Abstract
To reduce high mortality and morbidity rates, timely and proper treatment of methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection is required. A multiplex polymerase reaction (mPCR)-based DNA lateral flow assay (MBDLFA) was developed as a point-of-care diagnostic for simultaneous identification of S. aureus, methicillin resistance, and vancomycin resistance directly from blood or blood cultures. A mPCR was developed to detect nuc, mecA, and vanA/B; its sensitivity, specificity, and limit of detection (LOD) were determined. The developed reaction was further modified for use in MBDLFA and its sensitivity for detection of target genes from artificially inoculated blood samples was checked. The optimized mPCR successfully detected nuc, mecA, and vanA/B from genomic DNA of bacterial colonies with LODs of 107, 107, and 105 CFU/mL, respectively. The reaction was sensitive and specific. The optimized mPCR was used in MBDLFA that detected nuc, mecA, and vanA/B with LODs of 107, 108, and 104 CFU/mL, respectively, directly from artificially inoculated blood. The developed MBDLFA can be used as a rapid, cheap point-of-care diagnostic for detecting S. aureus, MRSA, and vancomycin resistance directly from blood and blood cultures in ~2 h with the naked eye. This will reduce morbidity, mortality, and treatment cost in S. aureus bacteremia.
1. Introduction
Bacteremia or bloodstream infection is a term used to define the presence of bacteria in blood with the development of infection signs [1]. It is usually associated with high rates of morbidity and mortality. Bloodstream infection rates were estimated to reach two million episodes yearly in North America and Europe combined, with an estimated one-quarter of a million deaths [2]. Escherichia coli has the highest incidence, whereas Staphylococcus aureus and Pseudomonas aeruginosa cause higher mortality rates [3].
S. aureus ranked second among pathogens causing bacteremia; S. aureus bacteremia (SAB) is associated with a mortality rate reaching 30% [4]. This high mortality rate usually arises from the serious associated complications such as endocarditis, osteomyelitis, and metastatic abscesses [5]. Several factors affect the mortality rates of bacteremia. Some of these factors are related to the patients themselves, such as age, immune status, ethnicity, and comorbidities. Other factors are related to host–pathogen interaction, such as persistent bacteremia, bacteriuria, or shock. The mortality rate of SAB is also affected by toxin production, S. aureus clonal type, and the antimicrobial resistance against methicillin, where methicillin-resistant S. aureus (MRSA) is associated with a significant increase in mortality [6,7]. Recently, a higher mortality rate is associated with SAB in patients with COVID-19 infection [8].
This increase in mortality in MRSA bacteremia may arise from increased virulence caused by the presence of SCCmec-associated virulence factors [7] or from the delayed receipt of appropriate antimicrobials or improper therapy [9]. Management of SAB depends mainly on the antimicrobial resistance pattern of S. aureus, i.e., whether it is methicillin-sensitive S. aureus (MSSA) or MRSA. MSSA is better treated with semisynthetic penicillins or first-generation cephalosporins rather than vancomycin. However, vancomycin remains the drug of choice for treating MRSA bacteremia and is used as empirical therapy when bloodstream infection is suspected [10,11]. Treatment failure associated with vancomycin use has also been reported, although not at a high rate [11].
Proper antibiotic administration timing is another factor affecting SAB’s outcome [7]. Using rapid diagnostic testing to identify the causative agents and their susceptibilities has reduced the mortality risk in bloodstream infections [12]. Therefore, proper and timely identification of S. aureus and its antimicrobial susceptibility pattern is critical in reducing the mortality and morbidity associated with SAB. Unfortunately, using culture-dependent methods for identification and susceptibility testing is time-consuming and the identification results may not be available for three days. Blood culturing is the gold standard for bacteremia diagnosis; however, the blood culture results may not be available before the next day of testing, considering the working hours for sample processing and reporting [6].
Polymerase chain reaction (PCR)-based tests have provided a rapid means for microbial identification without the need for timely culture methods [13]. However, post-PCR steps, in their simplest form, using gel electrophoresis, are time-consuming and labor-intensive, especially when used for many samples, besides using the carcinogenic ethidium bromide for visualization [14]. Other molecular techniques for microbial identification include pyrosequencing and fluorescent-probe hybridization; however, these methods are expensive, labor-intensive, and require specific instrumentation unavailable in the laboratories of countries with limited resources [13]. Many techniques were developed to simplify the post-PCR steps such as DNA biosensors, where a DNA probe is immobilized onto the sensor’s surface. In these techniques, PCR can be carried out with primers containing specific oligonucleotide sequences or immunogenic substances bound to their 5′ or 3′ ends followed by hybridization to the DNA probe and DNA detection using optical, electrochemical, or gravimetric means [15], where labels such as colored latex particles, gold nanoparticles, or florescent compounds are used for detection [16].
The development of point-of-care diagnostics has gained great attention. They allow for optimized treatment and minimize the cost and time required for sample transport to central laboratories as they need simple instrumentation [17]. The nucleic acid lateral flow assay (LFA) is an example of a DNA biosensor that can be used as a point-of-care diagnostic allowing the rapid and specific DNA identification at the patient’s site. It is user-friendly, operates at a low cost, requires simple instrumentation, and allows for a one-step analysis. Several types and techniques are available for the nucleic acid lateral flow assay. These assays have many applications, such as clinical analysis and pathogen and toxin detection, as well as identification of various proteins, pesticides, and heavy metals in medicine and the environment [16]. Lateral flow assays were developed for microbial identification and detection of their resistance to antimicrobials in stool [18]. They were also used in environmental DNA detection [19].
DNA lateral flow assays depending on single-tag hybridization technology were developed in the form of dipstick strips for the detection of PCR amplicons. Single-tag hybridization depends on using biotinylated oligonucleotides and a single-stranded tag–spacer sequence for preparing the PCR amplicons that bind streptavidin-coated blue latex particles. The product is then hybridized to a complementary probe immobilized on the membrane strip, producing a colored line [20]. The membrane test strip is a nitrocellulose paper with different pads that allow capillary sample flow. The sample is applied onto a sample pad and moves by capillary flow to reach the conjugate pad, where it reacts with the complementary probe, thus allowing product visualization. Using a multiplex format allows the detection of different products on a single strip using different tags, which is advantageous in terms of time and cost savings [16].
Here, we developed a multiplex polymerase reaction (mPCR)-based DNA lateral flow assay (MBDLFA) as a point-of-care diagnostic for the simultaneous identification of S. aureus, methicillin resistance, and vancomycin resistance directly from blood samples or blood cultures of suspected bacteremia patients.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Clinical isolates of methicillin-resistant S. aureus (S31, S43) from the culture collection of the Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, and vancomycin-resistant Enterococcus faecalis (E25, Y3), kindly provided by Dr. Yomna Hashem, were used in this study. Other standard microbial species were used in the specificity testing: S. aureus ATCC 25923, Acinetobacter baumannii ATCC 19606, E. coli ATCC 25922, Enterococcus faecium ATCC 27270, Enterococcus faecalis ATCC 19433, Klebsiella pneumoniae ATCC 10031, and P. aeruginosa ATCC 27856. All microbial strains were stored in Luria Bertani broth containing 25% glycerol at –70 °C; when required, they were retrieved from the frozen stock by subculturing onto Luria Bertani agar plates and incubating at 37 °C for 24 h.
2.2. Primer Design
Primer pairs were designed to allow partial amplification of conserved regions in nuc, mecA, and vancomycin-resistance gene variants A and B (vanA/B). The nuc gene encodes for a thermonuclease specific for S. aureus [21]; mecA encodes for penicillin-binding protein 2 that is responsible for methicillin resistance [22], and vanA/B encodes for vancomycin-resistance determinants type A and B [23]. Different sequences of the target genes were downloaded from the National Center for Biotechnology Information (NCBI); the list of accession numbers of the used sequences is provided in Table S1. The sequences of each gene were aligned and primers were designed in the conserved regions [24,25] (Table 1). The primers were designed so that their parameters and melting temperatures allowed their use in a multiplex amplification reaction with product sizes that differ by at least 100 bp. Primer specificity was confirmed using the Primer-BLAST tool (NCBI, https://www.ncbi.nlm.nih.gov/, last accessed January 2022). All primers used in the uniplex and the conventional multiplex polymerase chain reactions (mPCR) were commercially synthesized by Macrogen, Korea.

Table 1.
Primers used in the study and the PCR amplicon size.
2.3. Conventional Multiplex PCR Development
The DNA was extracted from the test isolates (S31 and E25) using DNeasy blood and tissue kit (Qiagen, Germany) according to the manufacturer’s instructions. Initially, the designed primers were checked in a uniplex PCR (25 µL) consisting of: 1 µL of extracted DNA, 5 µL of 5X GoTaq Flexi Reaction Buffer, 1.7 mM MgCl2, 0.2 mM dNTP mix, 20 μM primer (Table 1), and 0.625 U GoTaq DNA Polymerase. All PCR reagents were from Promega (Madison, WI, USA). The reaction was performed in Veriti 96-Well Fast thermal cycler (Applied Biosystems TM, San Francisco, CA, USA) using initial denaturation at 94 °C for 3 min, then 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 1 min, followed by a final extension step at 72 °C for 10 min. The amplicons were detected by electrophoresis in ethidium bromide-stained agarose gels (1.5%). The PCR products were purified using Gene JET™ PCR Purification Kit (Thermo Fisher Scientific, Vilnius, Lithuania, EU) and sequenced using an ABI3730XL sequencer (Macrogen, Seoul, Korea). The resulting nucleotide sequences were checked for similarity to confirm their specificity using the BlastN tool available at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=tblastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome, accessed on August 2022) and were deposited in Genbank.
The primers were then used to develop a conventional mPCR. Optimizing mPCR involved testing different Mg2+ concentrations and a gradient of annealing temperatures ranging from 55–60 °C. The optimum mPCR reaction mixture (25 µL) contained 1 µL of extracted DNA, 5 µL of 5X Green GoTaq Flexi Reaction Buffer, 1.7 mM MgCl2, 0.2 mM dNTP mix, 20 μM of each primer (Table 1), and 0.625 U GoTaq DNA Polymerase. The cycling parameters were as follows: initial denaturation at 94 °C for 3 min, then 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 1 min, followed by a final extension step at 72 °C for 10 min. The mPCR amplicons were visualized by electrophoresis on 1.5% agarose gels stained with ethidium bromide.
2.4. Sensitivity and Limit of Detection (LOD) of the Developed mPCR
The total aerobic viable counts of overnight cultures of S31 and E25 isolates were determined. The genomic DNA was extracted from different dilutions of S31 and E25 suspensions (from 107 to 103 CFU/mL in phosphate-buffered saline) using the DNeasy Blood and Tissue Kit (Qiagen, Germany), according to manufacturer’s instructions. The optimized mPCR reaction was then performed on the extracted DNA from each dilution, and the PCR products were visualized by electrophoresis using a 1.5% agarose gel stained with ethidium bromide. The LOD of the mPCR was determined as the smallest concentration of cells with detectable products [26].
2.5. Specificity of the Developed mPCR
The specificity of the developed mPCR was tested using S. aureus ATCC 25923, MRSA strain S43, vancomycin-resistant E. faecalis Y3, as well as other bacteremia-causing pathogens (A. baumannii ATCC 19606, E. coli ATCC 25922, E. faecium ATCC 27270, E. faecalis ATCC 19433, K. pneumoniae ATCC 10031, and P. aeruginosa ATCC 27856). Genomic DNA was extracted from the tested strains using the DNeasy Blood and Tissue Kit, according to the manufacturer’s instructions. The optimized mPCR was performed on the extracted DNA as described earlier. Also, the assay was performed on pooled extracted DNA from S31 and E25.
2.6. Development of the mPCR-Based DNA Lateral Flow Assay (MBDLFA)
The primers for the MBDLFA were synthesized using the sequences of the previously designed primers for conventional mPCR. The 5′ terminus of the forward primer contained an oligonucleotide sequence complementary to the oligonucleotide immobilized on the corresponding line of the custom-designed chromatography printed array strip (C-PAS), and the 5′ terminus of the reverse primer was biotinylated (Table 1). All primers, buffers, and test strips were commercially synthesized by TBA Co., Japan. Optimizing the mPCR for use in the MBDLFA involved testing different primers and Mg2+ concentrations, a gradient of annealing temperatures ranging from 55–60 °C, and high and low concentrations of DNA. The optimized reaction (25 µL) contained 1 µL of extracted DNA, 5 µL of 5X GoTaq Flexi Reaction Buffer, 2 mM MgCl2, 0.2 mM dNTP mix, 7.5 μM of each of Tag1-NucF and Biotin-NucR primer, 5 μM of each of Tag2-mecAF and Biotin-mecAR primer, 20 μM each of Tag3-VanF and Biotin-VanR primers, and 0.625 U GoTaq DNA Polymerase and used the same cycling parameters as the conventional mPCR. The mPCR products for the MBDLFA (10 µL) were mixed with 10 µL dilution buffer, and 1 µL of latex solution containing avidin-coated blue beads. According to the manufacturer’s protocol, optimizing the lateral flow dilution buffer involved testing dilution buffers with different NaCl concentrations (Modi; 0 mM NaCl incl, 150 mM NaCl incl, and 300 mM NaCl incl); using Modi; 0 mM NaCl was optimum. The designed C-PAS was immersed in the mixture and the results were visualized after a 5 min incubation period at room temperature via the presence of blue-colored lines corresponding to each tested gene (Figure 1). The MBDLFA was tested on the DNA extracted from bacterial colonies of S31 and E25 as well as on pooled DNA of S31 and E25. The specificity of the developed MBDLFA was confirmed as previously described under conventional mPCR using different Gram-positive and Gram-negative pathogens.
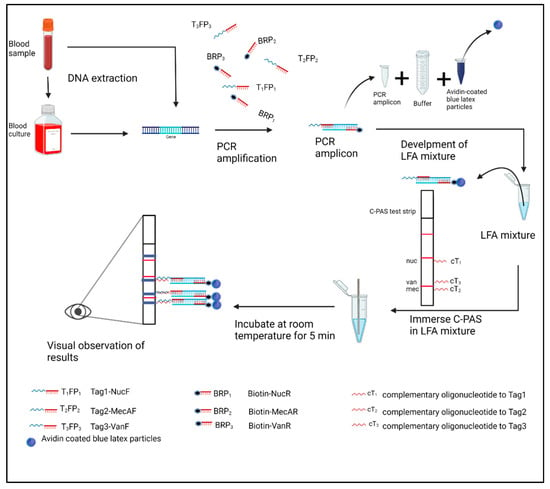
Figure 1.
Schematic diagram of the of the multiplex polymerase chain reaction (PCR)-based DNA lateral flow assay (MBDLFA) workflow; created with biorender.com. The designed chromatography-printed array strip (C-PAS) containing complementary oligonucleotides to each single-stranded tag and indicating the expected positions of the generated colored line specific to each tag is shown. The red lines in strip represent position markers that help localization of test lines. Tag1-NucF, Tag2-MecAF, Tag3-VanF: single-stranded oligonucleotides-tagged forward primers for amplification of nuc, mecA, and van genes, respectively; Biotin-NucR, Biotin-MecAR, Biotin-VanR: biotin-labelled reverse primers for amplification of nuc, mecA, and van genes, respectively.
2.7. Detection of MRSA and Vancomycin Resistance in Artificially Spiked Blood Samples Using the MBDLFA
A defibrinated human blood sample was artificially inoculated with either S31 or E25 [27]. Briefly, overnight cultures of S31 or E25 were diluted to reach a viable count equivalent to 107 CFU/mL. Ten-fold serial dilutions were made in Luria Bertani broth, 1 mL of different dilutions of the bacterial culture was centrifuged at 13,000 rpm for 5 min, and the pellet was suspended in 100 µL defibrinated blood. DNA was extracted from each inoculated blood sample using the DNeasy Blood and Tissue Kit, according to the manufacturer’s protocol. The developed MBDLFA was performed using 1 µL of each extracted DNA sample, as described previously. The results of the developed MBDLFA were visualized via the naked eye by two independent technicians to avoid any bias during result interpretation. The products were also visualized by electrophoresis using 1.5% agarose gel stained with ethidium bromide.
3. Results
3.1. Development of mPCR for the Detection of S. aureus, Methicillin Resistance and Vancomycin Resistance
The designed primers were tested in three separate uniplex PCRs; they successfully detected nuc, mecA, and vanA/B genes as specific markers for S. aureus, methicillin resistance, and vancomycin resistance. Amplification products of the predicted sizes (192, 310, and 420 bp) were detectable (Figure 2A). To further confirm the specificity of the designed primers, the amplified PCR products were sequenced, and the resulting nucleotide sequences had 99.53% similarity to S. aureus nuc MZ816763.1, 99.24% similarity to S. aureus mecA EF600988.1, and 99.49% similarity to S. aureus vanA MK214489.1. They were deposited in GenBank under accession numbers ON934435, ON934436, and ON934437, respectively. The mPCR was then checked using the three primer pairs; it successfully detected S. aureus (nuc) and methicillin resistance (mecA) with LODs of 107 CFU/mL and vancomycin resistance (vanA/B) with an LOD of 105 CFU/mL (Figure 2B, Table 2).
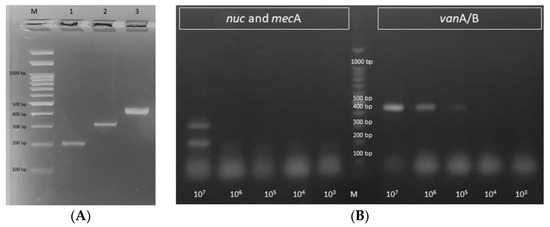
Figure 2.
Agarose gel visualization of the amplification products of the developed: (A) uniplex PCR, where: M is a 100 bp DNA ladder, lanes 1–3 show the amplification products of nuc, mecA, and vanA/B genes with the expected product sizes of 192, 310, and 420 bp, respectively, and (B) mPCR performed on the genomic DNA extracted from different dilutions of S31 and E25 (from 107 to 103 CFU/mL). It successfully detected S. aureus (nuc; 192 bp) and methicillin resistance (mecA; 310 bp) with a LODs of 107 CFU/mL and vancomycin resistance (vanA/B; 420 bp) with an LOD of 105 CFU/mL. M: 100 bp DNA ladder.

Table 2.
Limit of detection, time for detection, and specificity of the tested methods.
3.2. The Developed mPCR Is Specific
Other microbial strains were tested to confirm the specificity of the developed mPCR. The assay successfully detected S. aureus ATCC 25923 with one single band of 192 bp, corresponding to the partial amplification of the nuc gene (Figure S1). The MRSA strain S43 produced two bands of 192 bp and 310 bp, corresponding to the partial amplification of the nuc and mecA genes; the vancomycin-resistant E. faecalis strain Y3 produced a single band of 420 bp, corresponding to the partial amplification of vanA/B (Figure S1). No PCR amplification products were detected on agarose gel when testing A. baumannii ATCC 19606, E. coli ATCC 25922, E. faecium ATCC 27270, E. faecalis ATCC 19433, K. pneumoniae ATCC 10031, or P. aeruginosa ATCC 27856 (Figure S1). The developed mPCR specifically detected the three genes simultaneously in pooled DNA from S31 and E25 (Figure 3).
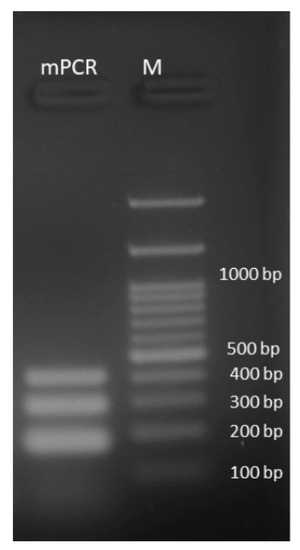
Figure 3.
Agarose gel visualization of the amplification products of the developed mPCR performed on the pooled genomic DNA of S31 and E25. The developed mPCR specifically detected the three genes simultaneously, (nuc: 192 bp; mecA: 310 bp, and vanA/B: 420 bp). M: 100 bp DNA ladder.
3.3. Detection of MRSA and Vancomycin Resistance Using the MBDLFA
The optimized mPCR was modified to develop the MBDLFA. The developed MBDLFA was specific and successfully detected nuc, mecA, and vanA/B from the DNA extracted from pure bacterial colonies as well as from pooled DNA of S31 and E25, without nonspecific products under all tested conditions (Figure 4). No products were detectable when the developed MBDLFA was applied to other Gram-positive and Gram-negative pathogens and one single band corresponding to the nuc gene was detectable when the DNA extracted from S. aureus ATCC 25923 was tested (Figure S1).

Figure 4.
The developed multiplex polymerase reaction (mPCR)-based DNA lateral flow assay (MBDLFA) was specific and successfully detected: (A) nuc and mecA from genomic DNA extracted from Staphylococcus aureus S31; (B) vanA/B from genomic DNA extracted from Enterococcus faecalis E25; and (C) nuc, mecA, and vanA/B from pooled genomic DNA of S31 and E25, using the designed chromatography-printed assay strip (C-PAS). The red lines on the strip represent position markers that help localization of the test lines.
3.4. Detection of MRSA and Vancomycin Resistance in Artificially Inoculated Blood Using the MBDLFA
The DNA was extracted from blood samples artificially inoculated with either S31 or E25 and the developed MBDLFA was performed on the extracted DNA. The assay successfully detected S. aureus, methicillin resistance, and vancomycin resistance in the DNA extracted directly from the artificially inoculated blood samples with LODs of 107 CFU/mL, 108 CFU/mL, and 104 CFU/mL for nuc, mecA, and vanA/B genes, respectively (Figure 5A,B, Table 2), using the designed C-PAS. When the products were visualized on 1.5% agarose gel stained with ethidium bromide, the LODs of the assay from artificially inoculated blood samples was 105 CFU/mL for both the nuc and mecA genes and 104 CFU/mL for the vanA/B genes (Figure 5C, Table 2).
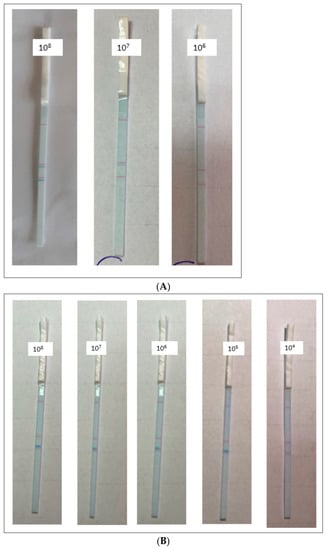
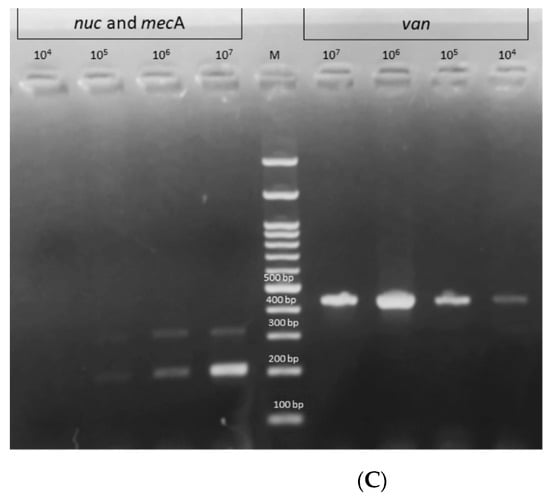
Figure 5.
The limit of detection (LOD) of the developed multiplex polymerase reaction (mPCR)-based DNA lateral flow assay (MBDLFA) using the DNA extracted from blood samples artificially inoculated with either Staphylococcus aureus S31 or Enterococcus faecalis E25. The assay successfully detected: (A) S. aureus (nuc) and methicillin resistance (mecA) in S31 with LODs of 107 CFU/mL and 108 CFU/mL, respectively, using the designed chromatography-printed assay strip (C-PAS), and (B) vancomycin resistance (vanA/B) in E25 with an LOD of 104 CFU/mL, using the designed C-PAS. (C) The products were visualized on a 1.5% agarose gel stained with ethidium bromide, the LODs of the assay from artificially inoculated blood samples was 105 CFU/mL for both the nuc and mecA genes and 104 CFU/mL for the vanA/B genes. The red lines on the strips in (A,B) represent position markers that help the localization of test lines.
4. Discussion
SAB is accompanied by high morbidity and mortality rates [4] that are affected by several factors, including the antimicrobial resistance pattern and proper timing of antimicrobial administration [11,28]. The available bacteremia diagnosis methods depend on blood culturing, which is usually time-consuming and subject to possible failure [6,28].
In this study, nuc, mecA, and vanA/B genes encoding the thermonuclease enzyme, penicillin-binding protein 2a (PBP2a), and D-Ala-D-Lac ligase were used as markers for S. aureus identification, methicillin resistance, and vancomycin resistance, respectively. The nuc gene is used as a specific marker for S. aureus identification using molecular techniques [21]. The PCR detection of mecA is the gold-standard technique for MRSA identification [22]. Several studies have designed mPCRs for identifying MRSA using primers specific for the nuc and mecA genes [29,30]. Other genes have also been used for PCR identification of S. aureus, such as 16S rRNA and vicK [31,32]. Sometimes, other target genes, such as femB, are incorporated into the mPCR to enhance the reaction specificity and detectability of methicillin resistance [33]. vanA is the common detectable ligase-encoding gene in S. aureus that is responsible for vancomycin resistance, whereas vanB is rarely detected [23,34].
The designed primers were checked in uniplex PCR reactions. Single bands of the expected size were produced, and sequencing of the purified PCR products further confirmed their specificity. Combining the primers in a mPCR and testing different strains of MRSA and vancomycin-resistant enterococci resulted in the expected products without non-specific bands. The specificity of the developed mPCR was confirmed by testing other bacteremia-causing Gram-positive and Gram-negative pathogenic species. The use of mPCR is advantageous over the uniplex format in terms of time and money during diagnosis and screening reactions.
The developed mPCR reaction successfully detected MRSA and vancomycin resistance with LODs of 107, 107, and 105 CFU/mL for the nuc, mecA, and van A/B genes, respectively, in the DNA extracted from the tested isolates. A lower LOD was reported in some studies where an mPCR was developed for identifying S. aureus and methicillin resistance using nuc and mecA [35,36]. However, our LOD (105 CFU/PCR) is nearly similar to that reported in other studies (104 to 106 CFU/PCR) [37,38]. A previous mPCR developed by Okolie and colleagues [39] detected methicillin and vancomycin resistance as well as other S. aureus species-specific genes with an LOD of 104 CFU/mL, which is slightly lower than our detected LOD.
Using a DNA-based lateral flow assay to detect PCR amplicons is an easy, fast, and user-friendly method for application as a point-of -care diagnostic [16], besides being safe due to the lack of use of the carcinogenic ethidium bromide. We successfully developed an MBDLFA capable of the specific detection of S. aureus, MRSA, and vancomycin resistance. The MBDLFA successfully detected nuc, mecA, and vanA/B in the DNA extracted directly from artificially inoculated blood samples with LODs of 107, 108, and 104 CFU/mL (equivalent to 104, 105, and 10 CFU/reaction), respectively. The lower LOD detected for the vanA/B genes in all cases compared with the nuc and mecA genes may be attributed to the available genetic copies for the tested genes where vanA/B are sometimes plasmid encoded. This LOD was higher than that for the same reactions visualized on an agarose gel stained with ethidium bromide (105, 105, and 104 CFU/mL, equivalent to 102, 102, and 10 CFU/reaction for nuc, mecA, and vanA/B, respectively). A nested uniplex PCR developed by Banada and colleagues [40] detected S. aureus directly from blood samples with LODs of 10 CFU/mL and 50 CFU/mL for sodA and nuc genes, respectively. The higher LOD in our reaction may be due to the multiplex nature of the reaction, where the sensitivity of PCR decreases as the number of amplified genes increases, and the use of the nested PCR technique in Banada et al. study. The enhanced sensitivity of the nested PCR arises from the increased total number of cycles used in the two reaction rounds [41]. Other studies designed real-time PCR reactions to detect S. aureus and/or methicillin resistance directly from blood samples with a lower detection limit of 103 CFU/mL [42,43]. The real-time PCR technique is not available in all laboratories, especially in developing countries, in addition to its high cost and the need for more trained personnel compared with conventional PCR. Several PCR-based lateral flow assays have been developed to detect different pathogens, resistance genes, and microbial toxins [18,20,44,45,46]. A previous study reported the use of a multiple cross displacement amplification reaction coupled with a lateral flow assay to detect S. aureus and identify MRSA with an LOD as low as 103 CFU/mL. However, multiple cross displacement amplification reactions use a large set of primers per reaction and suffer from false positive results due to contamination [47], which is not the case with our developed MBDLFA. Another developed PCR-based strip detected MRSA from positive blood cultures using a DNA lateral flow technique [45]. However, blood culture bottles are positive only when the microbial count exceeds 107–108 CFU/mL [48].
The developed MBDLFA allows for the simultaneous detection of S. aureus, MRSA, and vancomycin resistance directly from blood samples and/or cultures in two hours. Rapid diagnosis is a critical requirement for the proper treatment of SAB [7]. Bacteremia diagnosis depends mainly on the blood culture results; blood cultures require at least 5 h to become positive and may need 15 h depending on the initial bacterial load and antibiotic treatment at the sampling time [43,49,50]. After confirmation of positive blood cultures, antimicrobial testing is performed. The results of identification and antimicrobial susceptibility are only available after 72 h [50]. During that time, empirical treatment is usually administered, which could involve the unnecessary use of vancomycin as an anti-MRSA agent [10]. This may alter the patient’s microbiome leading to opportunistic infections, subject the patient to drug-related toxicity, and increase the cost of hospitalization. In addition, improper antibiotic use can select for resistant strains [28,51]. Using the developed MBDLFA on DNA extracted from positive blood cultures will also save the time required for traditional testing of MRSA and vancomycin resistance.
The developed MBDLFA permits the use of as little as 100 µL of blood for DNA extraction; a higher blood volume may be used to enhance the LOD of the assay. Other blood culture techniques that require higher blood volumes allow for higher sensitivity [42] but can be problematic when it is difficult to obtain large blood volumes, as in pediatric cases [28].
Many studies regarding molecular-based methods for pathogen identification and antimicrobial resistance marker detection depend on positive blood cultures [52]. Before culturing, the initial bacterial count in blood is less than 1 CFU/mL in half of the patients with bacteremia [53]. The minimum number of bacteria present in blood culture before a culture is positive is 108 CFU/mL [48], which is similar or slightly higher than the detection limit of the developed MBDLFA. After a positive blood culture, the suggested tests are either in situ hybridization-based methods, DNA-microarray-based hybridization technologies, nucleic-acid-amplification-based methods, or combined platforms [52]. Although in situ hybridization and DNA-microarray based methods are as fast as PCR-based techniques, they have a higher or similar detection limit (106 CFU/mL). These methods imply the need for expensive instrumentation and highly trained personnel, which may not be available in developing countries as a point-of-care test. In contrast to the developed MBDLFA, the in situ hybridization method does not provide information about the antimicrobial susceptibility [6,52]. It is worth mentioning that about 50% of bacteremia cases occur with a negative blood culture result due to antibiotic use or a low blood bacterial load, which will delay the introduction of proper treatment [28]. Molecular methods performed directly on blood allow the detection of bacteremia even if the blood culture is negative, especially after the start of antibiotic treatment [42,54].
Some PCR-based systems are commercially available but are intended mainly for identification purposes; some tests allow for MRSA detection directly from blood [6]. These blood-based MRSA detection tests depend mainly on real-time PCR or conventional PCR followed by sequencing, which requires expensive kits and instrumentation and trained personnel, which is not readily available in lower-resourced places.
All PCR-based assays suffer false positive results due to contaminating DNA from the used reagents or environment, DNA from dead bacteria (DNAemia), or infections already controlled by the immune system. Quantitative real-time PCR helps better interpret positive results, as the low abundance of bacteria usually characterizes contamination [28]. It is always a good practice to make parallel use of blood culture as the gold-standard method for diagnosis of bacteremia with PCR-based assays [51]. This will allow for the rapid detection of bacteremia with a high blood bacterial load and commencement of proper treatment within the first 2 h of sampling. The blood culture results can be used as a confirmatory tool. Using PCR-based methods for MRSA identification in bacteremia patients resulted in a timely shift to effective therapy, with shorter hospital stays and lower hospitalization costs [55].
5. Conclusions
The developed MBDLFA represents a rapid and cheap point-of-care diagnostic for simultaneous MRSA and vancomycin detection in bacteremia patients at the admission site and, consequently, prompt and proper treatment. This will reduce the morbidity and mortality rates associated with SAB and the hospitalization time and cost.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12112691/s1, Table S1: List of accession numbers of genomes used for primer design with the location of genes used. Figure S1: The developed assay is specific and successfully detected (A) Staphylococcus aureus ATCC 25923 with one single band at 192 bp, and (B) methicillin-resistant S. aureus strain S43 with two bands at 192 bp and 310 bp, and Enterococcus faecalis strain Y3 with a single band at 420 bp. (C) No PCR amplification products were detectable on agarose gel when testing other pathogens. Also, the developed multiplex-polymerase-reaction-based DNA lateral flow assay was specific and successfully detected (D) the nuc gene from the genomic DNA extracted from S. aureus ATCC 25923. No products were detectable when the assay was applied to other Gram-positive and Gram-negative pathogens as indicated in (E), where the assay was performed on genomic DNA extracted from Escherichia coli ATCC 25922. M: 100 bp DNA ladder, 1: S. aureus ATCC 25923, 2: methicillin-resistant S. aureus strain S43, 3: E. faecalis strain Y3, 4: Acinetobacter baumannii ATCC 19606, 5: E. coli ATCC 25922, 6: Enterococcus faecium ATCC 27270, 7: E. faecalis ATCC 19433, 8: Klebsiella pneumoniae ATCC 10031, and 9: Pseudomonas aeruginosa ATCC 27856.
Author Contributions
Conceptualization, O.M.H. and M.T.K.; methodology, O.M.H. and M.T.K.; investigation, O.M.H. and M.T.K.; resources, O.M.H. and M.T.K.; writing—original draft preparation, O.M.H. and M.T.K.; writing—review and editing, O.M.H. and M.T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Faculty of Pharmacy Cairo University-Cairo-Egypt (protocol code MI (2867) and approved on 28-12-2020).
Informed Consent Statement
Patient consent was waived due to collection of blood samples from healthy volunteers (author of the study).
Data Availability Statement
The data presented in this study are available in the article text and Supplementary Material.
Acknowledgments
The authors thank Yomna Hashem for providing the vancomycin-resistant E. faecalis strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laupland, K.B. Incidence of Bloodstream Infection: A Review of Population-Based Studies. Clin. Microbiol. Infect. 2013, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Al-Hasan, M.N. Overall Burden of Bloodstream Infection and Nosocomial Bloodstream Infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kern, W.V.; Rieg, S. Burden of Bacterial Bloodstream Infection—A Brief Update on Epidemiology and Significance of Multidrug-Resistant Pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, K.; Seifert, H.; Rieg, S.; Kern, W.V.; Scarborough, M.; Gordon, N.C.; Bin Kim, H.; Song, K.-H.; Tilley, R.; Gott, H.; et al. Survival Following Staphylococcus aureus Bloodstream Infection: A Prospective Multinational Cohort Study Assessing the Impact of Place of Care. J. Infect. 2018, 77, 516–525. [Google Scholar] [CrossRef] [PubMed]
- del Rio, A.; Cervera, C.; Moreno, A.; Moreillon, P.; Miró, J.M. Patients at Risk of Complications of Staphylococcus aureus Bloodstream Infection. Clin. Infect. Dis. 2009, 48, S246–S253. [Google Scholar] [CrossRef]
- Buonomini, A.R.; Riva, E.; di Bonaventura, G.; Gherardi, G. Rapid Detection of Methicillin-Resistant Staphylococcus aureus Directly from Blood for the Diagnosis of Bloodstream Infections: A Mini-Review. Diagnostics 2020, 10, 830. [Google Scholar] [CrossRef]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of Mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Dupper, A.C.; Malik, Y.; Gavioli, E.M.; Banga, J.; Caban, A.B.; Nadkarni, D.; Obla, A.; Vasa, C.V.; Mazo, D.; et al. Staphylococcus aureus Bacteremia in Patients Infected With COVID-19: A Case Series. Open Forum Infect. Dis. 2020, 7, ofaa518. [Google Scholar] [CrossRef]
- Kaasch, A.J.; Barlow, G.; Edgeworth, J.D.; Fowler, V.G.; Hellmich, M.; Hopkins, S.; Kern, W.V.; Llewelyn, M.J.; Rieg, S.; Rodriguez-Baño, J.; et al. Staphylococcus aureus Bloodstream Infection: A Pooled Analysis of Five Prospective, Observational Studies. J. Infect. 2014, 68, 242–251. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Kimmig, A.; Hagel, S.; Weis, S.; Bahrs, C.; Löffler, B.; Pletz, M.W. Management of Staphylococcus aureus Bloodstream Infections. Front. Med. 2021, 7, 616524. [Google Scholar] [CrossRef] [PubMed]
- Timbrook, T.T.; Morton, J.B.; McConeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 64, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Castro-Escarpulli, G.; AlonsoAguilar, N.M.; Sánchez, G.R.; Bocanegra-Garcia, V.; Guo, X.; Juárez-Enríquez, S.R.; Luna-Herrera, J.; Martínez, C.M.; Guadalupe, A.-A.M. Identification and Typing Methods for the Study of Bacterial Infectons: A Brief Review and Mycobacterial as Case of Study. Arch. Clin. Microbiol. 2015, 7, 1–10. [Google Scholar]
- Sharp, P.A.; Sugden, B.; Sambrook, J. Detection of Two Restriction Endonuclease Activities in Haemophilus Parainfluenzae Using Analytical Agarose-Ethidium Bromide Electrophoresis. Biochemistry 1973, 12, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Sato, T.; Niwa, K.; Kawase, M.; Tanner, A.C.R.; Takahashi, N. Rapid and Sensitive PCR-Dipstick DNA Chromatography for Multiplex Analysis of the Oral Microbiota. Biomed. Res. Int. 2014, 2014, 180323. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, Formats and Applications of Lateral Flow Assay: A Literature Review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Kosack, C.S.; Page, A.-L.; Klatser, P.R. A Guide to Aid the Selection of Diagnostic Tests. Bull World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef]
- Henderson, W.A.; Xiang, L.; Fourie, N.H.; Abey, S.K.; Ferguson, E.G.; Diallo, A.F.; Kenea, N.D.; Kim, C.H. Simple Lateral Flow Assays for Microbial Detection in Stool. Anal. Methods 2018, 10, 5358–5363. [Google Scholar] [CrossRef]
- Doyle, J.; Uthicke, S. Sensitive Environmental DNA Detection via Lateral Flow Assay (Dipstick)—A Case Study on Corallivorous Crown-of-thorns Sea Star (Acanthaster Cf. Solaris) Detection. Environ. DNA 2021, 3, 323–342. [Google Scholar] [CrossRef]
- Shanmugakani, R.K.; Akeda, Y.; Yamamoto, N.; Sakamoto, N.; Hagiya, H.; Yoshida, H.; Takeuchi, D.; Sugawara, Y.; Kodera, T.; Kawase, M.; et al. PCR-Dipstick Chromatography for Differential Detection of Carbapenemase Genes Directly in Stool Specimens. Antimicrob. Agents Chemother. 2017, 61, e00067-17. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by Polymerase Chain Reaction Amplification of the Nuc Gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.B.; Agrawal, P.; Kumar, S.; Kapila, K. Comparison of Cefoxitin Disc Diffusion Test, Oxacillin Screen Agar, and PCR for MecA Gene for Detection of MRSA. Indian J. Med. Microbiol. 2009, 27, 27–29. [Google Scholar] [CrossRef]
- Saber, T.; Samir, M.; El-Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.A.; Tartor, Y.H. Methicillin- and Vancomycin-Resistant Staphylococcus aureus From Humans and Ready-To-Eat Meat: Characterization of Antimicrobial Resistance and Biofilm Formation Ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef]
- Igbinosa, E.; Beshiru, A.; Akporehe, L.; Oviasogie, F.; Igbinosa, O. Prevalence of Methicillin-Resistant Staphylococcus aureus and Other Staphylococcus Species in Raw Meat Samples Intended for Human Consumption in Benin City, Nigeria: Implications for Public Health. Int. J. Environ. Res. Public Health 2016, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Vali, L.; Davies, S.E.; Lai, L.L.G.; Dave, J.; Amyes, S.G.B. Frequency of Biocide Resistance Genes, Antibiotic Resistance and the Effect of Chlorhexidine Exposure on Clinical Methicillin-Resistant Staphylococcus aureus Isolates. J. Antimicrob. Chemother. 2008, 61, 524–532. [Google Scholar] [CrossRef]
- Helmy, O.M.; Ragab, Y.M.; Hussein, M.M.M. Multiplex Polymerase Chain Reaction (PCR) for the Detection of Diarrheagenic Escherichia coli and Shigella Directly from Stool. Afr. J. Microbiol. Res. 2013, 7, 4368–4372. [Google Scholar]
- Mohamed, S.A.; Samir, T.M.; Helmy, O.M.; Elhosseiny, N.M.; Ali, A.A.; El-Kholy, A.A.; Attia, A.S. A Novel Surface-Exposed Polypeptide Is Successfully Employed as a Target for Developing a Prototype One-Step Immunochromatographic Strip for Specific and Sensitive Direct Detection of Staphylococcus aureus Causing Neonatal Sepsis. Biomolecules 2020, 10, 1580. [Google Scholar] [CrossRef]
- Opota, O.; Jaton, K.; Greub, G. Microbial Diagnosis of Bloodstream Infection: Towards Molecular Diagnosis Directly from Blood. Clin. Microbiol. Infect. 2015, 21, 323–331. [Google Scholar] [CrossRef]
- Maes, N.; Magdalena, J.; Rottiers, S.; de Gheldre, Y.; Struelens, M.J. Evaluation of a Triplex PCR Assay To Discriminate Staphylococcus aureus from Coagulase-Negative Staphylococci and Determine Methicillin Resistance from Blood Cultures. J. Clin. Microbiol. 2002, 40, 1514–1517. [Google Scholar] [CrossRef]
- Larsen, A.R.; Stegger, M.; Sørum, M. Spa Typing Directly from a MecA, Spa and Pvl Multiplex PCR Assay—A Cost-Effective Improvement for Methicillin-Resistant Staphylococcus aureus Surveillance. Clin. Microbiol. Infect. 2008, 14, 611–614. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Kochem, A.-J.; Disqué, C.; Mühl, H.; Gebert, S.; Winter, J.; Matten, J.; Sakka, S.G. Diagnosis of Bacteremia in Whole-Blood Samples by Use of a Commercial Universal 16S RRNA Gene-Based PCR and Sequence Analysis. J. Clin. Microbiol. 2009, 47, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Arunrut, N.; Kiatpathomchai, W.; Ananchaipattana, C. Multiplex PCR Assay and Lyophilization for Detection of Salmonella Spp., Staphylococcus aureus and Bacillus cereus in Pork Products. Food Sci. Biotechnol. 2018, 27, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Ji, Y. PCR-Based Approaches for the Detection of Clinical Methicillin-Resistant Staphylococcus aureus. Open Microbiol. J. 2016, 10, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global Prevalence and Distribution of Vancomycin Resistant, Vancomycin Intermediate and Heterogeneously Vancomycin Intermediate Staphylococcus aureus Clinical Isolates: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, T.T.; Martins, K.B.; Martins, P.Y.F.; de Oliveira, R.A.; Mondelli, A.L.; Fortaleza, C.M.C.B.; Cunha, M.D.L.R.D.S.D. Detection of the Mec A Gene and Identification of Staphylococcus Directly from Blood Culture Bottles by Multiplex Polymerase Chain Reaction. Braz. J. Infect. Dis. 2018, 22, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wiriyachaiporn, S.; Howarth, P.H.; Bruce, K.D.; Dailey, L.A. Evaluation of a Rapid Lateral Flow Immunoassay for Staphylococcus aureus Detection in Respiratory Samples. Diagn. Microbiol. Infect. Dis. 2013, 75, 28–36. [Google Scholar] [CrossRef]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New Quadriplex PCR Assay for Detection of Methicillin and Mupirocin Resistance and Simultaneous Discrimination of Staphylococcus aureus from Coagulase-Negative Staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef]
- McClure, J.-A.; Conly, J.M.; Obasuyi, O.; Ward, L.; Ugarte-Torres, A.; Louie, T.; Zhang, K. A Novel Assay for Detection of Methicillin-Resistant Staphylococcus aureus Directly from Clinical Samples. Front. Microbiol. 2020, 11, 1295. [Google Scholar] [CrossRef]
- Okolie, C.E.; Wooldridge, K.G.; Turner, D.P.J.; Cockayne, A.; James, R. Development of a Heptaplex PCR Assay for Identification of Staphylococcus aureus and CoNS with Simultaneous Detection of Virulence and Antibiotic Resistance Genes. BMC Microbiol. 2015, 15, 157. [Google Scholar] [CrossRef]
- Banada, P.P.; Chakravorty, S.; Shah, D.; Burday, M.; Mazzella, F.M.; Alland, D. Highly Sensitive Detection of Staphylococcus aureus Directly from Patient Blood. PLoS ONE 2012, 7, e31126. [Google Scholar] [CrossRef]
- Hirschhorn, J.W.; Schandl, C.A.; Nolte, F.S. Polymerase Chain Reaction And Other Nucleic Acid Amplification Technology. In Henry’s Clinical Diagnosis and Management by Laboratory Methods; McPherson, R.A., Pincus, M.R., Eds.; Elsevier: Philadelphia, PA, USA, 2022; pp. 1387–1400. [Google Scholar]
- Peters, R.P.H.; van Agtmael, M.A.; Gierveld, S.; Danner, S.A.; Groeneveld, A.B.J.; Vandenbroucke-Grauls, C.M.J.E.; Savelkoul, P.H.M. Quantitative Detection of Staphylococcus aureus and Enterococcus Faecalis DNA in Blood To Diagnose Bacteremia in Patients in the Intensive Care Unit. J. Clin. Microbiol. 2007, 45, 3641–3646. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Siegel, D.; Gebert, S.; Winter, J. Rapid Detection of Staphylococcus aureus Bacteremia and Methicillin Resistance by Real-Time PCR in Whole Blood Samples. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Rohrman, B.A.; Leautaud, V.; Molyneux, E.; Richards-Kortum, R.R. A Lateral Flow Assay for Quantitative Detection of Amplified HIV-1 RNA. PLoS ONE 2012, 7, e45611. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Miyagi, C.; Tamaki, Y.; Mizuno, T.; Ezaki, T. Development of a Rapid Diagnostic Method for Identification of Staphylococcus aureus and Antimicrobial Resistance in Positive Blood Culture Bottles Using a PCR-DNA-Chromatography Method. J. Infect. Chemother. 2016, 22, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Noguera, P.; Posthuma-Trumpie, G.A.; van Tuil, M.; van der Wal, F.J.; de Boer, A.; Moers, A.P.H.A.; van Amerongen, A. Carbon Nanoparticles in Lateral Flow Methods to Detect Genes Encoding Virulence Factors of Shiga Toxin-Producing Escherichia coli. Anal. Bioanal. Chem. 2011, 399, 831–838. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Fu, S.; Hu, S.; Wang, Y.; Xu, J.; Ye, C. Multiple Cross Displacement Amplification Coupled With Nanoparticles-Based Lateral Flow Biosensor for Detection of Staphylococcus aureus and Identification of Methicillin-Resistant S. aureus. Front. Microbiol. 2018, 9, 907. [Google Scholar] [CrossRef]
- Mason, W.J.; Blevins, J.S.; Beenken, K.; Wibowo, N.; Ojha, N.; Smeltzer, M.S. Multiplex PCR Protocol for the Diagnosis of Staphylococcal Infection. J. Clin. Microbiol. 2001, 39, 3332–3338. [Google Scholar] [CrossRef]
- Khatib, R.; Riederer, K.; Saeed, S.; Johnson, L.B.; Fakih, M.G.; Sharma, M.; Tabriz, M.S.; Khosrovaneh, A. Time to Positivity in Staphylococcus aureus Bacteremia: Possible Correlation with the Source and Outcome of Infection. Clin. Infect. Dis. 2005, 41, 594–598. [Google Scholar] [CrossRef]
- Afshari, A.; Schrenzel, J.; Ieven, M.; Harbarth, S. Bench-to-Bedside Review: Rapid Molecular Diagnostics for Bloodstream Infection—A New Frontier? Crit. Care 2012, 16, 222. [Google Scholar] [CrossRef]
- Chen, K.; Malik, A.A.; Sheng, Y.-J.; Ahmed, S.; Sun, C.; Deng, C.-L.; Ojha, S.C. Clinical Utility of Molecular Tests for Guiding Therapeutic Decisions in Bloodstream Staphylococcal Infections: A Meta-Analysis. Front. Pediatr. 2021, 9, 713447. [Google Scholar] [CrossRef]
- Peker, N.; Couto, N.; Sinha, B.; Rossen, J.W. Diagnosis of Bloodstream Infections from Positive Blood Cultures and Directly from Blood Samples: Recent Developments in Molecular Approaches. Clin. Microbiol. Infect. 2018, 24, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Towns, M.L.; Jarvis, W.R.; Hsueh, P.-R. Guidelines on Blood Cultures. J. Microbiol. Immunol. Infect. 2010, 43, 347–349. [Google Scholar] [CrossRef]
- Wheeler, J.; Murphy, O.M.; Freeman, R.; Kearns, A.M.; Steward, M.; Lee, M.J.S. PCR Can Add to Detection of Pneumococcal Disease in Pneumonic Patients Receiving Antibiotics at Admission. J. Clin. Microbiol. 2000, 38, 3907. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; West, J.E.; Balada-Llasat, J.; Pancholi, P.; Stevenson, K.B.; Goff, D.A. An Antimicrobial Stewardship Program’s Impact with Rapid Polymerase Chain Reaction Methicillin-Resistant Staphylococcus aureus/S. aureus Blood Culture Test in Patients with S. aureus Bacteremia. Clin. Infect. Dis. 2010, 51, 1074–1080. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).