the Fetus: Diagnosis, Prevention, and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1118981529. [Google Scholar]
- Lam, K.W.G.; Jiang, P.; Liao, G.J.W.; Chan, K.C.A.; Leung, T.Y.; Chiu, R.W.K.; Lo, Y.M.D. Noninvasive prenatal diagnosis of monogenic diseases by targeted massively parallel sequencing of maternal plasma: Application to β-thalassemia. Clin. Chem. 2012, 58, 1467–1475. [Google Scholar] [CrossRef]
- Chiu, E.K.L.; Hui, W.W.I.; Chiu, R.W.K. cfDNA screening and diagnosis of monogenic disorders—Where are we heading? Prenat. Diagn. 2018, 38, 52–58. [Google Scholar] [CrossRef]
- Vora, N.L.; Hui, L. Next-generation sequencing and prenatal ’omics: Advanced diagnostics and new insights into human development. Genet. Med. 2018, 20, 791–799. [Google Scholar] [CrossRef]
- Bhatti, G.; Romero, R.; Gomez-Lopez, N.; Pique-Regi, R.; Pacora, P.; Jung, E.; Yeo, L.; Hsu, C.D.; Kavdia, M.; Tarca, A.L. The amniotic fluid cell-free transcriptome in spontaneous preterm labor. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Moufarrej, M.N.; Wong, R.J.; Shaw, G.M.; Stevenson, D.K.; Quake, S.R. Investigating Pregnancy and Its Complications Using Circulating Cell-Free RNA in Women’s Blood During Gestation. Front. Pediatr. 2020, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.K.; Lo, Y.M.D. Prenatal diagnosis innovation: Genome sequencing of maternal plasma. Annu. Rev. Med. 2016, 67, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.Y.; Chan, K.Y.K.; Hui, P.W.; Au, P.K.C.; Tam, W.K.; Li, S.K.M.; Leung, G.K.C.; Fung, J.L.F.; Chan, M.C.Y.; Luk, H.M.; et al. Cost-effectiveness analysis of chromosomal microarray as a primary test for prenatal diagnosis in Hong Kong. BMC Pregnancy Childbirth 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).