Diagnostics of Ovarian Tumors in Postmenopausal Patients
Abstract
1. Introduction
2. Materials and Methods
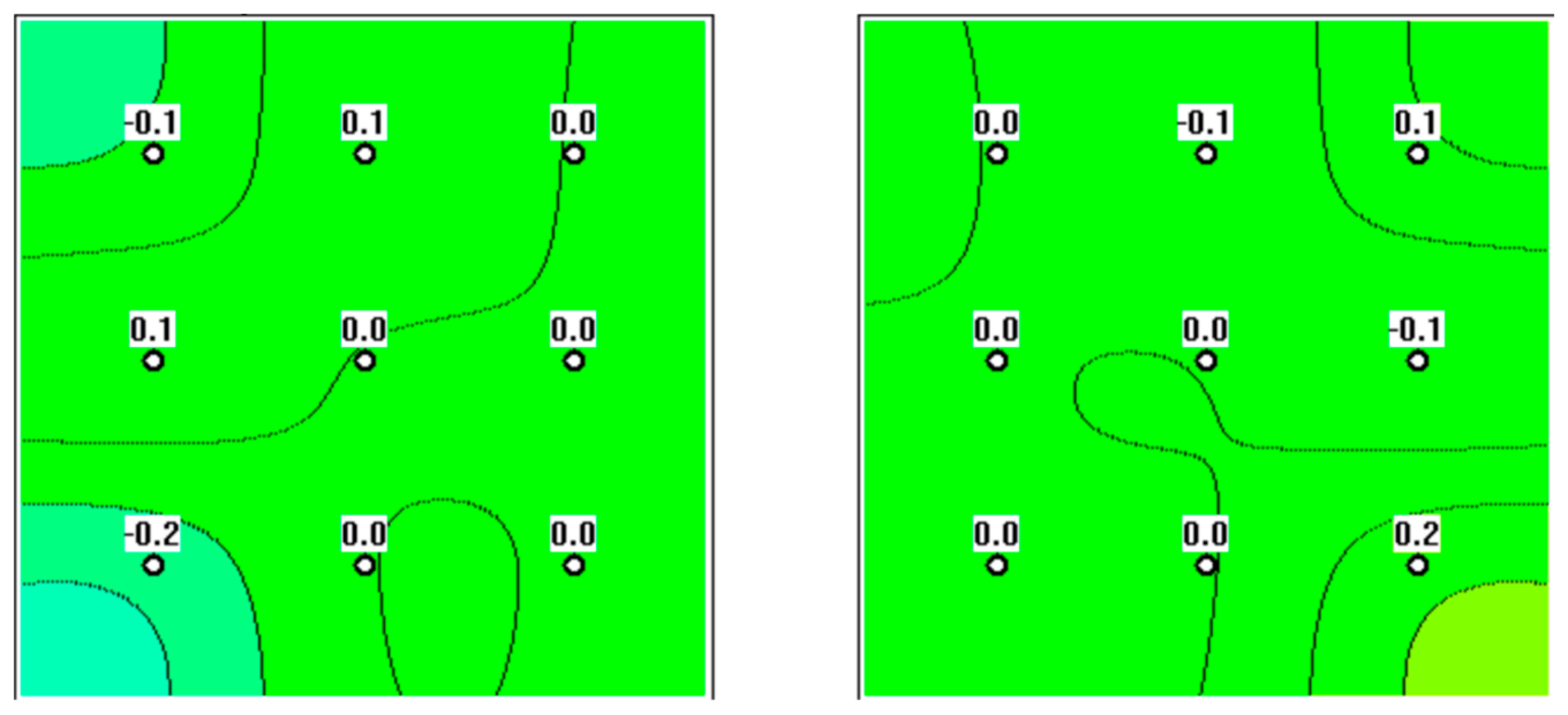
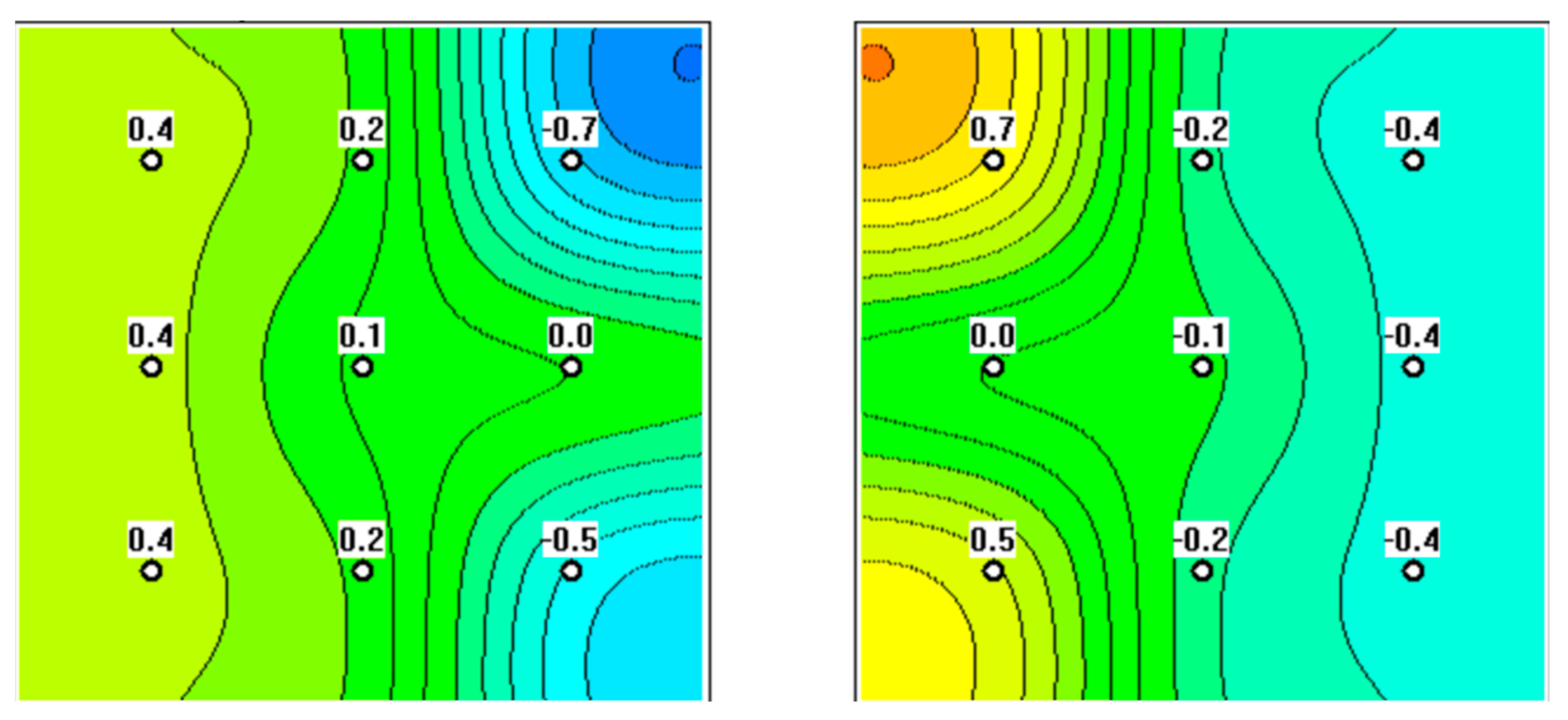
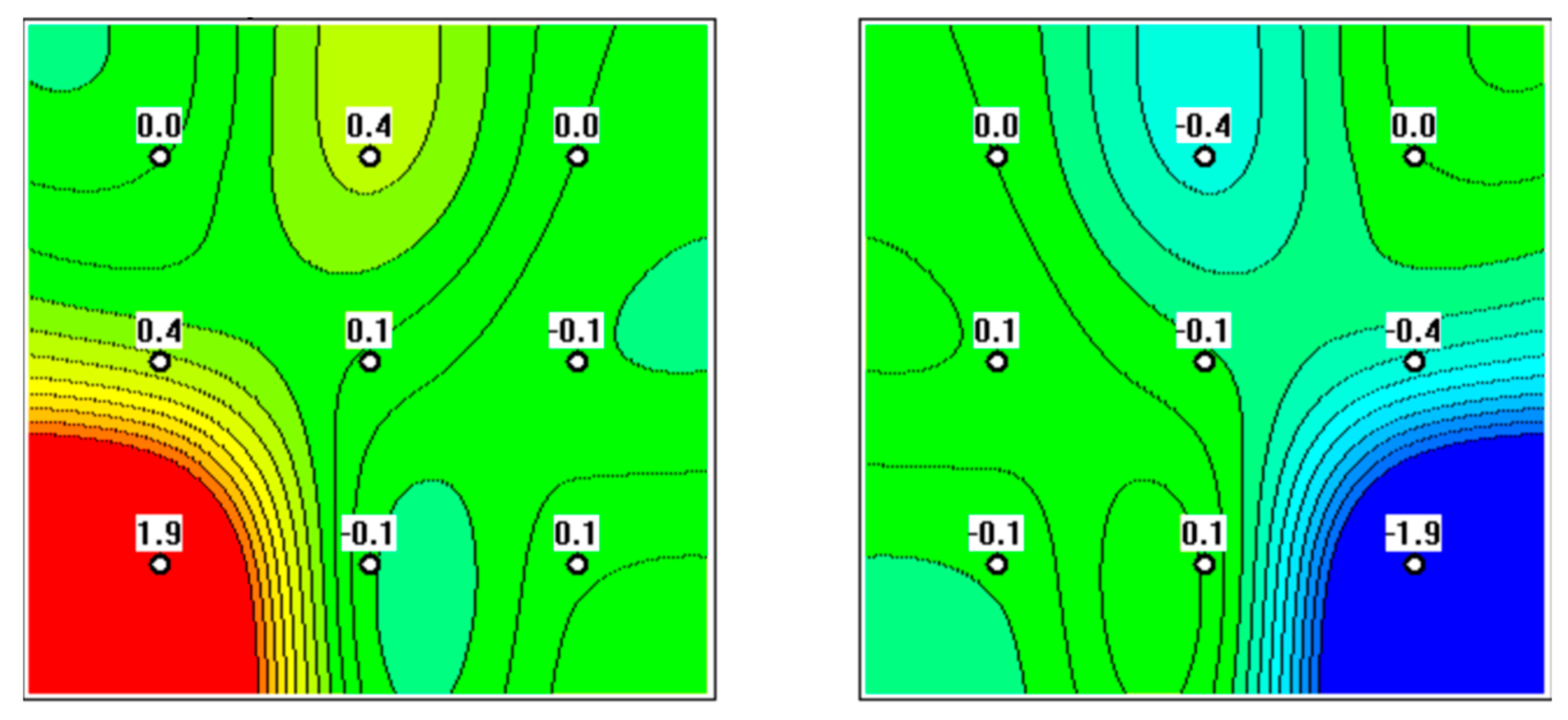
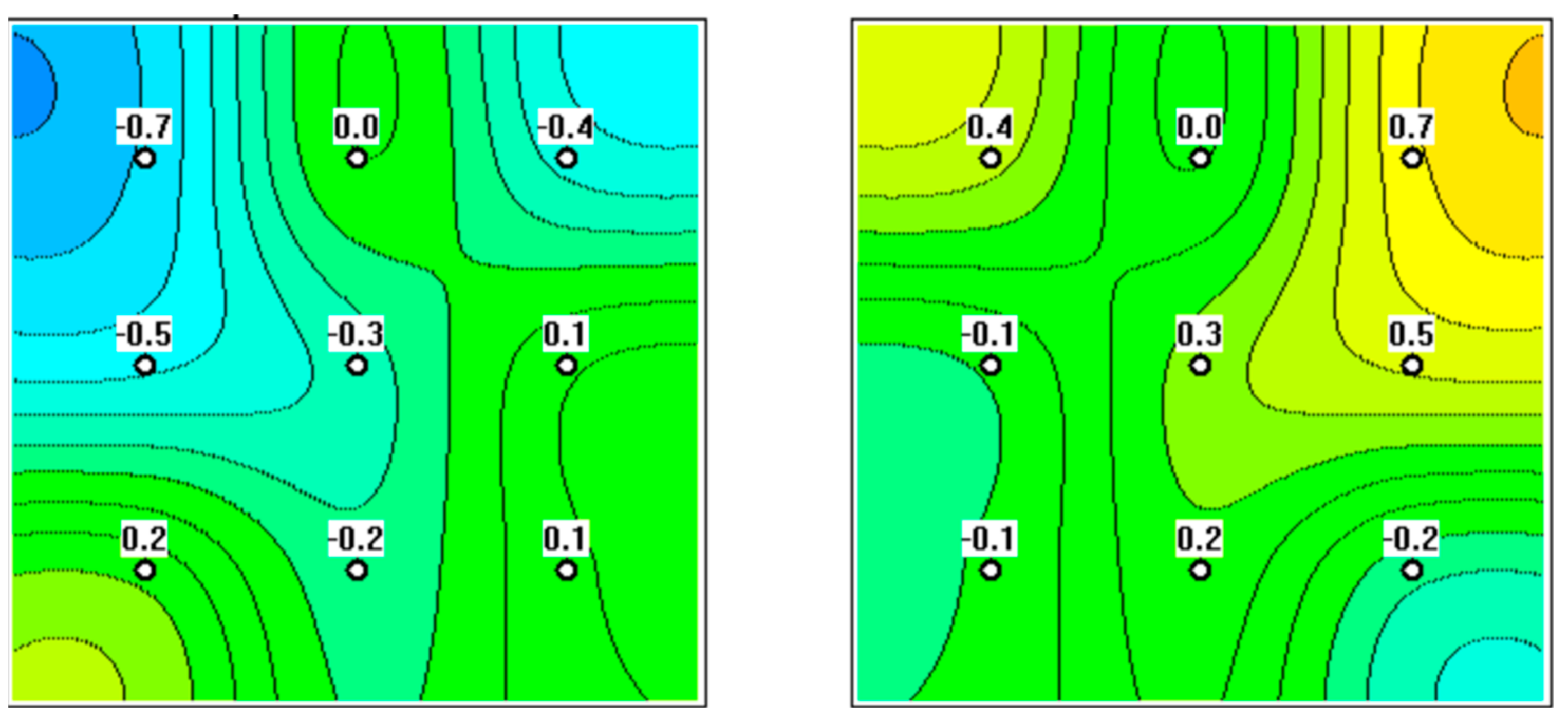
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urmancheeva, A.F.; Kutusheva, G.F.; Ulrikh, E.A. Ovarian Tumors: Clinical Picture, Diagnosis and Treatment; Publishing House N-L: Saint Petersburg, Russia, 2012. [Google Scholar]
- Egunova, M.A.; Kutsenko, I.G. Differential diagnosis of benign and malignant neoplasms of the ovaries (history of the issue). J. Obstet. Women’s Dis. 2016, 65, 68–78. [Google Scholar] [CrossRef]
- Ionescu, C.A.; Matei, A.; Navolan, D.; Dimitriu, M.; Bohâltea, R.; Neacsu, A.; Ilinca, C.; Ples, L. Correlation of ultrasound features and the Risk of Ovarian Malignancy Algorithm score for different histopathological subtypes of benign adnexal masses. Medicine 2018, 97, e11762. [Google Scholar] [CrossRef]
- Kaprin, A.D.; Starinskiy, V.V.; Petrova, G.V. The State of Cancer Care for the Population of RUSSIA; P.A. Herzen Branch of the National Medical Research Center of Radiology of the Ministry of Health of Russia: Moscow, Russia, 2018; ISBN 978-5-85502-237-7. [Google Scholar]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef]
- Yureneva, S.V.; Ermakova, E.I. The management of women with menopausal disorders (review of clinical guidelines). Probl. reproduktsii 2017, 23, 115–122. [Google Scholar] [CrossRef]
- Guraslan, H.; Dogan, K. Management of unilocular or multilocular cysts more than 5 centimeters in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Committee Opinion No. 716: The Role of the Obstetrician-Gynecologist in the Early Detection of Epithelial Ovarian Cancer in Women at Average Risk. Obstet. Gynecol. 2017, 130, e146–e149. [CrossRef]
- Alibakhshikenari, M.; Virdee, B.S.; Shukla, P.; Parchin, N.O.; Azpilicueta, L.; See, C.H.; Abd-Alhameed, R.A.; Falcone, F.; Huynen, I.; Denidni, T.A.; et al. Metamaterial-Inspired Antenna Array for Application in Microwave Breast Imaging Systems for Tumor Detection. IEEE Access 2020, 8, 174667–174678. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, A.V.; Protasova, A.E. General questions of screening. J. Pract. Oncol. 2022, 2, 53–59. [Google Scholar]
- Urmancheeva, A.F.; Kutusheva, G.F.; Ulrikh, E.A.; Efimova, O.A. Complex radiation diagnostics of ovarian tumor formations at the preoperative stage. Povolzhsky Oncol. Bull. 2017, 3, 61–64. [Google Scholar]
- Zola, P.; Macchi, C.; Cibula, D.; Colombo, N.; Kimmig, R.; Maggino, T.; Reed, N.; Kesic, V. Follow-up in Gynecological Malignancies: A State of Art. Int. J. Gynecol. Cancer 2015, 25, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Clinical Recommendations of the Ministry of Health of The Russian Federation “Diagnostics and Treatment of Benign Ovarian Neoplasms from the Perspective of Cancer Prevention”. 2018. Available online: http://zdrav.spb.ru (accessed on 27 October 2022).
- Stewart, B.W.; Wild, C.P. World Cancer Report, 2014; IARC: Lyon, France, 2014; 916p. [Google Scholar]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.K.; MacKay, E.V. Carcinoembryonic antigen (CEA) in ovarian cancer: Factors influencing its incidence and changes which occur in response to cytotoxic drugs. BJOG Int. J. Obstet. Gynaecol. 1976, 83, 753–759. [Google Scholar] [CrossRef]
- Bast, R.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Benjapibal, M.; Neungton, C. Pre-operative prediction of serum CA125 level in women with ovarian masses. J. Med. Assoc. Thai. 2007, 90, 1986–1991. [Google Scholar]
- Wilbaux, M.; Hénin, E.; Oza, A.; Colomban, O.; Pujade-Lauraine, E.; Freyer, G.; Tod, M.; You, B. Prediction of tumour response induced by chemotherapy using modelling of CA-125 kinetics in recurrent ovarian cancer patients. Br. J. Cancer 2014, 110, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tie, R.; Chang, K.; Wang, F.; Deng, S.; Lu, W.; Yu, L.; Chen, M. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and ca125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer 2012, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, B.; Kou, X.J.; Liu, T.Z.; Ming, L. Clinical significance of combined detection of serum tumor markers in diagnosis of patients with ovarian cancer. Asian Pac. J. Cancer Prev. 2013, 14, 6241–6243. [Google Scholar] [CrossRef][Green Version]
- Robati, M.; Ghaderi, A.; Mehraban, M.; Shafizad, A.; Nasrolahi, H.; Mohammadianpanah, M. Vascular endothelial growth factor (VEGF) improves the sensitivity of CA125 for differentiation of epithelial ovarian cancers from ovarian cysts. Arch. Gynecol. Obs. 2013, 288, 859–865. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Whitehead, C.M.; Rubatt, J.M.; Cheek, R.L.; Groelke, J.; He, Q.; Malinowski, D.P.; Fischer, T.J.; Berchuck, A. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol. Oncol. 2008, 110, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qin, J.; Sangvatanakul, V. Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 167, 81–85. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Skates, S.; Lokshin, A.; Moore, R.G. Differential diagnosis of pelvic mass: Improved algorithms and novel biomarkers. Int. J. Gynecol. Cancer IGC 2012, 22 (Suppl. 1), S5–S8. [Google Scholar] [CrossRef]
- Zhen, S.; Bian, L.H.; Chang, L.L.; Gao, X. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: A meta-analysis. Mol. Clin. Oncol. 2014, 2, 559–566. [Google Scholar] [CrossRef]
- Wu, C.M.; Li, X.L.; Li, L.; Yin, L.L.; Li, Q. Combined detection of tumor makers in the diagnosis of ovarian tumors. Int. J. Lab. Med. 2014, 35, 724–725. [Google Scholar]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obs. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Jabre-Raughley, M.; Brown, A.K.; Robison, K.M.; Miller, M.C.; Allard, W.J.; Kurman, R.J.; Bast, R.C.; Skates, S.J. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am. J. Obstet. Gynecol. 2010, 203, 228.e1–228.e6. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E.; Ruzzenente, O.; Bresciani, V.; Nuzzo, T.; Gelati, M.; Salvagno, G.; Franchi, M.; Lippi, G.; Guidi, G. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: Is it really useful? Clin. Chem. Lab. Med. 2011, 49, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Dayyani, F.; Uhlig, S.; Colson, B.; Simon, K.; Rolny, V.; Morgenstern, D.; Schlumbrecht, M. Diagnostic performance of risk of ovarian malignancy algorithm against CA-125 and HE4 in connection with ovarian cancer: A meta-analysis. Int. J. Gynecol. Cancer 2016, 26, 1586. [Google Scholar] [CrossRef] [PubMed]
- Kaijser, J.; Bourne, T.; Valentin, L.; Sayasneh, A.; Van Holsbeke, C.; Vergote, I.; Testa, A.C.; Franchi, D.; Van Calster, B.; Timmerman, D. Improving strategies for diagnosing ovarian cancer: A summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet. Gynecol. 2013, 41, 9–20. [Google Scholar] [CrossRef]
- Mehdi, G.; Maheshwari, V.; Afzal, S.; Ansari, H.A.; Ansari, M. Image-guided fine-needle aspiration cytology of ovarian tumors: An assessment of diagnostic efficacy. J. Cytol. 2010, 27, 91–95. [Google Scholar]
- Dubrovskaya, K.S. Diagnostics, Treatment and Prediction of the Outcomes of Pelvic Neoplasms in Gynecological Patients Abstract of the Dissertation for the Degree of Candidate of Medical Sciences; Sechenov University: Moscow, Russia, 2018. [Google Scholar]
- Borisova, E.A. Comprehensive differential diagnosis of tumors of the uterine appendages. In Abstract of the Dissertation of the Candidate of Medical Sciences; Krasnoyarsk State Medical University: Irkutsk, Russia, 2018. [Google Scholar]
- Suh-Burgmann, E.; Flanagan, T.; Osinski, T.; Alavi, M.; Herrinton, L. Prospective Validation of a Standardized Ultrasonography-Based Ovarian Cancer Risk Assessment System. Obstet. Gynecol. 2018, 132, 1101–1111. [Google Scholar] [CrossRef]
- Valentin, L.; Ameye, L.; Savelli, L.; Leone, F.P.G.; Czekierdowski, A.; Lissoni, A.A.; Fischerova, D.; Guerriero, S.; Van Holsbeke, C.; Van Huffel, S.; et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: Logistic regression models do not help. Ultrasound Obstet. Gynecol. 2011, 38, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Meys, E.M.J.; Kaijser, J.; Kruitwagen, R.; Slangen, B.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. 2021, 58, 148–168. [Google Scholar] [CrossRef]
- Ngu, S.F.; Chai, Y.K.; Choi, K.M.; Leung, T.W.; Li, J.; Kwok, G.S.T.; Chu, M.M.Y.; Tse, K.Y.; Cheung, V.Y.T.; Ngan, H.Y.S.; et al. Diagnostic Performance of Risk of Malignancy Algorithm (ROMA), Risk of Malignancy Index (RMI) and Expert Ultrasound Assessment in a Pelvic Mass Classified as Inconclusive by International Ovarian Tumour Analysis (IOTA) Simple Rules. Cancers 2022, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Dieguez, N.; Glickman, A.; Munmany, M.; Casanovas, G.; Agustí, N.; Díaz-Feijoo, B.; Saco, A.; Sánchez, B.; Gaba, L.; Angeles, M.A.; et al. Comparison of HE4, CA125, ROMA and CPH-I for Preoperative Assessment of Adnexal Tumors. Diagnostics 2022, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Rick, C.; Dowling, F.; Au, P.; Snell, K.; Rai, N.; Champaneria, R.; Stobart, H.; Neal, R.; Davenport, C.; et al. Refining Ovarian Cancer Test accuracy Scores (ROCkeTS): Protocol for a prospective longitudinal test accuracy study to validate new risk scores in women with symptoms of suspected ovarian cancer. BMJ Open 2016, 6, e010333. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerová, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef]
- Froyman, W.; Landolfo, C.; De Cock, B.; Wynants, L.; Sladkevicius, P.; Testa, A.C.; Van Holsbeke, C.; Domali, E.; Fruscio, R.; Epstein, E.; et al. Risk of complications in patients with conservatively managed ovarian tumors (IOTA5): A 2-year interim analysis of a multicenter, prospective, cohort study. Lancet Oncol. 2019, 20, 448–458. [Google Scholar] [CrossRef]
- The Management of Ovarian Cysts in Postmenopausal Women, RCOG Greentop Guisline No. July 2016. Available online: https://www-temp.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/ovarian-cysts-in-postmenopausal-women-green-top-guideline-no-34/ (accessed on 27 October 2022).
- Final Recommendation Statement: Ovarian Cancer: Screening. U.S. Preventive Services Task Force (USPSTF). December 2016. Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening#:~:text=The%20USPSTF%20does%20not%20recommend,been%20evaluated%20in%20screening%20studies (accessed on 27 October 2022).
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Ovarian Cancer: US Preventive Services Task Force Recommendation Statement. US Preventive Services Task Force. JAMA 2018, 319, 588–594. [Google Scholar]
- Davenport, C.; Rai, N.; Sharma, P.; Deeks, J.J.; Berhane, S.; Mallett, S.; Saha, P.; Champaneria, R.; Bayliss, S.E.; Snell, K.I.; et al. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. Cochrane Database Syst. Rev. 2022, 7, CD011964. [Google Scholar] [CrossRef]
- Watrowski, R.; Obermayr, E.; Wallisch, C.; Aust, S.; Concin, N.; Braicu, E.I.; Van Gorp, T.; Hasenburg, A.; Sehouli, J.; Vergote, I.; et al. Biomarker-Based Models for Preoper-ative Assessment of Adnexal Mass: A Multicenter Validation Study. Cancers 2022, 14, 1780. [Google Scholar] [CrossRef]
- Funston, G.; Hamilton, W.; Abel, G.; Crosbie, E.J.; Rous, B.; Walter, F.M. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: A population-based cohort study. PLoS Med. 2020, 17, e1003295. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Gautherie, M. Temperature and Blood Flow Patterns in Breast Cancer During Natural Evolution and Following Radiotherapy. Biomed. Thermol. 1982, 107, 21–64. [Google Scholar]
- Goryanin, I.; Karbainov, S.; Shevelev, O.; Tarakanov, A.; Redpath, K.; Vesnin, S.; Ivanov, Y. Passive microwave radiometry in biomedical studies. Drug Discov. Today 2020, 25, 757–763. [Google Scholar] [CrossRef]
- Vesnin, S.; Turnbull, A.K.; Dixon, J.M.; Goryanin, I. Modern microwave thermometry for breast cancer. J. Mol. Imaging Dyn. 2017, 7, 136. [Google Scholar]
- Li, J.; Galazis, C.; Popov, L.; Ovchinnikov, L.; Kharybina, T.; Vesnin, S.; Losev, A.; Goryanin, I. Dynamic Weight Agnostic Neural Networks and Medical Microwave Radiometry (MWR) for Breast Cancer Diagnostics. Diagnostics 2022, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, C.K.; Pak, E.V. Method for Screening Diagnostics of Malignant Neoplasms of the Ovaries in Postmenopausal Women. Patent RU 2616989, C1, 10 May 2016. [Google Scholar]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Ovarian Cancer. 2013. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 27 October 2022).
- Starinsky, V.V.; Sergeeva, N.S.; Marshutina, N.V.; Korneeva, I.A. Problems of early diagnosis and screening of ovarian cancer: Reality and prospects. Oncol. J. Them. PA 2013, 1, 56–62. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer Statistics, 2012. Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Rein, B.J.; Gupta, S.; Dada, R.; Safi, J.; Michener, C.; Agarwal, A. Potential markers for detection and monitoring of ovarian cancer. J. Oncol. 2011, 2011, 475983. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Kataoka, M.; Pandit-Taskar, N.; Ishill, N.; Mironov, S.; Moskowitz, C.S.; Mironov, O.; Collins, M.A.; Chi, D.S.; Larson, S.; et al. Recurrent ovarian cancer: Use of contrast-enhanced CT and PET/CT to accurately localize tumor recurrence and to predict patients’ survival. Radiology 2010, 257, 125–134. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Nikolovski, I.; Long Roche, K.; Lakhman, Y. CT of Ovarian Cancer for Primary Treatment Planning: What the Surgeon Needs to Know-Radiology In Training. Radiology 2022, 304, 516–526. [Google Scholar] [CrossRef]
- Feng, S.; Xia, T.; Ge, Y.; Zhang, K.; Ji, X.; Luo, S.; Shen, Y. Computed Tomography Imaging-Based Radiogenomics Analysis Reveals Hypoxia Patterns and Immunological Characteristics in Ovarian Cancer. Front. Immunol. 2022, 13, 868067. [Google Scholar] [CrossRef]
- Van de Vrie, R.; Rutten, M.J.; Asseler, J.; Leeflang, M.M.G.; Kenter, G.G.; Mol, B.J.; Buist, M. Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer. Cochrane Database Syst. Rev. 2019, 2019, CD009786. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.N.; Shmeliova, D.V.; Boos, N.A. Phantom to control the thermal ablation process. Russ. Technol. J. 2021, 9, 73–78. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F. Globocan Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10; International Agency for Research on Cancer: Lyon, France, 2008. [Google Scholar]
- Dodge, J.E.; Covens, A.; Lacchetti, C.; Elit, L.M.; Le, T.; Devries–Aboud, M.; Fung-Kee-Fung, M. Management of a Suspicious Adnexal Mass: A Clinical Practice Guideline. Curr. Oncol. 2012, 19, 244–257. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafin, C.; Vesnin, S.; Turnbull, A.; Dixon, M.; Goltsov, A.; Goryanin, I. Diagnostics of Ovarian Tumors in Postmenopausal Patients. Diagnostics 2022, 12, 2619. https://doi.org/10.3390/diagnostics12112619
Mustafin C, Vesnin S, Turnbull A, Dixon M, Goltsov A, Goryanin I. Diagnostics of Ovarian Tumors in Postmenopausal Patients. Diagnostics. 2022; 12(11):2619. https://doi.org/10.3390/diagnostics12112619
Chicago/Turabian StyleMustafin, Chingis, Sergey Vesnin, Arran Turnbull, Michael Dixon, Alexey Goltsov, and Igor Goryanin. 2022. "Diagnostics of Ovarian Tumors in Postmenopausal Patients" Diagnostics 12, no. 11: 2619. https://doi.org/10.3390/diagnostics12112619
APA StyleMustafin, C., Vesnin, S., Turnbull, A., Dixon, M., Goltsov, A., & Goryanin, I. (2022). Diagnostics of Ovarian Tumors in Postmenopausal Patients. Diagnostics, 12(11), 2619. https://doi.org/10.3390/diagnostics12112619






