The Outcome- or Cost-Effectiveness Analysis of LUS-Based Care or CXR-Based Care of Neonatal Lung Diseases: The Clinical Practice Evidence from a Level Ⅲ NICU in China
Abstract
1. Introduction
2. Objects and Methods
2.1. Ethics Approval
2.2. Objects
2.3. Observation Index
2.4. Statistical Methods
3. Results
3.1. Influence on Ventilator Utilization Rate, Duration Time of Ventilator and the Frequency of Ventilator Weaning Failure
3.2. Influence of LUS-Based Care on the Misdiagnosis Rate of RDS
3.3. Influence of LUS-Based Care on the Frequency and Dosage of PS
3.4. Influence of LUS-Based Care on the Incidence of BPD in Premature Infants
3.5. Effect of LUS-Based Care on the Fatality Rate of RDS
3.6. Effect of LUS-Based Care on the Fatality Rate of Pulmonary Hemorrhage
3.7. Effect of LUS-Based Care on the Fatality Rate of Pneumothorax
3.8. Effect of LUS-Based Care on the Prognosis of Very Low Birth Weight (VLBW) Infants
3.9. Effect of LUS-Based Care on the Total Mortality of Hospitalized Patients
4. Discussion
4.1. Reducing the Frequency of Ventilator Use, Shortening the Duration of Ventilation, and Avioding the Ventilator Weaning Failure
4.2. Avoiding RDS Misdiagnosis and Reducing PS Dosage
4.3. BPD May Be a Preventable Disease, and the Management of Lung Diseases under Ultrasound Monitoring May Prevent the Occurrence of BPD
4.4. Changes in Traditional Pulmonary Disease Management Strategies Greatly Reducing the Mortality of Very Low Birth Weight and Hospitalized Infants
4.5. Cost Savings—The Inevitable Side Effects of LUS-Based Care
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bahreyni Toosi, M.; Malekzadeh, M. Radiation dose to new-borns in neonatal intensive care units. Iran. J. Radiol. 2012, 9, 145–149. [Google Scholar] [CrossRef]
- Linet, M.S.; Kim, K.P.; Rajaraman, P. Children’s exposure to diagnostic medical radiation and cancer risk: Epidemiologic and dosimetric considerations. Pediatr. Radiol. 2009, 39, S4–S26. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.; Ancelet, S.; Dreuil, S.; Caër-Lorho, S.; Le Pointe, H.D.; Brisse, H.; Chateil, J.F.; Lee, C.; Leuraud, K.; Bernier, M. Childhood cancer risks estimates following CT scans: An update of the French CT cohort study. Eur. Radiol. 2022, 32, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Migliaro, F.; Salomè, S.; Corsini, I.; De Luca, D.; Capasso, L.; Gragnaniello, D.; Raimondi, F.; NeoLUS Collaborative Study Group. Neonatal lung ultrasound: From paradox to diagnosis … and beyond. Early Hum. Dev. 2020, 150, 105184. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Stoller, J.Z.; Fraga, M.V. Point-of-care ultrasound in the neonatal ICU. Curr. Opin. Pediatr. 2020, 32, 216–227. [Google Scholar] [CrossRef]
- Corsini, I.; Parri, N.; Ficial, B.; Dani, C. Lung ultrasound in the neonatal intensive care unit: Review of the literature and future perspectives. Pediatr. Pulmonol. 2020, 55, 1550–1562. [Google Scholar] [CrossRef]
- Mazmanyan, P.; Kerobyan, V.; Shankar-Aguilera, S.; Yousef, N.; De Luca, D. Introduction of point-of-care neonatal lung ultrasound in a developing country. Eur. J. Pediatr. 2020, 179, 1131–1137. [Google Scholar] [CrossRef]
- Lovrenski, J. Pediatric lung ultrasound—Pros and potentials. Pediatr. Radiol. 2020, 50, 306–313. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Jordan, I.; Balaguer, M.; Batista-Muñoz, A.; Ramon, M.; Bobillo-Perez, S. Early surfactant replacement guided by lung ultrasound in preterm newborns with RDS: The ULTRASURF randomised controlled trial. Eur. J. Pediatr. 2020, 179, 1913–1920. [Google Scholar] [CrossRef]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Dolce, P.; Pierri, L.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Lung Ultrasound Score Progress in Neonatal Respiratory Distress Syndrome. Pediatrics 2021, 147, e2020030528. [Google Scholar] [CrossRef]
- Liu, J.; Lovrenski, J.; Hlaing, A.Y.; Kurepa, D. Neonatal lung diseases: Lung ultrasound or chest X-ray. J. Matern.-Fetal Neonatal Med. 2021, 34, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, H.Y.; Wang, X.L.; Xiao, L.J. The Significance and the Necessity of Routinely Performing Lung Ultrasound in the Neonatal Intensive Care Units. J. Matern.-Fetal Neonatal Med. 2016, 29, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Qiu, R.X.; Liu, J.; Zhang, L.; Ren, X.L.; Qin, S.J. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: A 3-year clinical practice report from a neonatal intensive care unit in China. J. Matern.-Fetal Neonatal Med. 2022, 35, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Liu, J.; Xia, R.M.; Ren, X.L.; Li, J.J. The new application of point-of-care lung ultrasound in guiding or assisting neonatal severe lung disease treatment based on a case series. J. Matern.-Fetal Neonatal Med. 2020, 33, 3907–3915. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tong, X.M. Risk factors for the first ventilator weaning failure in preterm infants receiving invasive mechanical ventilation. Chin. J. Contemp. Pediatr. 2021, 23, 569–574. [Google Scholar] [CrossRef]
- He, Y.; Yuan, L.; Zhang, X.J.; Chen, C.; Zhou, J.G. Analysis of risk factors and related poor outcomes of first endotracheal extubation failure in early intubated very low birth weight and extremely low birth weight infants. Chin. J. Appl. Clin. Pediatr. 2020, 35, 824–828. [Google Scholar] [CrossRef]
- Rocha, G.; Rodrigues, M.; Guimarães, H. Respiratory distress syndrome of the preterm neonate--placenta and necropsy as witnesses. J. Matern.-Fetal Neonatal Med. 2011, 24, 148–151. [Google Scholar] [CrossRef]
- Alhassen, Z.; Vali, P.; Guglani, L.; Lakshminrusimha, S.; Ryan, R.M. Recent Advances in Pathophysiology and Management of Transient Tachypnea of Newborn. J. Perinatol. 2021, 41, 6–16. [Google Scholar] [CrossRef]
- Liu, J.; Cao, H.Y.; Wang, H.W.; Kong, X.Y. The Role of Lung Ultrasound in Diagnosis of Respiratory Distress Syndrome in Newborn Infants. Iran. J. Pediatr. 2015, 25, e323. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.X.; Ren, X.L. The Ultrasonic Imaging Characteristics of Neonatal Respiratory Distress Syndrome (RDS): The New Concept of Lung Ultrasound to Diagnose RDS. Chest 2020, 157, A318. [Google Scholar] [CrossRef]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattaross, L.; Zhang, H.; Hwang, M.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. 2019, 145, e58990. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Fu, W.; Yang, C.S.; Huang, J.J. The diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine 2014, 93, e19. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.X.; Ren, X.L.; Li, J.J.; Xia, R.M.; Chi, J.H. The differentiation diagnosis of respiratory distress syndrome and transient tachypnea of the newborn by lung ultrasound. Chest 2019, 155 (Suppl. 4), 232A. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.X.; Li, X.W.; Wang, Y.; Chen, S.W.; Fu, W. Lung Ultrasonography to Diagnose Transient Tachypnea of the Newborn. Chest 2016, 149, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.; Liu, Y.; Wang, H.W.; Feng, Z.C. Lung ultrasonography for the diagnosis of severe pneumonia of the newborn. Chest 2014, 146, 483–488. [Google Scholar] [CrossRef]
- Liu, J.; Cao, H.Y.; Fu, W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J. Int. Med. Res. 2016, 44, 1534–1542. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, C.Z. Risk factors for bronchopulmonary dysplasia in extremely preterm infants. Chin. J. Perinat. Med. 2017, 20, 824–828. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.Q. Analysis of factors influencing bronchopulmonary dysplasia in extremely preterm infants. Chin. J. Child Health Care 2019, 27, 1059–1061, 1067. [Google Scholar] [CrossRef]
- Qi, X.H.; Wu, M.; Chen, T.Q.; Zhang, L.; Yu, B.M.; Dai, J.J. Risk Factors Analysis of Bronchopulmonary Dysplasia in Very (extremely) Low Birth Weight Pre-mature Infants. J. Clin. Res. 2020, 37, 810–813. [Google Scholar] [CrossRef]
- Collaborative Study Group for Extremely Preterm and Extremely Low Birth Weight Infants. A study on the clinical outcomes of extremely preterm infants and extremely low birth weight infants. Chin. J. Neonatol. 2020, 35, 108–112. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhu, H.; Liu, S.J. Analysis of influencing factors of bronchopulmonary dysplasia in extremely premature infants. Fujian Med. J. 2020, 42, 36–38. [Google Scholar]
- Han, F.; Yi, J.M.; Shi, X.Y.; Long, H.; Wang, G.Q. Risk factors for bronchopulmonary dysplasia in preterm infants younger than 32 weeks. Acad. J. Chin. PLA Med. Sch. 2019, 40, 321–324, 327. [Google Scholar]
- Xu, S.; Li, X.; Xu, J.; Yin, X.; Xi, H.; Yang, P.; Ma, L. Effects of early nutrition on bronchopulmonary dysplasia in premature infants. Chin. J. Clin. Nutr. 2021, 29, 148–156. [Google Scholar] [CrossRef]
- Li, Y.; Miao, X.L.; Guo, L.M.; Cui, C.D.; Zhou, Y. Risk factors of bronchopulmonary dysplasia in preterm infants under 32 weeks of gestational age. J. Med. Postgrad. 2020, 33, 924–927. [Google Scholar] [CrossRef]
- Guan, J.; Feng, X.X. Risk factors and readmission of bronchopulmonary dysplasia in premature infants. Henan Med. Res. 2020, 29, 6379–6381. [Google Scholar] [CrossRef]
- Xu, D.D.; Gao, H.; Zhang, J.L.; Wang, Y. Risk factors and early prediction of bronchopulmonary dysplasia in extremely low birth weight infants with gestational age < 32 weeks. Anhui Med. J. 2021, 42, 713–717. [Google Scholar] [CrossRef]
- Ji, L.; Feng, S.H.; Yang, Y.; Wang, H.J. Incidence and lung function of bronchopulmonary dysplasia in premature infants with different gestational age. J. Clin. Pediatr. 2018, 36, 505–509. [Google Scholar] [CrossRef]
- Nie, Z.Q.; Chen, X.Y. Valu of different pulmonary surfactant combined with mechanical ventilation in neonatal respiratory distress syndrome. Anhui Med. Pharm. J. 2019, 23, 1222–1225. [Google Scholar] [CrossRef]
- Wu, H.L.; Xu, Y.; Lou, L.L.; Wang, J. Comparison of the clinical efectiveness of nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in the treatment of respiratory distress syndrome in premature infants. J. Xuzhou Med. Univ. 2021, 41, 209–213. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, L.H.; Chen, Q. Prognostic value of DcR3 combined with SNAPPE-Ⅱ in neonatal respiratory distress syndrome. Int. J. Lab. Med. 2021, 42, 1735–1738. [Google Scholar] [CrossRef]
- Wu, X.J.; Wang, D.Y. Clinical study of high frequency oscillatory ventilation in the treatment of neonatal pulmonary hemorrhage. Chin. J. Prim. Med. Pharm. 2017, 24, 1015–1019. [Google Scholar] [CrossRef]
- Yang, X.F.; Sun, B. Factors Associated with the Outcomes of Pulmonary Hemorrhage in the Neonates. Chin. J. Hemorheol. 2017, 27, 83–85. [Google Scholar] [CrossRef]
- Xu, B.L.; Liu, D.P.; Jin, J.; Li, Z.H.; Zhou, Y.J.; Li, R.; Wang, Y.Y.; Wang, C.J.; Kang, W.Q. Clinical risk factors of bilateral pneumothorax in neonates. Chin. J. Woman Child Health Res. 2020, 1503–1506. [Google Scholar] [CrossRef]
- Li, F.; Liu, C.X.; Mao, J.; Li, J. Characteristics and prognosis of neonatal pneumothorax:a clinical analysis of 150 cases. Int. J. Pediatr. 2021, 48, 710–714. [Google Scholar] [CrossRef]
- Meng, Q.K. Analysis of risk factors and prognosis of neonatal pneumothorax. World Latest Med. Inf. Electron. Version 2019, 19, 112. [Google Scholar] [CrossRef]
- Zu, J.N.; Yang, M. Study on the efficacy and safety of high-frequency oscillatory ventilation in the treatment of neonatal pneumothorax. World Latest Med. Inf. Electron. Version 2020, 20, 171–172, 174. [Google Scholar] [CrossRef]
- Collaborative Quality Improvement Research Group of Neonatal Intensive Care Units in China. Outcomes of very low birth weight infants at discharge: A multicentered cross-sectional study of 25 tertiary neonatal intensive care units in China. Chin. J. Perinat. Med. 2018, 21, 394–400. [Google Scholar] [CrossRef]
- Xu, D.F.; Kong, X.Y.; Feng, Z.C. Mortality rate and cause of death in hospitalized neonates: An analysis of 480 cases. Chin. J. Contemp. Pediatr. 2017, 19, 152–158. [Google Scholar] [CrossRef]
- Mmt, Y.; Mts, R. Analysis of 5-year mortality rate of neonates in a hospital and the composition of diseases causing death. China Health Care Nutr. 2019, 29, 71. [Google Scholar] [CrossRef]
- Dong, H.; Li, W.; Xu, F.; Li, D.; Li, L.; Liu, Q.; Liu, J.; Li, H.; Wang, X.; Guo, X.; et al. Investigation of in-patient neonatal death at 18 hospitals in Henan Province. Chin. J. Perinat. Med. 2019, 22, 412–419. [Google Scholar] [CrossRef]
- Chen, D.X.; Zhang, Q.; Lu, H.R.; Liu, H.F.; Zhao, S.H. Neonatal mortality and death causes in hospital patients from 2012 to 2016. J. Xi’an Jiaotong Univ. Med. Sci. 2018, 39, 106–110. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.M.; Liu, Y.L.; You, Y.Y.; Jin, L.H.; Chen, S.Q. An analysis of neonatal diseases and causes of death in a neonatal department from 2010 to 2017. J. Wenzhou Med. Univ. 2019, 49, 894–899, 904. [Google Scholar] [CrossRef]
- Manley, B.J.; Doyle, L.W.; Owen, L.S.; Davis, P.G. Extubating extremely preterm infants: Predictors of success and outcomes following failure. J. Pediatr. 2016, 173, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Maksić, H.; Heljić, S.; Maksić, S.; Jonuzi, F. Pulmonary complications during mechanical ventilation in the neonatal period. Med. Arch. 2000, 54, 271–272. [Google Scholar]
- Liu, G.; Wu, H.; Li, Z. Current views of complications associated with neonatal ventilation. Minerva Pediatr. 2020, 72, 60–64. [Google Scholar] [CrossRef]
- Bae, C.W.; Kim, C.Y.; Chung, S.H.; Choi, Y.S. History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea. J. Korean Med. Sci. 2019, 34, e175. [Google Scholar] [CrossRef]
- The Subspecialty Group of Neonatology; The Society of Pediatrics of Chinese Medical Association; The Editorial Board of Chinese Journal of Pediatrics. Consensus for pulmonary surfactant therapy in neonates in China (2021). Chin. J. Pediatr. 2021, 59, 627–632. [Google Scholar] [CrossRef]
- Jaberi, E.; Roksana, M. A study on preterm births during 2013–2015, Shiraz. Iran. J. Obstet. Gynaecol. 2018, 38, 22–26. [Google Scholar] [CrossRef]
- Alfarwati, T.W.; Alamri, A.A.; Alshahrani, M.A.; Al-Wassia, H. Incidence, Risk factors and Outcome of Respiratory Distress Syndrome in Term Infants at Academic Centre, Jeddah, Saudi Arabia. Med. Arch. 2019, 73, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhu, Y.; Chen, C. Incidence rate and trend of premature infants. Chin. J. Neonatol. 2021, 36, 74–77. [Google Scholar] [CrossRef]
- Yan, J.M.; Huang, H.; Li, Q.Q.; Deng, X.Y. A single-center study on the incidence and mortality of preterm infants from 2006 to 2016. Chin. J. Contemp. Pediatr. 2018, 20, 368–372. [Google Scholar] [CrossRef]
- Islam, J.Y.; Keller, R.L.; Aschner, J.L.; Hartert, T.V.; Moore, P.E. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.D.; Mezzacappa-Filho, F.; Severino, S.D.; Ribeiro, M.Â.; Marson, F.A.; Morcilo, A.M.; Toro, A.A.; Ribeiro, J.D. Association between clinical variables related to asthma in schoolchildren born with very low birth weight with and without bronchopulmonary dysplasia. Rev. Paul. Pediatr. 2016, 34, 271–280. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Principi, N.; Di Pietro, G.M.; Esposito, S. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 2018, 16, 36. [Google Scholar] [CrossRef]
- Tracy, M.K.; Berkelhamer, S.K. Bronchopulmonary Dysplasia and Pulmonary Outcomes of Prematurity. Pediatr. Ann. 2019, 48, e148–e153. [Google Scholar] [CrossRef]
- Silva, L.V.; de Araujo, L.B.; de Oliveira Azevedo, V.M.G. Assessment of the neuropsychomotor development in the first year of life of premature infants with and without bronchopulmonary dysplasia. Rev. Bras. Ter. Intensiv. 2018, 30, 174–180. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Doyle, L.W. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin. Perinatol. 2018, 42, 478–484. [Google Scholar] [CrossRef]
- Ye, C.X.; Chen, S.B.; Wang, T.T.; Zhang, S.M.; Qin, J.B.; Chen, L.Z. Risk factors for preterm birth: A prospective cohort study. Chin. J. Contemp. Pediatr. 2021, 23, 1242–1249. [Google Scholar] [CrossRef]
- Liu, J.; Ren, X.L.; Fu, W.; Liu, Y.; Xia, R.M. Bronchoalveolar Lavage for the Treatment of Neonatal Pulmonary Atelectasis under Lung Ultrasound Monitoring. J. Matern.-Fetal Neonatal Med. 2017, 30, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.X.; Ren, X.L.; Liu, J.; Li, J.J.; Gao, Y.Q.; Xia, R.M. Bronchoalveolar Lavage to Treat Neonatal Meconium Aspiration Syndrome Under Monitoring of Lung Ultrasound Based on a Prospective Case Series Study. Iran. J. Pediatr. 2019, 29, e90012. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.R.; Wei, H.L.; Chen, C.; Qiu, R.X.; Ren, X.L.; Zhang, L.; Gao, Y.Q. Efficacy of Bronchoalveolar Lavage as Adjunct Therapy in the Treatment of Neonatal Severe Pneumonia: A Prospective Case–Control Study. J. Trop. Pediatr. 2020, 66, 528–533. [Google Scholar] [CrossRef]
- Ren, X.L.; Fu, W.; Liu, J.; Liu, Y.; Xia, R.M. Lung ultrasonography to diagnose pulmonary hemorrhage of the newborn. J. Matern.-Fetal Neonatal Med. 2017, 30, 2601–2606. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.X.; Gao, Y.Q. Lung ultrasound for diagnosis of neonatal pulmonary hemorrhage. Chin. J. Perinat. Med. 2019, 22, 740–743. [Google Scholar] [CrossRef]
- Liu, J.; Kurepa, D.; Feletti, F.; Alonso-Ojembarrena, A.; Lovrenski, J.; Copetti, R.; Sorantin, E.; Rodriguez-Fanjul, J.; Katti, K.; Aliverti, A.; et al. International Expert Consensus and Recommendations for Neonatal Pneumothorax Ultrasound Diagnosis and Ultrasound-guided Thoracentesis Procedure. J. Vis. Exp. 2020, 157, e60836. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, R.-X.; Liu, Y. Neonatal Massive Pneumothorax Resulting in Compression Atelectasis Treated by Ultrasound-Guided Pleural Puncture Therapy: A Typical Case Based on Lung Ultrasound Finding. Front. Pediatr. 2021, 9, 779615. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Shan, R.; Deng, B.; Wang, Y.; Huang, L.; Zong, H.; Xu, Y.; Meng, Q.; Liu, Y.; et al. Ultrasound diagnosis and grading of neonatal respiratory distress syndrome: A multicenter prospective study. Chin. Pediatr. Emerg. Med. 2020, 27, 801–807. [Google Scholar] [CrossRef]
- Department of Planning, Development and Informatization, Health Commission, PRC. Statistical Bulletin on Health Development in China 2020. National Health Commission of the People’s Republic of China. Available online: http://www.nhc.gov.cn (accessed on 13 July 2021).


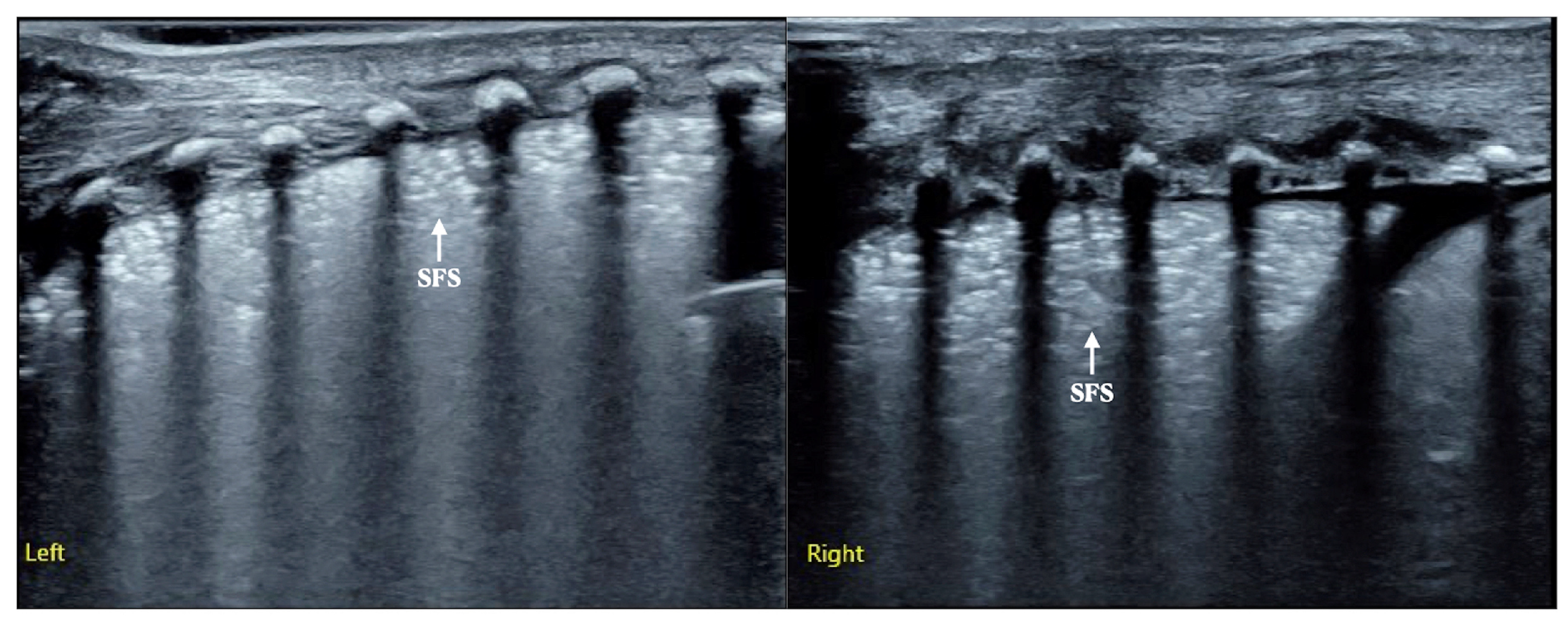
| Group | N | TDTV (Days) | T | P |
|---|---|---|---|---|
| CXR-based care | 672 | 15.03 ± 14.15 | 17.499 | 0.000 |
| LUS-based care | 357 | 4.88 ± 3.73 |
| Group | N | Death Cases | Fatality Rate (%) | x2 | P |
|---|---|---|---|---|---|
| CXR-based care | 282 | 40 | 14.2 | 42.19 | 0.000 |
| LUS-based care | 277 | 0 | 0 |
| Group | PH | Death Cases | Fatality Rate (%) | x2 | P |
|---|---|---|---|---|---|
| CXR-based care | 245 | 79 | 32.2 | 34.86 | 0.000 |
| LUS-based care | 82 | 0 | 0 |
| Group | Pneumothorax | Death Cases | Fatality Rate (%) | x2 | P |
|---|---|---|---|---|---|
| CXR-based care | 373 | 41 | 11.0 | 7.405 | 0.007 |
| LUS-based care | 61 | 0 | 0 |
| Group | VLBW | Poor Prognosis Cases | Poor Prognosis Rate (%) | x2 | P |
|---|---|---|---|---|---|
| CXR-based care | 2956 | 1373 | 46.5 | 51.595 | 0.000 |
| LUS-based care | 81 | 5 | 6.2 |
| Group | Hospitalized Patients | Death Cases | Mortality (‰) | x2 | P |
|---|---|---|---|---|---|
| CXR-based care | 126565 | 1244 | 9.83 | 40.137 | 0.000 |
| LUS-based care | 5027 | 5 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, X.; Wang, Y.; Li, J.; Yan, W.; Qin, S.-J.; Ren, X.-L.; Fu, W. The Outcome- or Cost-Effectiveness Analysis of LUS-Based Care or CXR-Based Care of Neonatal Lung Diseases: The Clinical Practice Evidence from a Level Ⅲ NICU in China. Diagnostics 2022, 12, 2790. https://doi.org/10.3390/diagnostics12112790
Liu J, Zhang X, Wang Y, Li J, Yan W, Qin S-J, Ren X-L, Fu W. The Outcome- or Cost-Effectiveness Analysis of LUS-Based Care or CXR-Based Care of Neonatal Lung Diseases: The Clinical Practice Evidence from a Level Ⅲ NICU in China. Diagnostics. 2022; 12(11):2790. https://doi.org/10.3390/diagnostics12112790
Chicago/Turabian StyleLiu, Jing, Xin Zhang, Yan Wang, Jie Li, Wei Yan, Sheng-Juan Qin, Xiao-Ling Ren, and Wei Fu. 2022. "The Outcome- or Cost-Effectiveness Analysis of LUS-Based Care or CXR-Based Care of Neonatal Lung Diseases: The Clinical Practice Evidence from a Level Ⅲ NICU in China" Diagnostics 12, no. 11: 2790. https://doi.org/10.3390/diagnostics12112790
APA StyleLiu, J., Zhang, X., Wang, Y., Li, J., Yan, W., Qin, S.-J., Ren, X.-L., & Fu, W. (2022). The Outcome- or Cost-Effectiveness Analysis of LUS-Based Care or CXR-Based Care of Neonatal Lung Diseases: The Clinical Practice Evidence from a Level Ⅲ NICU in China. Diagnostics, 12(11), 2790. https://doi.org/10.3390/diagnostics12112790







