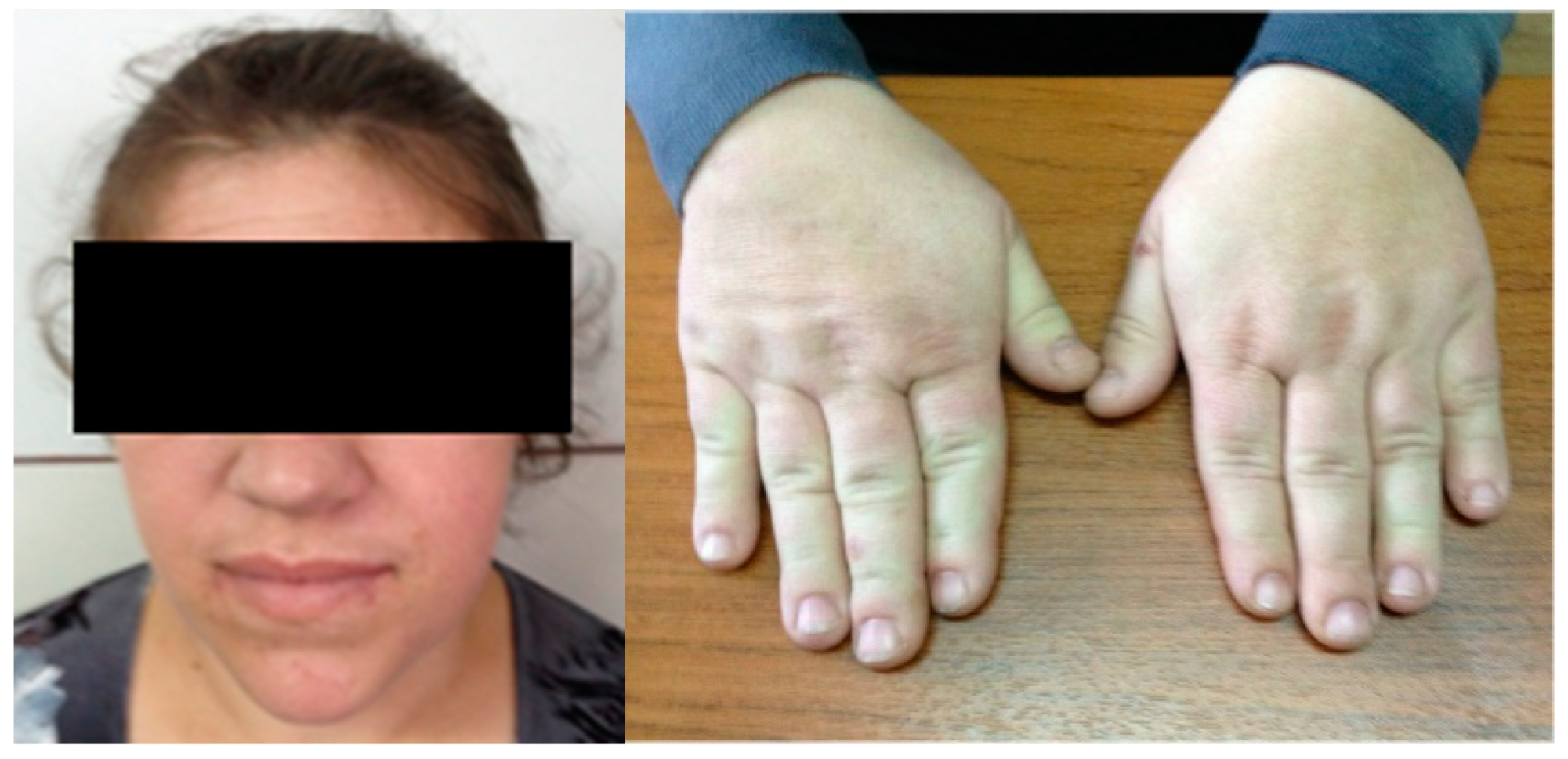
Approach of Acromegaly during Pregnancy
Abstract
1. Introduction
Aim
2. Methods
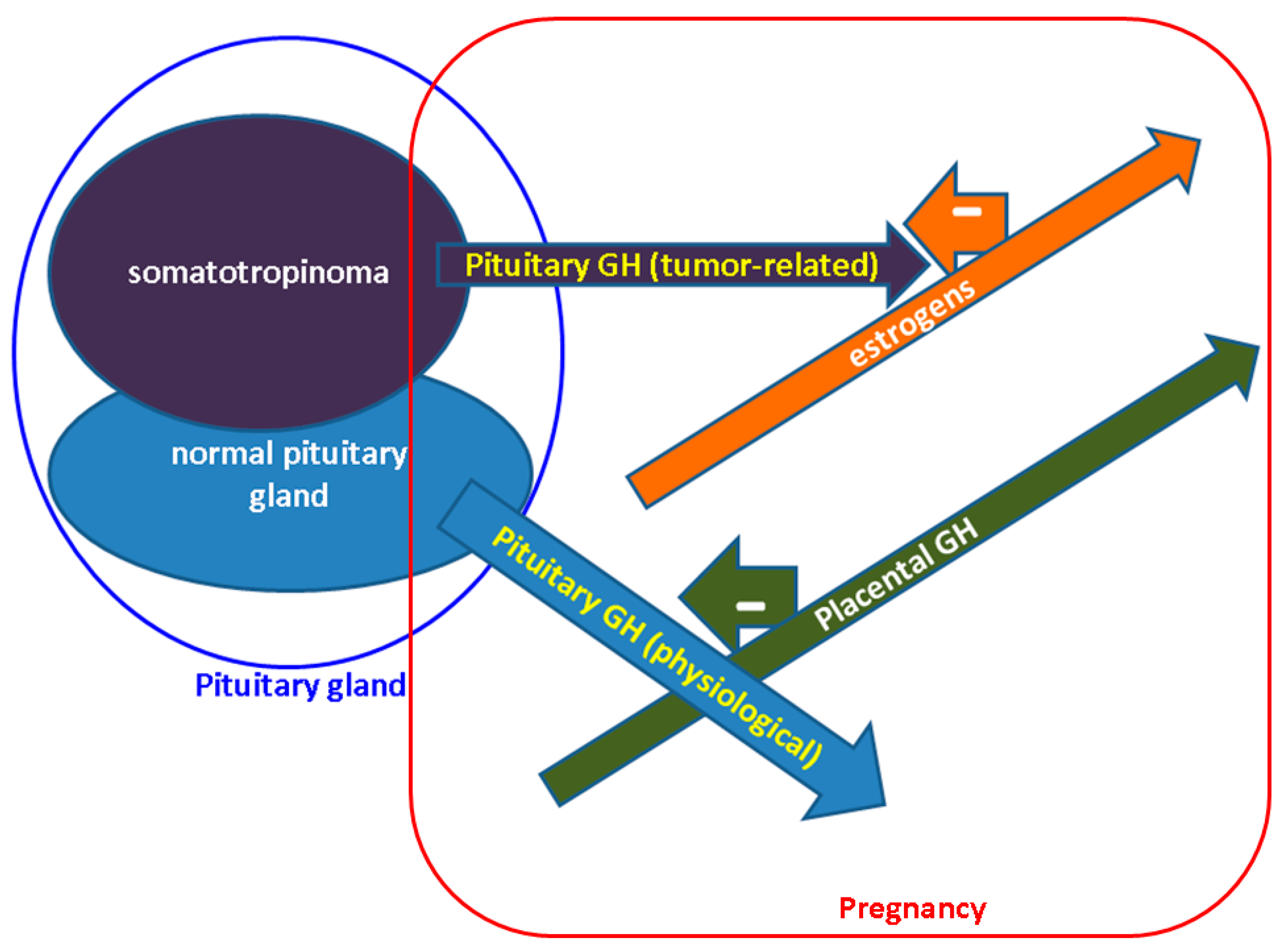
3. GH-IGF1 Axes during Pregnancy: Non-Acromegalic and Acromegalic Females
4. Sub/Infertility Issues in Acromegaly
5. Cardio-Metabolic Features in Pregnant Females with Acromegaly
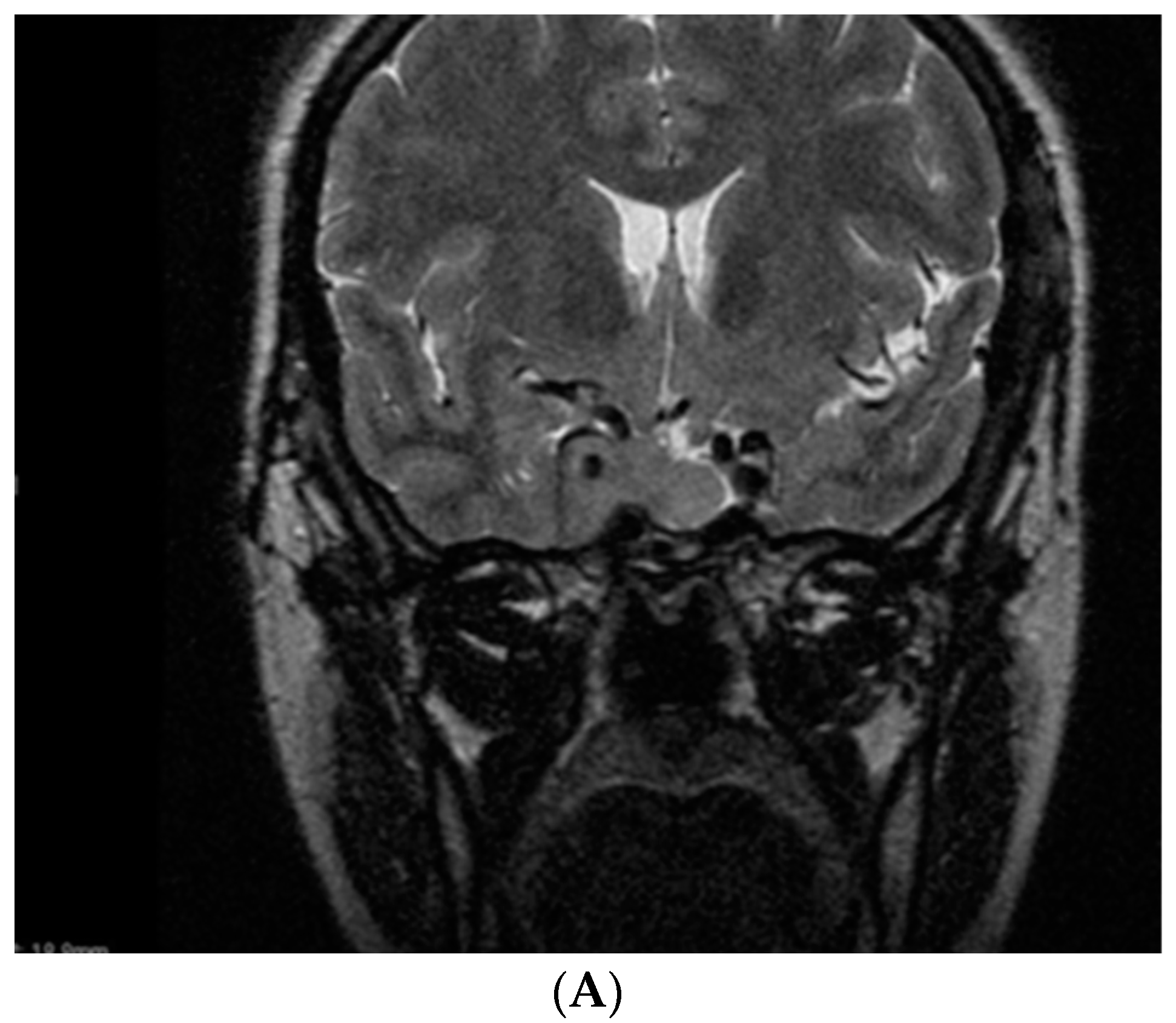
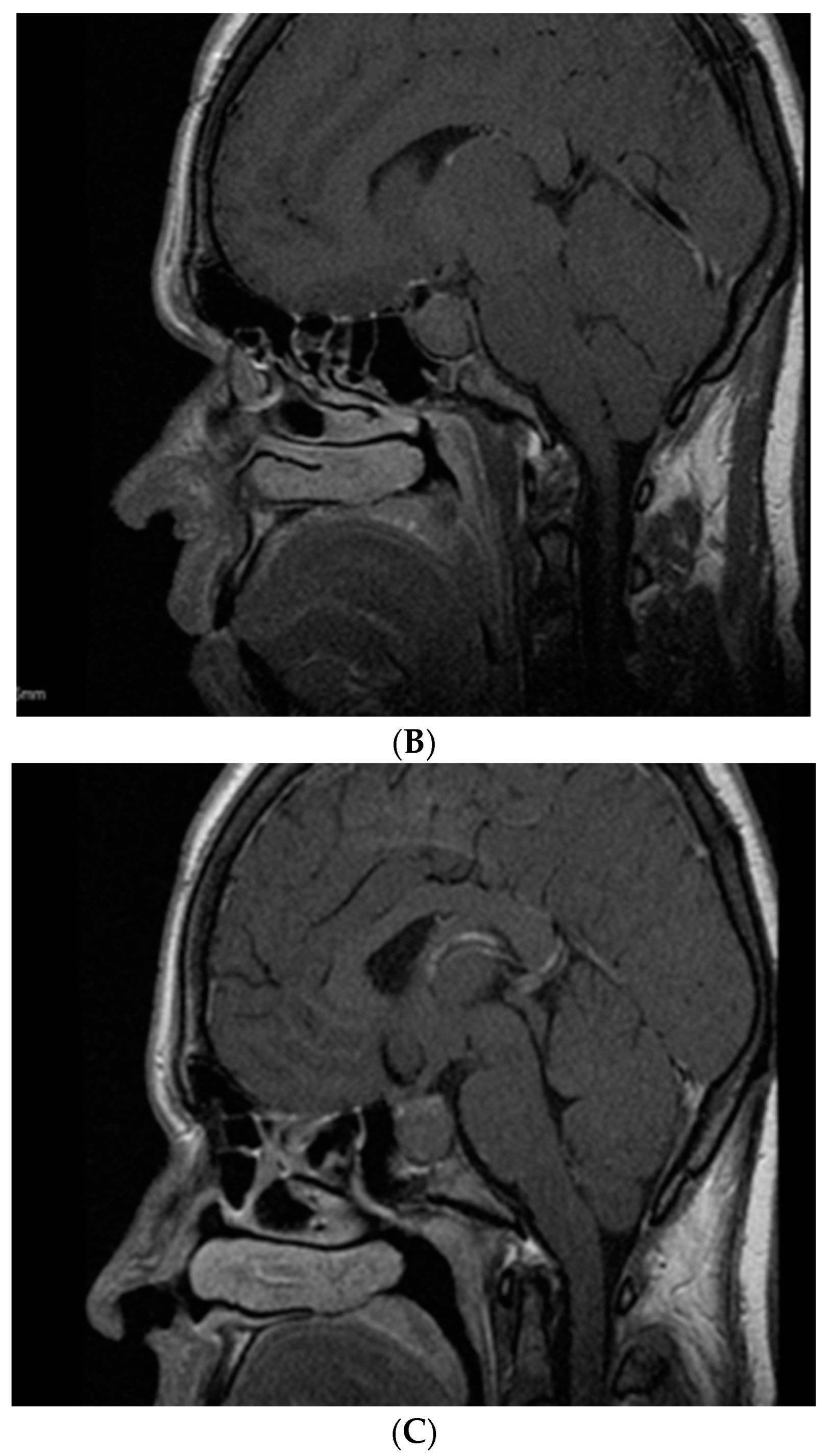
6. Somatotropinoma Evolution during Gestation
7. Specific Medical Therapy for Acromegaly during Pregnancy
8. Prolactin, DAs and Pregnancy
9. Pregnancy Outcome and Materno-Fetal Complications
10. Discussion
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIP | aryl hydrocarbon receptor-interacting protein |
| AMH | anti-Mullerian hormone |
| DA | dopamine analogues |
| DM | diabetes mellitus |
| FSH | Follicle Stimulating Hormone |
| GH | Growth Hormone |
| GnRH | Gonadotropin Releasing Hormone |
| GH-RH | GH Releasing Hormone |
| HBP | high blood pressure |
| IGF1 | Insulin-like Growth Factor |
| IGFBP | IGF Binding Protein |
| IQ | intelligence quotient |
| LH | Luteinizing Hormone |
| SSA | somatostatin analogues |
| SSTR | somatostatin receptors |
| SERM | selective estrogen receptor modulator |
References
- Daly, A.F.; Beckers, A. The Epidemiology of Pituitary Adenomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 347–355. [Google Scholar] [CrossRef]
- Lavrentaki, A.; Paluzzi, A.; Wass, J.A.; Karavitaki, N. Epidemiology of acromegaly: Review of population studies. Pituitary 2017, 20, 4–9. [Google Scholar] [CrossRef]
- Dal, J.; Feldt-Rasmussen, U.; Andersen, M.; Kristensen, L.Ø.; Laurberg, P.; Pedersen, L.; Dekkers, O.M.; Sørensen, H.T.; Jørgensen, J.O. Acromegaly incidence, prevalence, complications and long-term prognosis: A nationwide cohort study. Eur. J. Endocrinol. 2016, 175, 181–190. [Google Scholar] [CrossRef]
- Ganz, J.C. Pituitary adenomas. Prog. Brain Res. 2022, 268, 191–215. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Gagliardi, I.; Chiloiro, S.; Ferreira, A.G.; Bondanelli, M.; Giampietro, A.; Bianchi, A.; Marinis, L.; Fleseriu, M.; Zatelli, M.C. Acromegaly in the elderly patients. Endocrine 2020, 68, 16–31. [Google Scholar] [CrossRef]
- Lamas, C.; García-Martínez, A.; Cámara, R.; Fajardo-Montanana, C.; Viguera, L.; Aranda, I. Silent somatotropinomas. Minerva Endocrinol. 2019, 44, 137–142. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, E.J.; Seo, G.H.; Ku, C.R. Risk for Acromegaly-related Comorbidities by Sex in Korean Acromegaly. J. Clin. Endocrinol. Metab. 2020, 105, dgz317. [Google Scholar] [CrossRef]
- Guo, X.; Fu, H.; Pang, H.; Xing, B. Risk of left ventricular hypertrophy and diastolic and systolic dysfunction in Acromegaly: A meta-analysis. J. Clin. Neurosci. 2018, 48, 28–33. [Google Scholar] [CrossRef]
- Popescu, M.; Carsote, M.; Popescu, I.A.S.; Costache, A.; Ghenea, A.; Turculeanu, A.; Singer, C.E.; Iana, O.; Ungureanu, A. The role of the visual evoked potentials in diagnosing and monitoring pituitary adenomas. Reasearch Sci. Today 2021, 21, 27–38. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, L.; Yu, Y.; Wang, C.; Li, J. Acromegaly and non-parathyroid hormone-dependent hypercalcemia: A case report and literature review. BMC Endocr. Disord. 2021, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tan, H.; Huang, H.; Li, J. Advances in Research on the Cardiovascular Complications of Acromegaly. Front. Oncol. 2021, 11, 640999. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ram, N.; Masood, M.Q. Patterns of Abnormal Glucose Metabolism in Acromegaly and Impact of Treatment Modalities on Glucose Metabolism. Cureus 2021, 13, e13852. [Google Scholar] [CrossRef]
- Kamenický, P.; Maione, L.; Chanson, P. Cardiovascular complications of acromegaly. Ann. Endocrinol. 2021, 82, 206–209. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.; Cosentino, G.; Ganji, S.; Riera-Gonzalez, A.; Hsia, D.S. Endocrine Causes of Hypertension. Curr. Hypertens Rep. 2020, 22, 97. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, O.J.; Cheema, A.Y.; Khan, M.A.; Junaid, S.Z.S.; Erebo, J.K.; Ayirebi-Acquah, E.; Okpara, J.; Bofah, D.; Okon, J.G.; Munir, M.; et al. A Comprehensive Review of Four Clinical Practice Guidelines of Acromegaly. Cureus 2022, 14, e28722. [Google Scholar] [CrossRef]
- Fleseriu, M.; Biller, B.M.K.; Freda, P.U.; Gadelha, M.R.; Giustina, A.; Katznelson, L.; Molitch, M.E.; Samson, S.L.; Strasburger, C.J.; van der Lely, A.J.; et al. A Pituitary Society update to acromegaly management guidelines. Pituitary 2021, 24, 1–13. [Google Scholar] [CrossRef]
- Giustina, A.; Barkhoudarian, G.; Beckers, A.; Ben-Shlomo, A.; Biermasz, N.; Biller, B.; Boguszewski, C.; Bolanowski, M.; Bollerslev, J.; Bonert, V.; et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020, 21, 667–678. [Google Scholar] [CrossRef]
- Giustina, A.; Barkan, A.; Beckers, A.; Biermasz, N.; Biller, B.M.K.; Boguszewski, C.; Bolanowski, M.; Bonert, V.; Bronstein, M.D.; Casanueva, F.F.; et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J. Clin. Endocrinol Metab. 2020, 105, dgz096. [Google Scholar] [CrossRef]
- Chin, S.O.; Ku, C.R.; Kim, B.J.; Kim, S.W.; Park, K.H.; Song, K.H.; Oh, S.; Yoon, H.K.; Lee, E.J.; Lee, J.M.; et al. Medical Treatment with Somatostatin Analogues in Acromegaly: Position Statement. Endocrinol. Metab. 2019, 34, 53–62. [Google Scholar] [CrossRef]
- Ghemigian, A.; Cocolos, A.; Petrova, E.; Valea, A.; Dumitru, N.; Carsote, M. New cross-roads for second line medical therapy in acromegaly. Arch. Balk Med. Union. 2018, 53, 117–125. [Google Scholar]
- Kasuki, L.; Antunes, X.; Lamback, E.B.; Gadelha, M.R. Acromegaly: Update on Management and Long-Term Morbidities. Endocrinol. Metab. Clin. N. Am. 2020, 49, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chacko, A.G. Surgery for Acromegaly. Neurol. India 2020, 68, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A. Endocrine Society. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef] [PubMed]
- Bolfi, F.; Neves, A.F.; Boguszewski, C.L.; Nunes-Nogueira, V.S. Mortality in acromegaly decreased in the last decade: A systematic review and meta-analysis. Eur. J. Endocrinol. 2018, 179, 59–71. [Google Scholar] [CrossRef]
- Huang, W.; Molitch, M.E. Pituitary Tumors in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 569–581. [Google Scholar] [CrossRef]
- Araujo, P.B.; Vieira Neto, L.; Gadelha, M.R. Pituitary tumor management in pregnancy. Endocrinol. Metab. Clin. N. Am. 2015, 44, 181–197. [Google Scholar] [CrossRef]
- Laway, B.A.; Mir, S.A. Pregnancy and pituitary disorders: Challenges in diagnosis and management. Indian J. Endocrinol. Metab. 2013, 17, 996–1004. [Google Scholar] [CrossRef]
- Valassi, E. Acromegaly and pregnancy. Endocrinol. Nutr. 2013, 60, 1–3. [Google Scholar] [CrossRef]
- Valassi, E. Pituitary disease and pregnancy. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 184–195. [Google Scholar] [CrossRef]
- Szlapinski, S.K.; Hill, D.J. Metabolic Adaptations to Pregnancy in Healthy and Gestational Diabetic Pregnancies: The Pancreas-Placenta Axis. Curr. Vasc. Pharmacol. 2021, 19, 141–153. [Google Scholar] [CrossRef]
- Bowe, J.E.; Hill, T.G.; Hunt, K.F.; Smith, L.I.; Simpson, S.J.; Amiel, S.A.; Jones, P.M. A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight 2019, 4, e124540. [Google Scholar] [CrossRef] [PubMed]
- Moyce, B.L.; Dolinsky, V.W. Maternal β-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3467. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Muhlhausler, B.S.; Roberts, C.T.; Gatford, K.L. The growth hormone-insulin like growth factor axis in pregnancy. J. Endocrinol. 2021, 251, R23–R39. [Google Scholar] [CrossRef]
- Smith, A.; Woodside, B.; Abizaid, A. Ghrelin and the Control of Energy Balance in Females. Front. Endocrinol. 2022, 13, 904754. [Google Scholar] [CrossRef] [PubMed]
- Persechini, M.L.; Gennero, I.; Grunenwald, S.; Vezzosi, D.; Bennet, A.; Caron, P. Decreased IGF-1 concentration during the first trimester of pregnancy in women with normal somatotroph function. Pituitary 2015, 18, 461–464. [Google Scholar] [CrossRef]
- Muhammad, A.; Neggers, S.J.; van der Lely, A.J. Pregnancy and acromegaly. Pituitary 2017, 20, 179–184. [Google Scholar] [CrossRef]
- Dobolyi, A.; Lékó, A.H. The insulin-like growth factor-1 system in the adult mammalian brain and its implications in central maternal adaptation. Front. Neuroendocrinol. 2019, 52, 181–194. [Google Scholar] [CrossRef]
- Laway, B.A. Pregnancy in acromegaly. Adv. Endocrinol. Metab. 2015, 6, 267–272. [Google Scholar] [CrossRef]
- Dias, M.; Boguszewski, C.; Gadelha, M.; Kasuki, L.; Musolino, N.; Vieira, J.G.; Abucham, J. Acromegaly and pregnancy: A prospective study. Eur. J. Endocrinol. 2013, 170, 301–310. [Google Scholar] [CrossRef]
- Dias, M.L.; Vieira, J.G.; Abucham, J. Detecting and solving the interference of pregnancy serum, in a GH immunometric assay. Growth Horm. IGF Res. 2013, 23, 13–18. [Google Scholar] [CrossRef]
- Sperling, M.A. Traditional and novel aspects of the metabolic actions of growth hormone. Growth Horm. IGF Res. 2016, 28, 69–75. [Google Scholar] [CrossRef]
- Cheng, V.; Faiman, C.; Kennedy, L.; Khoury, F.; Hatipoglu, B.; Weil, R.; Hamrahian, A. Pregnancy and acromegaly: A review. Pituitary 2012, 15, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.E.; Blyth, A.J.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef] [PubMed]
- Blyth, A.; Ortiz, M.; Merriman, A.; Delaine, C.; Forbes, B. Determinants of IGF-II influencing stability, receptor binding and activation. Sci. Rep. 2022, 12, 4695. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, R.; Zhang, D.; Wang, Z.; Gao, L.; Yao, Y.; Deng, K.; Bao, X.; Feng, M.; Xu, Z.; et al. Hyperprolactinemia and Hypopituitarism in Acromegaly and Effect of Pituitary Surgery: Long-Term Follow-up on 529 Patients. Front. Endocrinol. 2022, 12, 807054. [Google Scholar] [CrossRef]
- Salvio, G.; Martino, M.; Balercia, G.; Arnaldi, G. Acromegaly and male sexual health. Rev. Endocr. Metab. Disord. 2022, 23, 671–678. [Google Scholar] [CrossRef]
- Salvio, G.; Martino, M.; Giancola, G.; Arnaldi, G.; Balercia, G. Hypothalamic-Pituitary Diseases and Erectile Dysfunction. J. Clin. Med. 2021, 10, 2551. [Google Scholar] [CrossRef]
- Raju, J.A.; Shipman, K.E.; Inglis, J.A.; Gama, R. Acromegaly Presenting as Erectile Dysfunction: Case Reports and Review of the Literature. Rev. Urol. 2015, 17, 246–249. [Google Scholar]
- Nishio, R.; Takeshita, A.; Uchida, T.; Herai, T.; Sakamoto, K.; Shimizu, Y.; Arai, M.; Tatsushima, K.; Fukuhara, N.; Okada, M.; et al. GH-induced LH hyporesponsiveness as a potential mechanism for hypogonadism in male patients with acromegaly. Endocr. J. 2021, 68, 953–968. [Google Scholar] [CrossRef]
- Marques, J.V.O.; Boguszewski, C.L. Fertility issues in aggressive pituitary tumors. Rev. Endocr. Metab. Disord. 2020, 21, 225–233. [Google Scholar] [CrossRef]
- Căpăþînă, C.; Radian, Ş.; Baciu, I.; Ghinea, A.; Deciu, D.; Dumitraşcu, A.; Ciubotaru, V.; Poiană, C. Spontaneous conception and term delivery in a woman with uncontrolled acromegaly and hypogonadotropic hypogonadism. Acta Endocrinol. 2016, 12, 481–484. [Google Scholar] [CrossRef]
- Schernthaner-Reiter, M.H.; Wolf, P.; Vila, G.; Luger, A. The Interaction of Insulin and Pituitary Hormone Syndromes. Front. Endocrinol. 2021, 12, 626427. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kalra, S.; Dutta, D.; Khandelwal, D.; Singla, R. The Interplay Between Pituitary Health and Diabetes Mellitus-The Need for ‘Hypophyseo-Vigilance’. Eur. Endocrinol. 2020, 16, 25–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogowicz-Frontczak, A.; Majchrzak, A.; Zozulińska-Ziółkiewicz, D. Insulin resistance in endocrine disorders-treatment options. Endokrynol. Pol. 2017, 68, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Giantini-Larsen, A.M.; Uribe-Cardenas, R.; Juthani, R.G. Acromegaly: Medical and Surgical Considerations. Otolaryngol. Clin. N. Am. 2022, 55, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jørgensen, N. Semen quality in hypogonadal acromegalic patients. Pituitary 2020, 23, 160–166. [Google Scholar] [CrossRef]
- Yilmaz, M.K.; Sulu, C.; Ozkaya, H.M.; Kadioglu, A.; Ortac, M.; Kadioglu, P. Evaluation of sex hormone profile and semen parameters in acromegalic male patients. J. Endocrinol. Investig. 2021, 44, 2799–2808. [Google Scholar] [CrossRef]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jørgensen, N. Semen quality in patients with pituitary disease and adult-onset hypogonadotropic hypogonadism. Endocr. Connect. 2018, 7, 523–533. [Google Scholar] [CrossRef]
- Duarte, F.H.; Jallad, R.S.; Bronstein, M.D. Clomiphene citrate for treatment of acromegaly not controlled by conventional therapies. J. Clin. Endocrinol. Metab. 2015, 100, 1863–1869. [Google Scholar] [CrossRef][Green Version]
- Palacios, J.D.; Komotar, R.J.; Kargi, A.Y. Successful Treatment of Acromegaly and Associated Hypogonadism with First-Line Clomiphene Therapy. Case Rep. Endocrinol. 2018, 2018, 7925019. [Google Scholar] [CrossRef]
- Duarte, F.H.; Jallad, R.S.; Bronstein, M.D. Estrogens and selective estrogen receptor modulators in acromegaly. Endocrine 2016, 54, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hur, K.Y.; Lee, J.H.; Lee, J.H.; Se, Y.B.; Kim, H.I.; Lee, S.H.; Nam, D.H.; Kim, S.Y.; Kim, K.W.; et al. Outcome of Endoscopic Transsphenoidal Surgery for Acromegaly. World Neurosurg. 2017, 104, 272–278. [Google Scholar] [CrossRef]
- Crespo, I.; Valassi, E.; Santos, A.; Webb, S.M. Health-related quality of life in pituitary diseases. Endocrinol. Metab. Clin. N. Am. 2015, 44, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.; Hunter, S.; Bradley, L.; Chahal, H.S.; Storr, H.L.; Akker, S.A.; Kumar, A.V.; Orme, S.M.; Evanson, J.; Abid, N.; et al. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. J. Clin. Endocrinol. Metab. 2014, 99, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.T.; Fleseriu, M. Personalized Medical Treatment of Patients With Acromegaly: A Review. Endocr. Pract. 2022, 28, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Costa, D.; Lauretta, R.; Mercuri, V.; Sbardella, E.; Samperi, I.; Appetecchia, M.; Bianchi, A.; Giampietro, A.; Gargiulo, P.; et al. Partial response to first generation SSA guides the choice and predict the outcome of second line therapy in acromegaly. Endocrine 2022, 78, 343–353. [Google Scholar] [CrossRef]
- Mondin, A.; Manara, R.; Voltan, G.; Tizianel, I.; Denaro, L.; Ferrari, M.; Barbot, M.; Scaroni, C.; Ceccato, F. Pasireotide-Induced Shrinkage in GH and ACTH Secreting Pituitary Adenoma: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 935759. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Kasuki, L.; Lim, D.S.T.; Fleseriu, M. Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update. Endocr. Rev. 2019, 40, 268–332. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Netea, M.G.; Riksen, N.P.; Hermus, A.R.M.M.; Netea-Maier, R.T. Acromegaly, inflammation and cardiovascular disease: A review. Rev. Endocr. Metab. Disord. 2020, 21, 547–568. [Google Scholar] [CrossRef]
- Jang, H.N.; Kim, Y.H.; Kim, J.H. Diabetes Mellitus Predicts Weight Gain After Surgery in Patients With Acromegaly. Front. Endocrinol. 2022, 13, 854931. [Google Scholar] [CrossRef]
- Sturma, J. Rare twin pregnancy and acromegalia. Cesk Gynekol. 1949, 14, 299–305. [Google Scholar] [PubMed]
- Abelove, W.A.; Rupp, J.J.; Paschkis, K.E. Acromegaly and pregnancy. J. Clin. Endocrinol. Metab. 1954, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Finkler, R.S. Acromegaly and pregnancy: Case report. J. Clin. Endocrinol. Metab. 1954, 14, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Maffezzoni, F.; Frara, S.; Doga, M.; Mazziotti, G.; Giustina, A. New medical therapies of acromegaly. Growth Horm. IGF Res. 2016, 30–31, 58–63. [Google Scholar] [CrossRef]
- Das, L.; Dutta, P.; Thirunavukkarasu, B.; Gupta, K.; Tripathi, M.; Gupta, P.; Aggarwal, N.; Rai, A.; Radotra, B.D.; Bhansali, A.; et al. Course and outcomes of pregnancy in women treated for acromegaly: Discerning a contemporary cohort. Growth Horm. IGF Res. 2021, 60–61, 101417. [Google Scholar] [CrossRef]
- Dogansen, S.C.; Tanrikulu, S.; Yalin, G.Y.; Yarman, S. Female gonadal functions and ovarian reserve in patients with acromegaly: Experience from a single tertiary center. Endocrine 2018, 60, 167–174. [Google Scholar] [CrossRef]
- Vialon, M.; Grunenwald, S.; Mouly, C.; Vezzosi, D.; Bennet, A.; Gourdy, P.; Caron, P.J. Gestational diabetes and acromegaly: Single-centre experience of 14 pregnancies. Clin. Endocrinol. 2019, 91, 805–809. [Google Scholar] [CrossRef]
- Jallad, R.S.; Shimon, I.; Fraenkel, M.; Medvedovsky, V.; Akirov, A.; Duarte, F.H.; Bronstein, M.D. Outcome of pregnancies in a large cohort of women with acromegaly. Clin. Endocrinol. 2018, 88, 896–907. [Google Scholar] [CrossRef]
- O’Shea, P.M.; Griffin, T.P.; Fitzgibbon, M. Hypertension: The role of biochemistry in the diagnosis and management. Clin. Chim Acta. 2017, 465, 131–143. [Google Scholar] [CrossRef]
- Blonde, L.; Umpierrez, G.E.; McGill, J.B.; Reddy, S.S.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan—2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef]
- Angras, K.; Sullivan, M.; Young, A.J.; Paglia, M.J.; Mackeen, A.D. A retrospective review of pregnancy outcomes in women with uncomplicated mild to moderate chronic hypertension. J. Matern. Fetal. Neonatal. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Affinati, A.H.; Auchus, R.J. Endocrine causes of hypertension in pregnancy. Gland Surg. 2020, 9, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Puig-Domingo, M.; Marazuela, M. Precision medicine in the treatment of acromegaly. Minerva Endocrinol. 2019, 44, 169–175. [Google Scholar] [CrossRef]
- Abreu, A.; Tovar, A.P.; Castellanos, R.; Valenzuela, A.; Giraldo, C.M.; Pinedo, A.C.; Guerrero, D.P.; Barrera, C.A.; Franco, H.I.; Ribeiro-Oliveira, A., Jr.; et al. Challenges in the diagnosis and management of acromegaly: A focus on comorbidities. Pituitary 2016, 19, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Valea, A.; Dumitrascu, M.C.; Albu, S.E.; Sandru, F. Pituitary non-functioning macroadenomas: If and when to recommend surgery. Rom. Med. J. 2019, 66, 430–433. [Google Scholar] [CrossRef]
- Hill, D.J. Placental control of metabolic adaptations in the mother for an optimal pregnancy outcome. What goes wrong in gestational diabetes? Placenta 2018, 69, 162–168. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Bandeira, D.B.; Olivatti, T.O.F.; Bolfi, F.; Boguszewski, C.L. Dos Santos Nunes-Nogueira, V. Acromegaly and pregnancy: A systematic review and meta-analysis. Pituitary 2022, 25, 352–362. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Berrocal, V.R.; Pascual-Corrales, E. Pituitary tumors: Epidemiology and clinical presentation spectrum. Hormones 2020, 19, 145–155. [Google Scholar] [CrossRef]
- Sammet, S. Magnetic resonance safety. Abdom Radiol. 2016, 41, 444–451. [Google Scholar] [CrossRef]
- Little, J.T.; Bookwalter, C.A. Magnetic Resonance Safety: Pregnancy and Lactation. Magn. Reson Imaging Clin. N. Am. 2020, 28, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bulas, D.; Egloff, A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013, 37, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Koshy, T.G.; Rajaratnam, S.; Mathews, J.E.; Rajshekhar, V. Acromegaly in pregnancy. Indian J. Endocrinol. Metab. 2012, 16, 1029–1031. [Google Scholar] [CrossRef]
- Kasuki, L.; Neto, L.V.; Takiya, C.M.; Gadelha, M.R. Growth of an aggressive tumor during pregnancy in an acromegalic patient. Endocr. J. 2012, 59, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Meoni, G.; Giommoni, E.; Petreni, P.; Pillozzi, S.; Mazzoni, F.; Pellegrini, E.; Brugia, M.; Lunghi, A.; Muto, A.; Antonuzzo, L. Somatostatin analogs in pregnant patients with neuroendocrine tumor. Anticancer Drugs 2020, 31, 1096–1098. [Google Scholar] [CrossRef] [PubMed]
- Hannon, A.M.; Frizelle, I.; Kaar, G.; Hunter, S.J.; Sherlock, M.; Thompson, C.J.; O’Halloran, D.J.; Irish Pituitary Database Group. Octreotide use for rescue of vision in a pregnant patient with acromegaly. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0019. [Google Scholar] [CrossRef] [PubMed]
- Dicuonzo, F.; Purciariello, S.; De Marco, A.; Guastamacchia, E.; Triggiani, V. Inoperable Giant Growth Hormone-secreting Pituitary Adenoma: Radiological Aspects, Clinical Management and Pregnancy Outcome. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 214–220. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M.; Ghervan, C.; Georgescu, C. Glycemic profile in patients with acromegaly treated with somatostatin analogue. J. Med. Life. 2015, 8, 79–83. [Google Scholar]
- Valea, A.; Ghervan, C.; Carsote, M.; Morar, A.; Iacob, I.; Tomesc, F.; Pop, D.D.; Georgescu, C. Effects of combination therapy: Somatostatin analogues and dopamine agonists on GH and IGF1 levels in acromegaly. Clujul Medical. 2015, 88, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Albarel, F.; Cuny, T.; Graillon, T.; Dufour, H.; Brue, T.; Castinetti, F. Preoperative Medical Treatment for Patients With Acromegaly: Yes or No? J. Endocr. Soc. 2022, 6, bvac114. [Google Scholar] [CrossRef] [PubMed]
- Inder, W.J.; Jang, C. Treatment of Prolactinoma. Medicina 2022, 58, 1095. [Google Scholar] [CrossRef] [PubMed]
- Teltayev, D.; Akshulakov, S.; Ryskeldiev, N.; Mustafin, K.; Vyacheslav, L. Pregnancy in women after successful acromegaly treatment, including surgical removal of pituitary adenoma and postoperative therapy using lanreotide acetate. Gynecol. Endocrinol. 2017, 33 (Suppl. S1), 50–51. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Bianchi, A.; Giampietro, A.; Pontecorvi, A.; Raverot, G.; Marinis, L. Second line treatment of acromegaly: Pasireotide or Pegvisomant? Best Pract. Res. Clin. Endocrinol. Metab. 2022, 101684. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Antonini, S.; Saladino, A.; Lavezzi, E.; Zampetti, B.; Cozzi, R. Clinical Management of Acromegaly: Therapeutic Frontiers and New Perspectives for Somatostatin Receptor Ligands (SRLs). Medicina 2022, 58, 794. [Google Scholar] [CrossRef] [PubMed]
- Gheorghisan-Galateanu, A.A.; Valea, A.; Carsote, M. Acromegaly profile on menopausal women after 36 months of medical therapy with somatostatin analogues. Med. Evol. 2016, XXII, 361–366. [Google Scholar]
- Bolanowski, M.; Kałużny, M.; Witek, P.; Jawiarczyk-Przybyłowska, A. Pasireotide-a novel somatostatin receptor ligand after 20 years of use. Rev. Endocr. Metab. Disord. 2022, 23, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Rass, L.; Rahvar, A.H.; Matschke, J.; Saeger, W.; Renné, T.; Aberle, J.; Flitsch, J.; Rotermund, R. Differences in somatostatin receptor subtype expression in patients with acromegaly: New directions for targeted therapy? Hormones 2022, 21, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Arnaldi, G.; Bogazzi, F.; Cannavò, S.; Colao, A.; De Marinis, L.; De Menis, E.; Degli Uberti, E.; Giorgino, F.; Grottoli, S.; et al. Pegvisomant in acromegaly: An update. J. Endocrinol. Investig. 2017, 40, 577–589. [Google Scholar] [CrossRef]
- Van der Lely, A.J.; Gomez, R.; Heissler, J.F.; Åkerblad, A.C.; Jönsson, P.; Camacho-Hübner, C.; Kołtowska-Häggström, M. Pregnancy in acromegaly patients treated with pegvisomant. Endocrine 2015, 49, 769–773. [Google Scholar] [CrossRef]
- Guarda, F.J.; Gong, W.; Ghajar, A.; Guitelman, M.; Nachtigall, L.B. Preconception use of pegvisomant alone or as combination therapy for acromegaly: A case series and review of the literature. Pituitary 2020, 23, 498–506. [Google Scholar] [CrossRef]
- Hummelshøj, N.E.; Dam, G.; Pedersen, L.H.; Hjelholt, A.; Villadsen, G.E. First-generation somatostatin ligand receptor treatment in a pregnant patient with a neuroendocrine tumor with liver metastases. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 21–0126. [Google Scholar] [CrossRef]
- Geilswijk, M.; Andersen, L.L.; Frost, M.; Brusgaard, K.; Beck-Nielsen, H.; Frederiksen, A.L.; Jensen, D.M. Octreotide therapy and restricted fetal growth: Pregnancy in familial hyperinsulinemic hypoglycemia. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 16–0126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skajaa, G.O.; Mathiesen, E.R.; Iyore, E.; Beck-Nielsen, H.; Jimenez-Solem, E.; Damm, P. Poor pregnancy outcome after octreotide treatment during pregnancy for familial hyperinsulinemic hypoglycemia: A case report. BMC Res. Notes 2014, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, F.N.; Gökçay Canpolat, A.; Şahin, M.; Çorapçioğlu, D. Determination of the frequency of hyperprolactinemia-related etiologies and the etiology-specific mean prolactin levels. Minerva Endocrinol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.K.; Chandrasekhar, Y.B.V.K.; Vooturi, S. Current Status of Surgery in Management of Prolactinomas. Neurol. India 2020, 68, S39–S43. [Google Scholar] [CrossRef]
- Petersenn, S.; Christ-Crain, M.; Droste, M.; Finke, R.; Flitsch, J.; Kreitschmann-Andermahr, I.; Luger, A.; Schopohl, J.; Stalla, G. Pituitary Disease in Pregnancy: Special Aspects of Diagnosis and Treatment? Geburtshilfe Frauenheilkd. 2019, 79, 365–374. [Google Scholar] [CrossRef]
- Glezer, A.; Jallad, R.S.; Machado, M.C.; Fragoso, M.C.; Bronstein, M.D. Pregnancy and pituitary adenomas. Minerva Endocrinol. 2016, 41, 341–350. [Google Scholar]
- Hannon, A.M.; O’Shea, T.; Thompson, C.A.; Hannon, M.J.; Dineen, R.; Khattak, A.; Gibney, J.; O’Halloran, D.J.; Hunter, S.; Thompson, C.J.; et al. Pregnancy in acromegaly is safe and is associated with improvements in IGF-1 concentrations. Eur. J. Endocrinol. 2019, 180, K21–K29. [Google Scholar] [CrossRef]
- Chanson, P.; Vialon, M.; Caron, P. An update on clinical care for pregnant women with acromegaly. Expert Rev. Endocrinol. Metab. 2019, 14, 85–96. [Google Scholar] [CrossRef]
- Karaca, Z.; Yarman, S.; Ozbas, I.; Kadioglu, P.; Akturk, M.; Kilicli, F.; Dokmetas, H.S.; Colak, R.; Atmaca, H.; Canturk, Z.; et al. How does pregnancy affect the patients with pituitary adenomas: A study on 113 pregnancies from Turkey. J. Endocrinol. Investig. 2018, 41, 129–141. [Google Scholar] [CrossRef]
- Lambert, K.; Rees, K.; Seed, P.T.; Dhanjal, M.K.; Knight, M.; McCance, D.R.; Williamson, C. Macroprolactinomas and Nonfunctioning Pituitary Adenomas and Pregnancy Outcomes. Obstet. Gynecol. 2017, 129, 185–194. [Google Scholar] [CrossRef]
- Tomczyk, K.; Rzymski, P.; Woźniak, J.; Wilczak, M. Pregnancy in a woman with acromegaly after transsphenoidal partial resection of pituitary macroadenoma-a case report. Pol. Merkur. Lekarski. 2017, 43, 268–271. [Google Scholar]
- Abucham, J.; Bronstein, M.D.; Dias, M.L. Management of endocrine disease: Acromegaly and pregnancy: A contemporary review. Eur. J. Endocrinol. 2017, 177, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Viani, S.; Zucchelli, G.; Paperini, L.; Soldati, E.; Segreti, L.; Di Cori, A.; Menichetti, F.; Coluccia, G.; Andreini, D.; Branchitta, G.; et al. Subcutaneous Implantable Defibrillator in an acromegalic pregnant woman for secondary prevention of sudden cardiac death: When (2) technologies save (2) lives. Int. J. Cardiol. 2016, 223, 313–315. [Google Scholar] [CrossRef]
- Wise-Oringer, B.K.; Zanazzi, G.J.; Gordon, R.J.; Wardlaw, S.L.; William, C.; Anyane-Yeboa, K.; Chung, W.K.; Kohn, B.; Wisoff, J.H.; David, R.; et al. Familial X-Linked Acrogigantism: Postnatal Outcomes and Tumor Pathology in a Prenatally Diagnosed Infant and His Mother. J. Clin. Endocrinol. Metab. 2019, 104, 4667–4675. [Google Scholar] [CrossRef]
- Gordon, R.J.; Bell, J.; Chung, W.K.; David, R.; Oberfield, S.E.; Wardlaw, S.L. Childhood acromegaly due to X-linked acrogigantism: Long term follow-up. Pituitary 2016, 19, 560–564. [Google Scholar] [CrossRef]
- Babinska, A.; Olszewska, H.; Sworczak, K. Safe treatment with somatostatin analogues in a woman with acromegaly whilst pregnant and lactating. Neuro Endocrinol. Lett. 2021, 42, 433–437. [Google Scholar]
- Hara, T.; Kanasaki, H.; Oride, A.; Moriyama, M.; Kyo, S. Case of a woman with acromegaly whose presenting complaint was prolonged post-partum amenorrhea. J. Obs. Gynaecol. Res. 2016, 42, 1379–1384. [Google Scholar] [CrossRef]
- Haliloglu, O.; Dogangun, B.; Ozcabi, B.; Kural, H.U.; Keskin, F.E.; Ozkaya, H.M.; Pamukcu, F.C.; Bektas, E.; Poyraz, B.C.; Buber, H.; et al. General health status and intelligence scores of children of mothers with acromegaly do not differ from those of healthy mothers. Pituitary 2016, 19, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.; Pivonello, R.; Grasso, L.F.S.; Cozzolino, A.; Colao, A. Growth hormone, prolactin, and sexuality. J. Endocrinol. Investig. 2012, 35, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Grasso, L.; Martinez-Orozco, J.A.; Al-Agha, R.; Pivonello, R.; Colao, A.; Ezzat, S. Pregnancy in acromegaly: Experience from two referral centers and systematic review of the literature. Clin. Endocrinol. 2012, 76, 264–271. [Google Scholar] [CrossRef]
- Assal, A.; Malcolm, J.; Lochnan, H.; Keely, E. Preconception counselling for women with acromegaly: More questions than answers. Obs. Med. 2016, 9, 9–14. [Google Scholar] [CrossRef]
- Bray, D.P.; Mannam, S.; Rindler, R.S.; Quillin, J.W.; Oyesiku, N.M. Surgery for acromegaly: Indications and goals. Front. Endocrinol. 2022, 13, 924589. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Sandru, F.; Rentea, D.E.; Mehedintu, C.; Zugravu, S.; Chirita, C.; Dumitrascu, M.C. Acromegaly without acral anomalies. Rom. J. Med. Pract. 2021, 16, 520–523. [Google Scholar] [CrossRef]
- Alam, S.; Kubihal, S.; Goyal, A.; Jyotsna, V.P. Spontaneous Remission of Acromegaly After Pituitary Apoplexy in a Middle-Aged Male. Ochsner. J. 2021, 21, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Sandru, F.; Rentea, D.E.; Zugravu, S.; Mehedintu, C.; Dumitrascu, A.; Dumitrascu, M.C. Particular aspects concerning acromegaly amid pandemic. Rom. J. Med. Pract. 2021, 16, 442–446. [Google Scholar] [CrossRef]
- Sanz-Sapera, E.; Sarria-Estrada, S.; Arikan, F.; Biagetti, B. Acromegaly remission, SIADH and pituitary function recovery after macroadenoma apoplexy. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19–0057. [Google Scholar] [CrossRef]
- Ghemigian, A.; Carsote, M.; Ghervan, C.; Dumitrascu, A.; Albu, S.E.; Georgescu, C.E.; Valea, A. Long-term follow-up after transcranial hypophysectomy in macroprolactinomas. J. Surg. Sci. 2016, 3, 44–50. [Google Scholar] [CrossRef]
- Zhang, R.C.; Mu, Y.F.; Dong, J.; Lin, X.Q.; Geng, D.Q. Complex effects of apoplexy secondary to pituitary adenoma. Rev. Neurosci. 2017, 28, 59–64. [Google Scholar] [CrossRef]
- Luger, A.; Broersen, L.H.A.; Biermasz, N.R.; Biller, B.M.K.; Buchfelder, M.; Chanson, P.; Jorgensen, J.O.L.; Kelestimur, F.; Llahana, S.; Maiter, D.; et al. ESE Clinical Practice Guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur. J. Endocrinol. 2021, 185, G1–G33. [Google Scholar] [CrossRef]
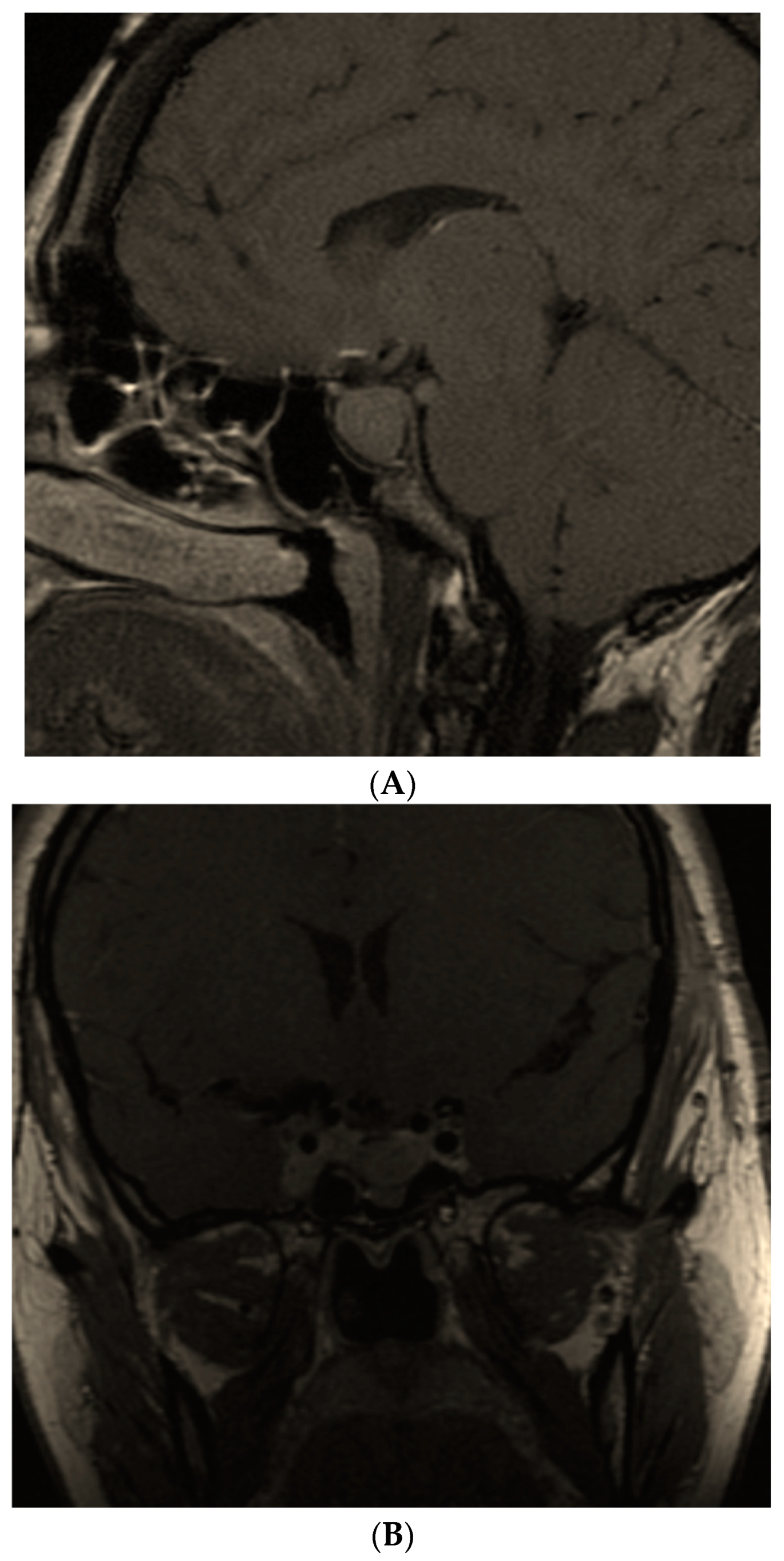
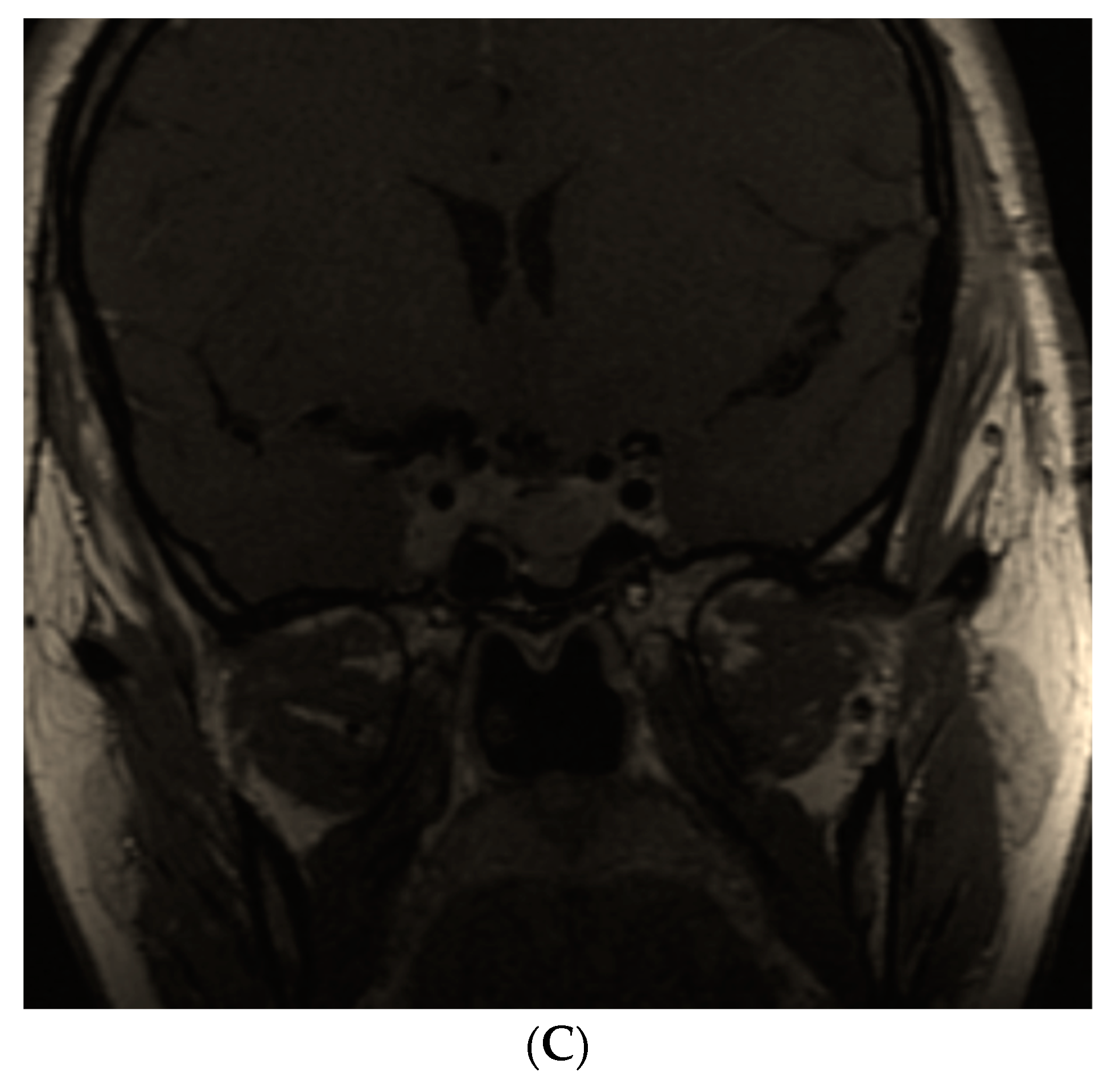
| First Author/ Year of Publication/ Reference No. | Type of Study | Studied Population | Results |
|---|---|---|---|
| Das L. 2021 [75] | Retrospective study | N = 14 pregnancies from 12 ACM women N1 = 5 active disease N2 = 9 controlled ACM | 100% had macroadenomas 2 years: median from hypophysectomy to pregnancy confirmation 13/14 term delivery, normal APGAR 1/14 HBP and preterm birth 0%: congenital malformations N1 = N2: materno-fetal outcomes N1 = N2: postpartum GH |
| Babinska A. 2021 [127] | Case report | 1 female case with ACM | 1 healthy new born → followed up for 15 years. |
| Meoni G. 2020 [95] | Retrospective study | N = 141 pregnancies from 127 ACM women N1 = 67 pregnancies in 62 females + SSAs N2 = 74 pregnancies in 65 females treated+ no SSAs | N1 = N2: materno-fetal outcomes Maternal: DM, HBP, headache, delivery mode) Fetal: birth term, height and weight of the new born 1/14 case of congenital malformation (N1)—ureteral stenosis |
| Guarda FJ. 2020 [111] | Case series | N = 4 pregnancies in 3 ACM females (one twin pregnancy) | N = 1 female (2 pregnancies)—exposure to PEG until 3 days before embryo transfer N = 2 females—exposure to PEG until the moment of pregnancy confirmation No materno-fetal outcomes |
| Vialon M. 2020 [77] | Retrospective study | N = 14 pregnancies in 11 ACM females | N = 7 (50%) had gestational DM |
| Wise-Oringer BK. 2019 [125] | Case report | 1 female with X-linked acrogigantism | Prenatal diagnostic of the same disease as the mother → successful pregnancy |
| Hannon AM. 2019 [96] | Case report | 1 female with ACM diagnostic within week 11 | Use of octreotide s.c. 100→150 µg/day to control tumor expansion in order to prevent visual loss (she refused surgery) |
| Hannon AM. 2019 [118] | Irish Pituitary Study | N = 17 pregnancies in 12 ACM females | 6/17: DA exposure among pregnancy (SSAs was stopped before gestation) Maternal outcomes: no tumor expansion, no visual field event, no case of gestational DM Fetal outcomes: 15/17: healthy newborns at term 1/17: pre-eclampsia—related emergency cesarean (week 32) 1/17: elective C-section for twin pregnancy (week 35) |
| Dicuonzo F. 2019 [97] | Case report | 1 female with giant, inoperable macroadenoma | 1 healthy newborn at term |
| Jallad RS. 2018 [78] | Retrospective study | N = 31 pregnancies in 20 ACM females | 4/31 abortion 45%: the rate of HBP worsening 32%: the rate of glucose profile worsening 0%: maternal-fetal death 2/27: congenital malformation 1/27: macrosomia |
| Dogansen SC. 2018 [76] | Retrospective study | N = 13 pregnancies in 45 ACM females |
7.7%: the rate of gestational DM and HBP 0%: tumor expansion |
| Karaca Z. 2018 [120] | Retrospective study | N = 21 pregnancies in ACM women (a cohort of 113 cases with pituitary tumors) | Congenital malformations: unilateral congenital cataract, craniosynostosis and microcephaly |
| Tomczyk K. 2017 [122] | Case report | 1 female with ACM with pre-conception partial hypophysectomy for macroadenoma | 1 healthy newborn at term |
| Lambert K. 2017 [121] | Prospective study | N = 3 pregnancies in ACM women (a cohort of 71 cases with pituitary tumors) | No pregnancies outcome: HBP, DM, preterm labor, stillbirth |
| Teltayev D. 2917 [102] | Case report | 1 female with ACM | 1 healthy newborn at term |
| Căpăþînă C. 2016 [51] | Case report | 1 female with ACM with pre-conception debulking hypophysectomy and gammaknife radiotherapy for macroadenoma | 1 healthy newborn at term |
| Viani S. 2016 [124] | Case report | 1 female with ACM | subcutaneous implantable defibrillator for secondary prevention of sudden cardiac death |
| Haliloglu O. 2016 [129] | Longitudinal study | N = 16 pregnancies in 6 ACM females | 2/16: large for gestational age 2/16: tall for the age during childhood. |
| Hara T. 2016 [128] | Case report | 1 female with ACM | 1 healthy newborn (diagnostic of acromegaly after 1 year of lactation) |
| van der Lely AJ. 2015 [109] | ACROSTUDY | N = 35 pregnancies with pegvisomant exposure (27/35 females with ACM) | Data obtained for 10 full-term healthy newborns 5 ACM females: elective abortion 2 ACM females: spontaneous abortion 1 ACM female: ectopic pregnancy |
| Dias M. 2013 [39] | Prospective study | N = 10 pregnancies in 8 ACM females Versus N1 = 64 control pregnancies | N = N1: 1 case of HBP/preeclampsia and 1 case of DM |
| Koshy TG. 2012 [93] | Case report | 1 female with ACM | Pituitary surgery (week 22) → cabergoline (1 mg/week) → full-term, healthy new born (cesarean) |
| Cheng S. 2012 [131] | Case series 343 | N = 13 pregnancies in ACM females | No teratogenic effect |
| Kasuki L. 2012 [94] | Case report | 1 female with ACM (pre-conception surgery) | 1 healthy newborn (week 37, cesarean) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, A.D.; Carsote, M.; Valea, A.; Nicola, A.G.; Dascălu, I.T.; Tircă, T.; Abdul-Razzak, J.; Țuculină, M.J. Approach of Acromegaly during Pregnancy. Diagnostics 2022, 12, 2669. https://doi.org/10.3390/diagnostics12112669
Popescu AD, Carsote M, Valea A, Nicola AG, Dascălu IT, Tircă T, Abdul-Razzak J, Țuculină MJ. Approach of Acromegaly during Pregnancy. Diagnostics. 2022; 12(11):2669. https://doi.org/10.3390/diagnostics12112669
Chicago/Turabian StylePopescu, Alexandru Dan, Mara Carsote, Ana Valea, Andreea Gabriela Nicola, Ionela Teodora Dascălu, Tiberiu Tircă, Jaqueline Abdul-Razzak, and Mihaela Jana Țuculină. 2022. "Approach of Acromegaly during Pregnancy" Diagnostics 12, no. 11: 2669. https://doi.org/10.3390/diagnostics12112669
APA StylePopescu, A. D., Carsote, M., Valea, A., Nicola, A. G., Dascălu, I. T., Tircă, T., Abdul-Razzak, J., & Țuculină, M. J. (2022). Approach of Acromegaly during Pregnancy. Diagnostics, 12(11), 2669. https://doi.org/10.3390/diagnostics12112669







