The Sub-Molecular and Atomic Theory of Cancer Beginning: The Role of Mitochondria
Abstract
1. Introduction
1.1. The Connection between Cell Nucleus and Mitochondria: The Possible Origin of Cancer
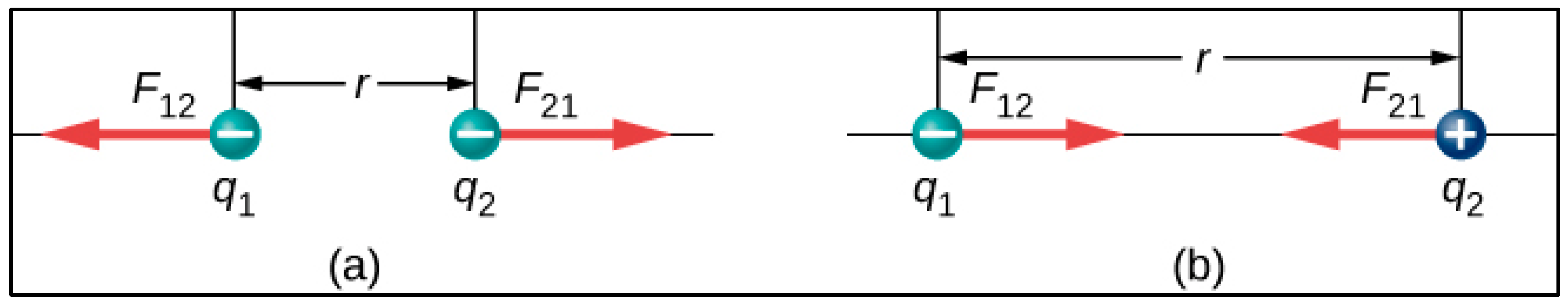
1.2. The Cancer Cell Structure Seen from Physics Perspective
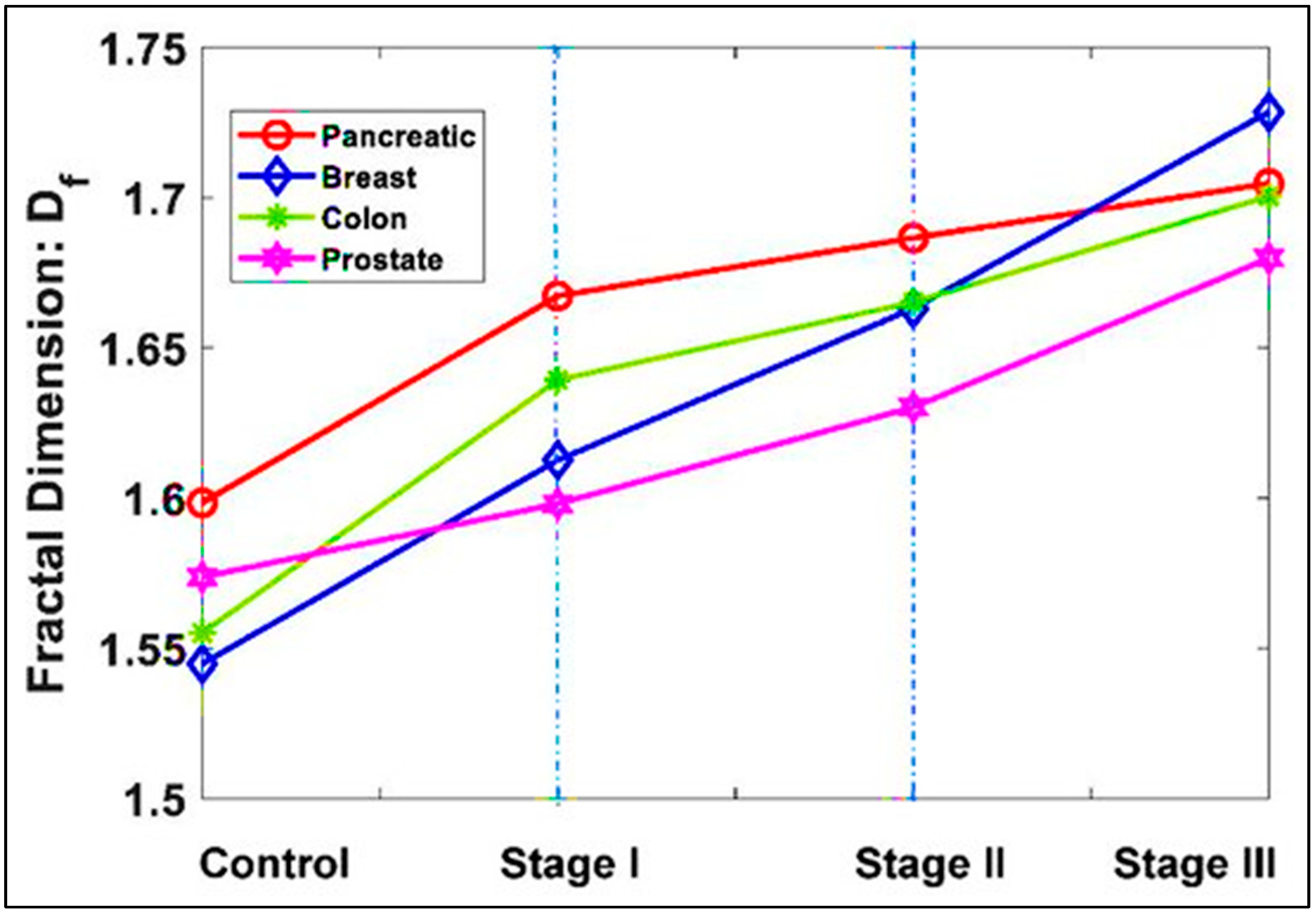
2. Cancer Seen as a Mitochondrial Survival Way-Out
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calvo, S.E.; Mootha, V.K. The mitochondrial proteome and human disease. Annu. Rev. Genom. Hum. Genet. 2010, 11, 25–44. [Google Scholar]
- Bonen, L.; Cunningham, R.S.; Gray, M.W.; Doolittle, W.F. Wheat embryo mitochondrial 18S ribosomal RNA: Evidence for its prokaryotic nature. Nucleic Acids Res. 1977, 4, 663–671. [Google Scholar]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Santi, T. Role of an Atomic-Level-Based Approach for Improving Cancer Therapy. Cancer Management and Therapy. In Cancer Management and Therapy; Hamza, A., Salem, N., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Helmenstine, A.M. How Many Atoms Are There in a Human Cell? ThoughtCo. 2022. Available online: https://www.thoughtco.com/how-many-atoms-in-human-cell-603882 (accessed on 16 September 2022).
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The chemical components of a cell. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Michor, F.; Liphardt, J.; Ferrari, M.; Widom, J. What does physics have to do with cancer? Nat. Rev. Cancer 2011, 11, 657–670. [Google Scholar] [CrossRef]
- Yin, Z. Gravitational-magnetic-electric field interaction. Results Phys. 2018, 10, 794–798. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Frieden, B.R. Coulomb interactions between cytoplasmic electric fields and phosphorylated messenger proteins optimize information flow in cells. PLoS ONE 2010, 5, e12084. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Szent Gyorgyi, A. The living state and Cancer. Proc. Natl. Acad. Sci. USA 1977, 7, 2844–2847. [Google Scholar]
- Basov, A.; Fedulova, L.; Vasilevskaya, E.; Dzhimak, S. Possible Mechanisms of Biological Effects Observed in Living Systems during 2H/1H Isotope Fractionation and Deuterium Interactions with Other Biogenic Isotopes. Molecules 2019, 24, 4101. [Google Scholar] [CrossRef]
- Sokolov, I. Fractals: A possible new path to diagnose and cure cancer? Future Oncol. 2015, 11, 3049–3051. [Google Scholar]
- Baish, J.W.; Jain, R.K. Fractals and cancer. Cancer Res. 2000, 60, 3683–3688. [Google Scholar]
- Dokukin, M.E.; Guz, N.V.; Woodworth, C.D.; Sokolov, I. Emerging of fractal geometry on surface of human cervical epithelial cells during progression towards Cancer. New J. Phys. 2015, 17, 033019. [Google Scholar]
- Iyer, S.; Gaikwad, R.M.; Subba-Rao, V.; Woodworth, C.D.; Sokolov, I. AFM detects differences in the surface brush on normal and cancerous cervical cells. Nat. Nanotechnol. 2009, 4, 389–393. [Google Scholar]
- Elkington, L.; Adhikari, P.; Pradhan, P. Fractal Dimension Analysis to Detect the Progress of Cancer Using Transmission Optical Microscopy. Biophysica 2022, 2, 59–69. [Google Scholar] [CrossRef]
- Le, W.; Chen, B.; Cui, Z.; Liu, Z.; Shi, D. Detection of cancer cells based on glycolytic-regulated surface electrical charges. Biophys. Rep. 2019, 5, 10–18. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Haber, D.A.; Gray, N.S.; Baselga, J. The evolving war on Cancer. Cell 2011, 145, 19–24. [Google Scholar] [CrossRef]
- Copeland, L.; Turner, J.F. The regulation of glycolysis and the pentose phosphate pathway. In Metabolism and Respiration; Davies, D.D., Ed.; Academic Press: Cambridge, MA, USA, 1980; pp. 279–316. [Google Scholar]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Shah, G.N.; Price, T.O.; Banks, W.A.; Morofuji, Y.; Kovac, A.; Ercal, N.; Sorenson, C.M.; Shin, E.S.; Sheibani, N. Pharmacological inhibition of mitochondrial carbonic anhydrases protects mouse cerebral pericytes from high glucose-induced oxidative stress and apoptosis. J. Pharmacol. Exp. Ther. 2013, 344, 637–645. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef]
- Orcutt, S.T.; Nguyen, T.; Harring, T.R.; Wosik, J.; Chang, A.; Lee, P.; Steinberg, M.; Bodei, L.; Paganelli, G.; Tomlinson, J.S.; et al. Subatomic Medicine and the Atomic Theory of Disease. Transl. Med. 2012, 2, 1–8. [Google Scholar]
- Müller, M.; Mentel, M.; van Hellemond, J.J.; Henze, K.; Woehle, C.; Gould, S.B.; Yu, R.Y.; van der Giezen, M.; Tielens, A.G.; Martin, W.F. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 2012, 76, 444–495. [Google Scholar] [CrossRef]
- Martin, W.; Mentel, M. The Origin of Mitochondria. Nat. Educ. 2010, 3, 58. [Google Scholar]
- Hendgen-Cotta, U.B.; Giorgio, V.; Hool, L. Mitochondria at the Crossroads of Survival and Demise. Oxidative Med. Cell. Longev. 2019, 2019, 2608187. [Google Scholar]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, electron transport chain. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar]
- Dzhimak, S.S.; Basov, A.A.; Volchenko, N.N.; Samkov, A.A.; Fedulova, L.V.; Baryshev, M.G. Changes in the functional activity of mitochondria isolated from the liver of rat that passed the preadaptation to ultra-low deuterium concentration. Dokl. Biochem. Biophys. 2017, 476, 323–325. [Google Scholar] [CrossRef]
- Zlatska, A.V.; Vasyliev, R.G.; Gordiienko, I.M.; Rodnichenko, A.E.; Morozova, M.A.; Vulf, M.A.; Zubov, D.O.; Novikova, S.N.; Litvinova, L.S.; Grebennikova, T.V.; et al. Effect of the deuterium on efficiency and type of adipogenic differentiation of human adipose-derived stem cells in vitro. Sci. Rep. 2020, 10, 5217. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Pham, V.H.; Tran, T.C.; Dipalma, G.; Inchingolo, F.; Tomassone, D.; Aityan, S.K.; Vergara, S.; et al. The Sub-Molecular and Atomic Theory of Cancer Beginning: The Role of Mitochondria. Diagnostics 2022, 12, 2726. https://doi.org/10.3390/diagnostics12112726
Balzanelli MG, Distratis P, Lazzaro R, Pham VH, Tran TC, Dipalma G, Inchingolo F, Tomassone D, Aityan SK, Vergara S, et al. The Sub-Molecular and Atomic Theory of Cancer Beginning: The Role of Mitochondria. Diagnostics. 2022; 12(11):2726. https://doi.org/10.3390/diagnostics12112726
Chicago/Turabian StyleBalzanelli, Mario G., Pietro Distratis, Rita Lazzaro, Van H. Pham, Toai C. Tran, Gianna Dipalma, Francesco Inchingolo, Diego Tomassone, Sergey K. Aityan, Sossio Vergara, and et al. 2022. "The Sub-Molecular and Atomic Theory of Cancer Beginning: The Role of Mitochondria" Diagnostics 12, no. 11: 2726. https://doi.org/10.3390/diagnostics12112726
APA StyleBalzanelli, M. G., Distratis, P., Lazzaro, R., Pham, V. H., Tran, T. C., Dipalma, G., Inchingolo, F., Tomassone, D., Aityan, S. K., Vergara, S., Nguyen, K. C. D., & Isacco, C. G. (2022). The Sub-Molecular and Atomic Theory of Cancer Beginning: The Role of Mitochondria. Diagnostics, 12(11), 2726. https://doi.org/10.3390/diagnostics12112726








