Detection and Morphological Analysis of Micro-Ruptured Cortical Arteries in Subdural Hematoma: Three-Dimensional Visualization Using the Tissue-Clearing Clear, Unobstructed, Brain/Body Imaging Cocktails and Computational Analysis Method
Abstract
1. Introduction
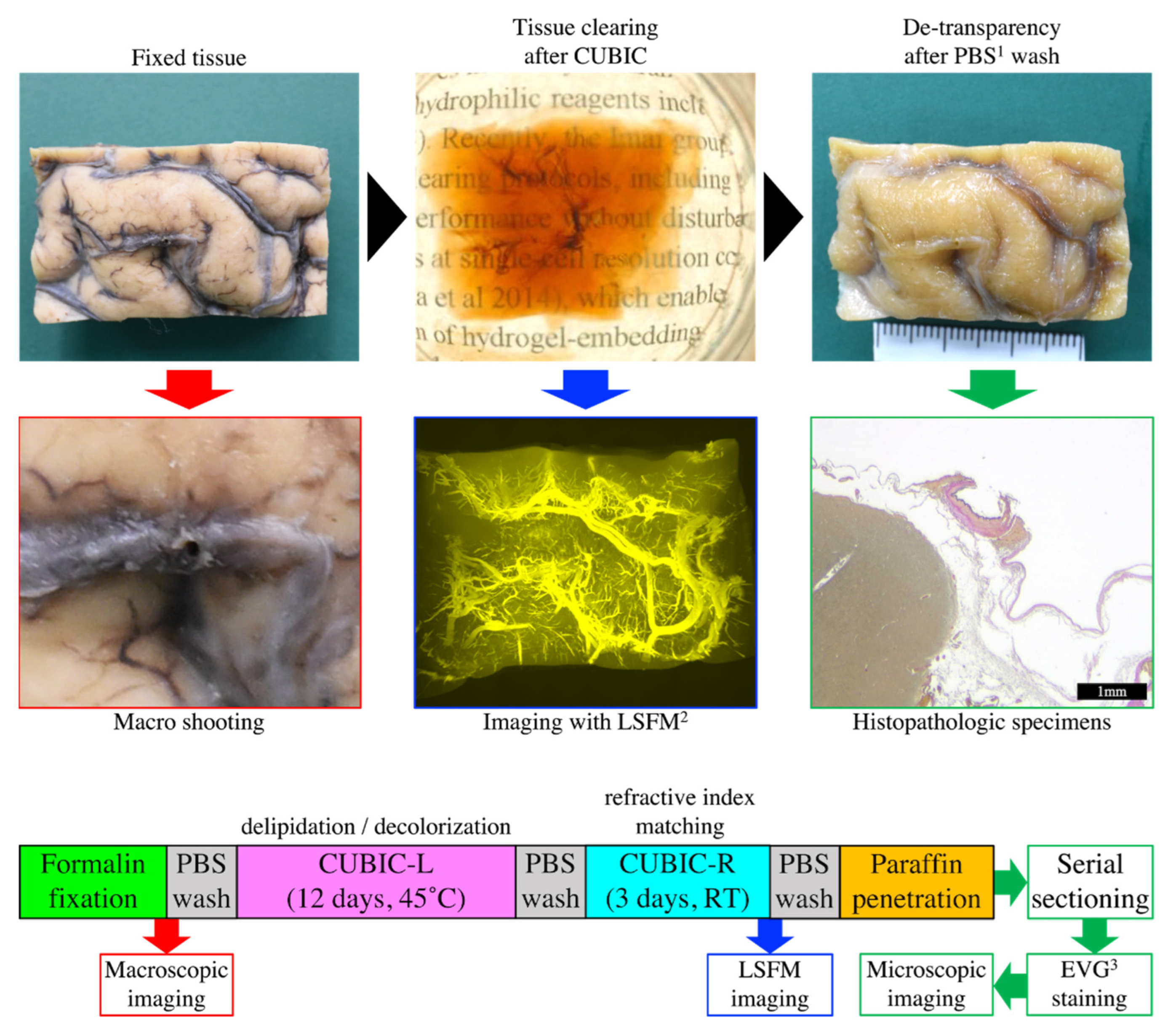
2. Materials and Methods
2.1. Human Brain Tissue
2.2. Macroscopic Imaging
2.3. CUBIC Tissue-Clearing Reagents
2.4. CUBIC Clearing of Brains
2.5. LSFM Imaging Conditions
2.6. Serial Sectioning and Histopathological Examination
2.7. Image Analysis
3. Results
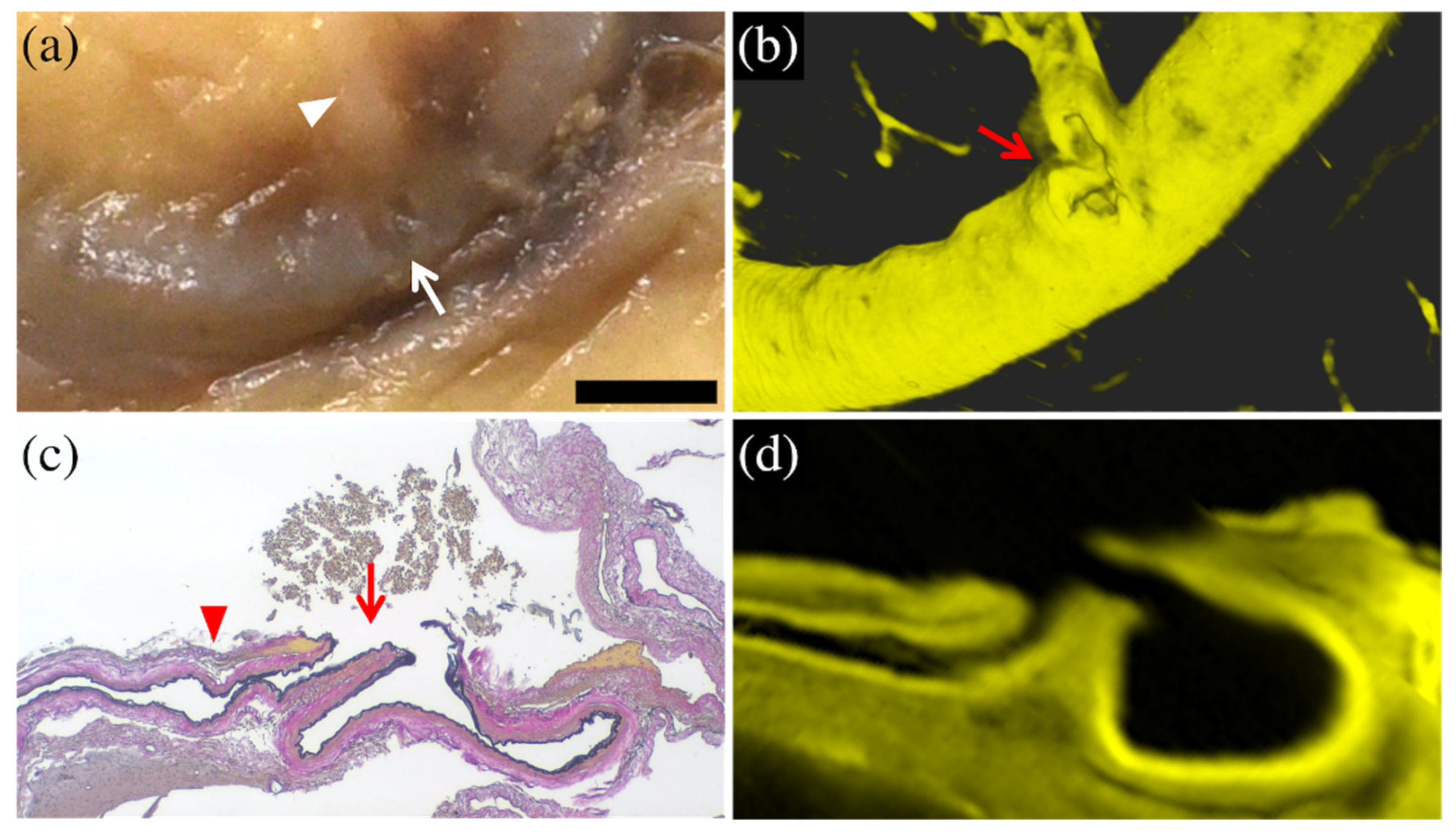
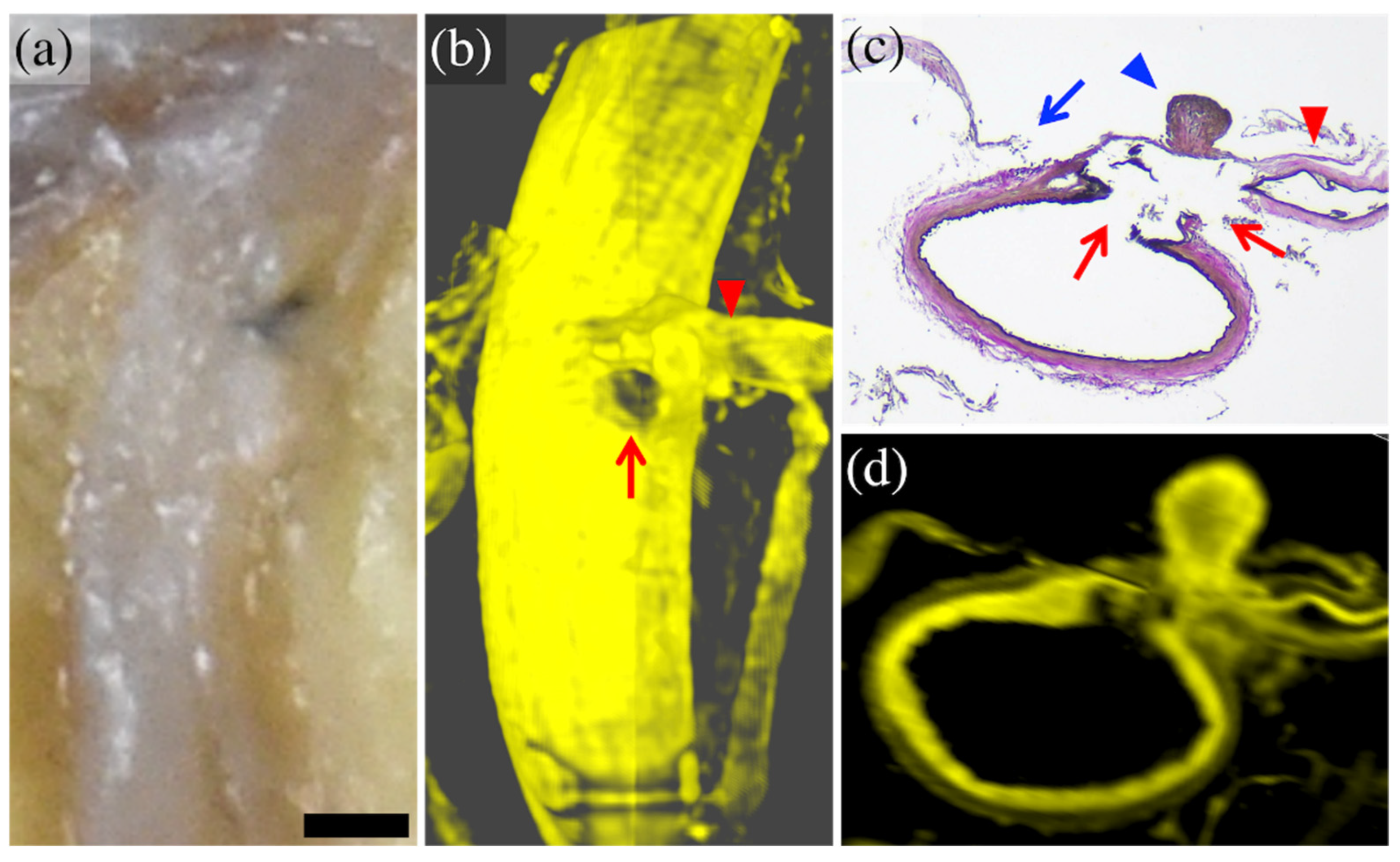
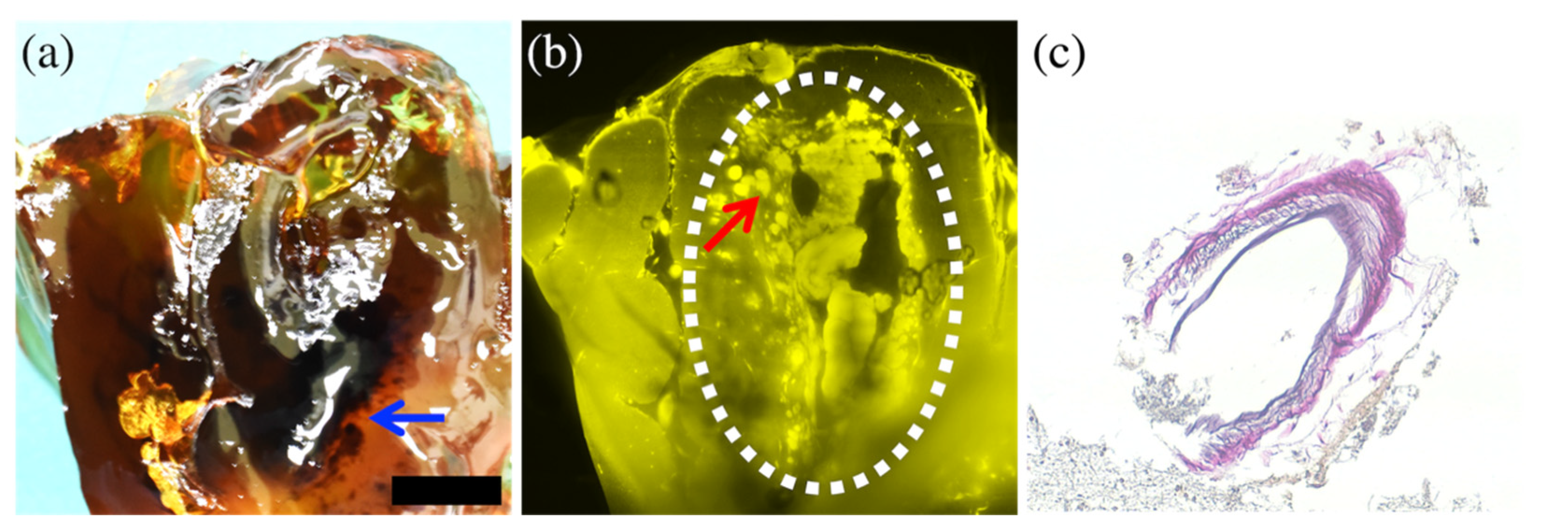
3.1. Results Per Case
3.2. Implications of CUBIC Processing on Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight, B. Forensic Pathology, 2nd ed.; Oxford University Press: New York, NY, USA, 1996; ISBN 978-03-4058-897-0. [Google Scholar]
- Whitwell, H. Intracranial haematomas: Extradural and subdural. In Forensic Neuropathology, 2nd ed.; Whitwell, H., Milroy, C., du Plessis, D., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-14-9870-616-2. [Google Scholar]
- Maxeiner, H.; Wolff, M. Pure subdural hematomas: A postmortem analysis of their form and bleeding points. Neurosurgery 2002, 50, 503–508; discussion 508–509. [Google Scholar] [CrossRef] [PubMed]
- Munro, D. The diagnosis and treatment of subdural hematoma. N. Engl. J. Med. 1934, 210, 1145–1160. [Google Scholar] [CrossRef]
- Talalla, A.; McKissock, W. Acute “spontaneous” subdural hemorrhage. An unusual form of cerebrovascular accident. Neurology 1971, 21, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Avis, S.P. Nontraumatic acute subdural hematoma. A case report and review of the literature. Am. J. Forensic Med. Pathol. 1993, 14, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Scott, M. Spontaneous nontraumatic subdural hematomas. J. Am. Med. Assoc. 1949, 141, 596–602. [Google Scholar] [CrossRef]
- Vance, B.M. Ruptures of surface blood vessels on cerebral hemispheres as a cause of subdural hemorrhage. AMA Arch. Surg. 1950, 61, 992–1006. [Google Scholar] [CrossRef]
- Drake, C.G. Subdural haematoma from arterial rupture. J. Neurosurg. 1961, 18, 597–601. [Google Scholar] [CrossRef]
- Smith, D.R.; Kempe, L.G. Cerebral false aneurysm formation in closed head trauma: Case report. J. Neurosurg. 1970, 32, 357–359. [Google Scholar] [CrossRef]
- Williams, B. Subdural haematoma of arterial origin. Lancet 1971, 297, 1074–1075. [Google Scholar] [CrossRef]
- Ito, J.; Ueki, K.; Ishikawa, H. Angiographic extravasation of contrast medium in acute traumatic subdural hematoma from arterial rupture: Case report. J. Neurosurg. 1972, 37, 226–228. [Google Scholar] [CrossRef]
- O’Brien, P.K.; Norris, J.W.; Tator, C.H. Acute subdural hematomas of arterial origin. J. Neurosurg. 1974, 41, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.S.; Patel, P.P. Spontaneous subdural hematoma of arterial origin: Report of two cases. Neurosurgery 1979, 5, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, V.; Brambilla, P.; Tonnarelli, G.P. Acute subdural hematoma. Spontaneous forms of arterial origin. Eur. Neurol. 1981, 20, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, H.A. Acute subdural hematoma. Review of 39 consecutive cases with high incidence of cortical artery rupture. J. Neurosurg. 1982, 57, 254–257. [Google Scholar] [CrossRef]
- Arai, H. Acute hypertensive subdural hematoma from arterial rupture shortly after the onset of cerebral subcortical hemorrhage: Leakage of contrast medium during angiography. Stroke 1983, 14, 281–285. [Google Scholar] [CrossRef]
- McDermott, M.; Fleming, J.F.; Vanderlinden, R.G.; Tucker, W.S. Spontaneous arterial subdural hematoma. Neurosurgery 1984, 14, 13–18. [Google Scholar] [CrossRef]
- Hesselbrock, R.; Sawaya, R.; Means, E.D. Acute spontaneous subdural hematoma. Surg. Neurol. 1984, 21, 363–366. [Google Scholar] [CrossRef]
- Yanai, Y.; Kohno, N.; Mitsui, T. Acute spontaneous subdural hematoma of arterial origin. Surg. Neurol. 1985, 23, 417–420. [Google Scholar] [CrossRef]
- Arienta, C.; Ceretti, L.; Caroli, M.; Villani, R. Acute spontaneous subdural hematomas. J. Neurosurg. Sci. 1986, 30, 197–204. [Google Scholar]
- Aoki, N. Acute subdural hematoma of arterial origin in a patient with a lumboperitoneal shunt. Neurol. Med. Chir. 1987, 27, 60–62. [Google Scholar] [CrossRef][Green Version]
- Tamaki, N.; Suyama, T.; Shirakuni, T.; Matsumoto, S. Acute subdural hematoma of arterial origin following ventriculoperitoneal shunting––report of two cases. Neurol. Med. Chir. 1987, 27, 643–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tokoro, K.; Nakajima, F.; Yamataki, A. Acute spontaneous subdural hematoma of arterial origin. Surg. Neurol. 1988, 29, 159–163. [Google Scholar] [CrossRef]
- Stephenson, G.; Gibson, R.M. Acute spontaneous subdural haematoma of arterial origin. Br. J. Neurosurg. 1989, 3, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Ranganadham, P.; Dinakar, I.; Mohandas, S.; Singh, A.K. Acute spontaneous subdural haematoma of arterial origin. Br. J. Clin. Pract. 1990, 44, 717–719. [Google Scholar]
- Borzone, M.; Altomonte, M.; Baldini, M.; Rivano, C. Pure subdural haematomas of arteriolar origin. Acta Neurochir. 1993, 121, 109–112. [Google Scholar] [CrossRef]
- Yasui, T.; Komiyama, M.; Kishi, H.; Yagura, H.; Fu, Y.; Nagata, Y.; Tamura, K. Angiographic extravasation of contrast medium in acute “spontaneous” subdural hematoma. Surg. Neurol. 1995, 43, 61–67. [Google Scholar] [CrossRef]
- Matsuyama, T.; Shimomura, T.; Okumura, Y.; Sakaki, T. Acute subdural hematomas due to rupture of cortical arteries: A study of the points of rupture in 19 cases. Surg. Neurol. 1997, 47, 423–427. [Google Scholar] [CrossRef]
- Koç, R.K.; Paşaoğlu, A.; Kurtsoy, A.; Oktem, I.S.; Kavuncu, I. Acute spontaneous subdural hematoma of arterial origin: A report of five cases. Surg. Neurol. 1997, 47, 9–11. [Google Scholar] [CrossRef]
- Murakami, M.; Kakita, K.; Hosokawa, Y. Ruptured traumatic aneurysm after trivial injury mimicking acute spontaneous subdural hematoma—Case Report—. Neurol. Med. Chir. 2003, 43, 130–133. [Google Scholar] [CrossRef]
- Depreitere, B.; Van Calenbergh, F.; van Loon, J. A clinical comparison of non-traumatic acute subdural haematomas either related to coagulopathy or of arterial origin without coagulopathy. Acta Neurochir. 2003, 145, 541–546; discussion 546. [Google Scholar] [CrossRef]
- Akioka, N.; Fukuda, O.; Takaba, M.; Kameda, H.; Saito, T.; Endo, S. Clinical investigation of acute spontaneous subdural hematoma cases. J. Stroke Cerebrovasc. Dis. 2007, 16, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Naama, O.; Belhachmi, A.; Ziadi, T.; Boulahroud, O.; Elasri, C.A.; Elmostarchid, B.; Boucetta, M. Acute spontaneous subdural hematoma: An unusual form of cerebrovacular accident. J. Neurosurg. Sci. 2009, 53, 157–159. [Google Scholar] [PubMed]
- Chhiber, S.S.; Singh, J.P. Acute spontaneous subdural hematoma of arterial origin: A report of four cases and review of literature. Neurol. India 2010, 58, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, J.C.; Boulos, A.S. Visualization of an actively bleeding cortical vessel into the subdural space by CT angiography. Clin. Neurol. Neurosurg. 2010, 112, 737–739. [Google Scholar] [CrossRef]
- Sung, S.K.; Kim, S.H.; Son, D.W.; Lee, S.W. Acute spontaneous subdural hematoma of arterial origin. J. Korean Neurosurg. Soc. 2012, 51, 91–93. [Google Scholar] [CrossRef]
- Dharsono, F.; Phatouros, C.C. Arterial origin subdural hematoma and associated pial pseudoaneurysm following minor head trauma. Clin. Imaging 2013, 37, 750–752. [Google Scholar] [CrossRef]
- Martins, W.A.; Teixeira, A.B.; Frigeri, T.M.; Paglioli, E. Spontaneous subdural hematoma associated to Duret hemorrhage. Interdiscip. Neurosurg. 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Awaji, K.; Inokuchi, R.; Ikeda, R.; Haisa, T. Nontraumatic pure acute subdural hematoma caused by a ruptured cortical middle cerebral artery aneurysm: Case report and literature review. NMC Case Rep. J. 2016, 3, 63–66. [Google Scholar] [CrossRef]
- Yoon, J.H.; Shim, Y.B.; Gwak, H.S.; Kwon, J.W.; Lee, S.H. Acute spontaneous subdural hematoma of arterial origin. Nerve 2017, 3, 12–14. [Google Scholar] [CrossRef]
- Shekarchizadeh, A.; Masih, S.; Reza, P.; Seif, B. Acute subdural hematoma and subarachnoid hemorrhage caused by ruptured cortical artery aneurysm: Case report and review of literature. Adv. Biomed. Res. 2017, 6, 46. [Google Scholar] [CrossRef]
- Mulcahy, M.J.; Chaganti, J.; Dower, A.; Al-Khawaja, D. Spontaneous acute arterial subdural hematoma. World Neurosurg. 2018, 110, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Uneda, A.; Hirashita, K.; Yabuno, S.; Kanda, T.; Suzuki, K.; Matsumoto, A.; Yunoki, M.; Yoshino, K. Repair of damaged cortical artery by direct micro-suture in surgical treatment of acute subdural hematoma: Technical note. Acta Neurochir. 2018, 160, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Verhey, L.H.; Wang, W.; Adel, J.G. True cortical saccular aneurysm presenting as an acute subdural hematoma. World Neurosurg. 2018, 113, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, S.; Takahara, K.; Nakaya, M.; Yoshida, K.; Mochizuki, Y.; Fukuchi, M.; Fujii, K. Neuroendoscopic hematoma removal with a small craniotomy for acute subdural hematoma. J. Clin. Neurosci. 2019, 61, 311–314. [Google Scholar] [CrossRef]
- Kuge, A.; Kondo, R.; Mitobe, Y.; Yamaki, T.; Sato, S.; Saito, S.; Sonoda, Y. Delayed acute subdural hematoma treated with endoscopic procedure: A case report. Surg. Neurol. Int. 2020, 11, 350. [Google Scholar] [CrossRef]
- Ciochon, U.M.; Steuble Brandt, E.G.; Stavngaard, T. Acute tentorial subdural hematoma caused by rupture of the posterior cerebral artery after minor trauma—A case report. Diagnostics 2020, 10, 175. [Google Scholar] [CrossRef]
- Abecassis, Z.A.; Nistal, D.A.; Abecassis, I.J.; Sen, R.D.; Levitt, M.R. Ghost aneurysms in acute subdural hematomas: A report of two cases. World Neurosurg. 2020, 139, e159–e165. [Google Scholar] [CrossRef]
- Shi, X.Y.; Zhang, J.X.; Tang, Z.X.; Sun, H.; Shen, Z. Severe spontaneous acute arterial subdural hematoma as an initial symptom of chronic myeloid leukemia. Br. J. Neurosurg. 2021, 19, 1–4. [Google Scholar] [CrossRef]
- Noufal, M.; Liang, C.W.; Yanez, P.; Calayag, M. Spontaneous chronic subdural hematoma due to cerebral cortical artery rupture: First case report and review of pertinent literature. Neuroradiol. J. 2021, 34, 688–691. [Google Scholar] [CrossRef]
- Funayama, K.; Harada, K.; Koyama, A.; Katsuragi-Go, R.; Nishikawa-Harada, N.; Higuchi, R.; Aoyama, T.; Watanabe, H.; Takahashi, N.; Takatsuka, H. The usefulness of postmortem computed tomography angiography for subdural hematoma caused by rupture of the cortical artery: A report of two autopsy cases and a literature review. Leg. Med. 2021, 53, 101941. [Google Scholar] [CrossRef]
- Yanagawa, T.; Yamashita, K.; Harada, Y.; Hatayama, T.; Kono, T. Location of hemorrhage with nontraumatic acute subdural hematoma due to ruptured microaneurysm. Surg. Neurol. Int. 2021, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Dodt, H.U.; Leischner, U.; Schierloh, A.; Jährling, N.; Mauch, C.P.; Deininger, K.; Deussing, J.M.; Eder, M.; Zieglgänsberger, W.; Becker, K. Ultramicroscopy: Three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 2007, 4, 331–336. [Google Scholar] [CrossRef]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79, Correction in Nat. Rev. Neurosci. 2020, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Dodt, H.U.; Osten, P.; Economo, M.N.; Chandrashekar, J.; Keller, P.J. Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron 2020, 106, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Susaki, E.A.; Tainaka, K.; Perrin, D.; Kishino, F.; Tawara, T.; Watanabe, T.M.; Yokoyama, C.; Onoe, H.; Eguchi, M.; Yamaguchi, S.; et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 2014, 157, 726–739. [Google Scholar] [CrossRef]
- Tainaka, K.; Kubota, S.I.; Suyama, T.Q.; Susaki, E.A.; Perrin, D.; Ukai-Tadenuma, M.; Ukai, H.; Ueda, H.R. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 2014, 159, 911–924. [Google Scholar] [CrossRef]
- Susaki, E.A.; Shimizu, C.; Kuno, A.; Tainaka, K.; Li, X.; Nishi, K.; Morishima, K.; Ono, H.; Ode, K.L.; Saeki, Y.; et al. Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat. Commun. 2020, 11, 1982. [Google Scholar] [CrossRef]
- Matsuo-Dapaah, J.; Lee, M.; Ishii, K.J.; Tainaka, K.; Coban, C. Using a new three-dimensional CUBIC tissue-clearing method to examine the brain during experimental cerebral malaria. Int. Immunol. 2021, 33, 587–594. [Google Scholar] [CrossRef]
- Nojima, S.; Susaki, E.A.; Yoshida, K.; Takemoto, H.; Tsujimura, N.; Iijima, S.; Takachi, K.; Nakahara, Y.; Tahara, S.; Ohshima, K.; et al. CUBIC pathology: Three-dimensional imaging for pathological diagnosis. Sci. Rep. 2017, 7, 9269. [Google Scholar] [CrossRef]
- Tainaka, K.; Murakami, T.C.; Susaki, E.A.; Shimizu, C.; Saito, R.; Takahashi, K.; Hayashi-Takagi, A.; Sekiya, H.; Arima, Y.; Nojima, S.; et al. Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep. 2018, 24, 2196–2210.e9. [Google Scholar] [CrossRef]
- Kubota, S.I.; Takahashi, K.; Nishida, J.; Morishita, Y.; Ehata, S.; Tainaka, K.; Miyazono, K.; Ueda, H.R. Whole-body profiling of cancer metastasis with single-cell resolution. Cell Rep. 2017, 20, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Wallace, J.; Kim, S.Y.; Kalyanasundaram, S.; Andalman, A.S.; Davidson, T.J.; Mirzabekov, J.J.; Zalocusky, K.A.; Mattis, J.; Denisin, A.K.; et al. Structural and molecular interrogation of intact biological systems. Nature 2013, 497, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.M.; Liu, A.; Ng, H.; Goldfinger, M.H.; Chau, T.W.; DeFelice, J.; Tilley, B.S.; Wong, W.M.; Wu, W.; Gentleman, S.M. Next, generation histology methods for three-dimensional imaging of fresh and archival human brain tissues. Nat. Commun. 2018, 9, 1066, Correction in Nat. Commun. 2018, 9, 2726. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, S.; Schueth, A.; Herrler, A.; Galuske, R.; Roebroeck, A. Scalable labeling for cytoarchitectonic characterization of large optically cleared human neocortex samples. Sci. Rep. 2019, 9, 10880. [Google Scholar] [CrossRef]
- Tanaka, N.; Kanatani, S.; Tomer, R.; Sahlgren, C.; Kronqvist, P.; Kaczynska, D.; Louhivuori, L.; Kis, L.; Lindh, C.; Mitura, P.; et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat. Biomed. Eng. 2017, 1, 769–806, Correction in Nat. Biomed. Eng. 2018, 2, 48. [Google Scholar] [CrossRef]
- Zhao, S.; Todorov, M.I.; Cai, R.; Al-Maskari, R.; Steinke, H.; Kemter, E.; Mai, H.; Rong, Z.; Warmer, M.; Stanic, K.; et al. Cellular and molecular probing of intact human organs. Cell 2020, 180, 796–812.e19. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Kafian, H.; Lalenejad, M.; Moradi-Mehr, S.; Birgani, S.A.; Abdollahpour, D. Light-sheet fluorescence microscopy with scanning non-diffracting beams. Sci. Rep. 2020, 10, 8501. [Google Scholar] [CrossRef]
- Tuchin, V.V. Tissue optics and photonics: Light-tissue interaction. J. Biomed. Photonics Eng. 2015, 1, 98–134. [Google Scholar] [CrossRef]
- Pan, C.; Cai, R.; Quacquarelli, F.P.; Ghasemigharagoz, A.; Lourbopoulos, A.; Matryba, P.; Plesnila, N.; Dichgans, M.; Hellal, F.; Ertürk, A. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 2016, 13, 859–867. [Google Scholar] [CrossRef]



| No. | Age (Years), Sex | Type of SDH 1 | Site of Arterial Rupture | Arterial Rupture Detection Using LSFM 2 | Macroscopic Findings of Arterial Rupture | Microscopic Findings of Arterial Rupture |
|---|---|---|---|---|---|---|
| 1 | 95 Female | Subacute | Right parietal lobe | Completely detected | Small recess at the bifurcation of a minor branch | Partial rupture of the minor branch at bifurcation |
| 2 | 94 Female | Acute | Right parietal lobe | Possible (Unclear visualization of distal rupture end) | Small recess | Complete rupture of the minor branch at bifurcation |
| 3 | 64 Female | Acute | Left parietal lobe | Completely detected | Localized bleeding adhesion | Two complete rupturesof the minor branch at and near bifurcation |
| 4 | 84 Female | Acute | Right temporal lobe | Completely detected | Normal | Partial rupture of the minor branch at bifurcation |
| 5 | 92 Male | Acute | Right parietal lobe | Not detected (Out of imaging range) | Arachnoid defect consistent with burr hole scar | Partial rupture of the minor branch at bifurcation |
| 6 | 91 Female | Encapsul-ated acute | Left parietal lobe | Not detected (Interference by bleeding) | Brain contusion with focal hematoma | Complete rupture inside focal hematoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funayama, K.; Tainaka, K.; Koyama, A.; Katsuragi-Go, R.; Nishikawa-Harada, N.; Higuchi, R.; Aoyama, T.; Watanabe, H.; Takahashi, N.; Takatsuka, H. Detection and Morphological Analysis of Micro-Ruptured Cortical Arteries in Subdural Hematoma: Three-Dimensional Visualization Using the Tissue-Clearing Clear, Unobstructed, Brain/Body Imaging Cocktails and Computational Analysis Method. Diagnostics 2022, 12, 2875. https://doi.org/10.3390/diagnostics12112875
Funayama K, Tainaka K, Koyama A, Katsuragi-Go R, Nishikawa-Harada N, Higuchi R, Aoyama T, Watanabe H, Takahashi N, Takatsuka H. Detection and Morphological Analysis of Micro-Ruptured Cortical Arteries in Subdural Hematoma: Three-Dimensional Visualization Using the Tissue-Clearing Clear, Unobstructed, Brain/Body Imaging Cocktails and Computational Analysis Method. Diagnostics. 2022; 12(11):2875. https://doi.org/10.3390/diagnostics12112875
Chicago/Turabian StyleFunayama, Kazuhisa, Kazuki Tainaka, Akihide Koyama, Rieka Katsuragi-Go, Natsumi Nishikawa-Harada, Ryoko Higuchi, Takashi Aoyama, Hiraku Watanabe, Naoya Takahashi, and Hisakazu Takatsuka. 2022. "Detection and Morphological Analysis of Micro-Ruptured Cortical Arteries in Subdural Hematoma: Three-Dimensional Visualization Using the Tissue-Clearing Clear, Unobstructed, Brain/Body Imaging Cocktails and Computational Analysis Method" Diagnostics 12, no. 11: 2875. https://doi.org/10.3390/diagnostics12112875
APA StyleFunayama, K., Tainaka, K., Koyama, A., Katsuragi-Go, R., Nishikawa-Harada, N., Higuchi, R., Aoyama, T., Watanabe, H., Takahashi, N., & Takatsuka, H. (2022). Detection and Morphological Analysis of Micro-Ruptured Cortical Arteries in Subdural Hematoma: Three-Dimensional Visualization Using the Tissue-Clearing Clear, Unobstructed, Brain/Body Imaging Cocktails and Computational Analysis Method. Diagnostics, 12(11), 2875. https://doi.org/10.3390/diagnostics12112875






