Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection
2.2. Characteristics of the Hematology Analyzers
2.3. Measured and Calculated Parameters
2.4. Data Analysis
2.4.1. Comparison between Horiba Micros ES 60 and Beckman Coulter DXH 800
2.4.2. Intra-Assay Agreement Horiba Micros ES 60
3. Results
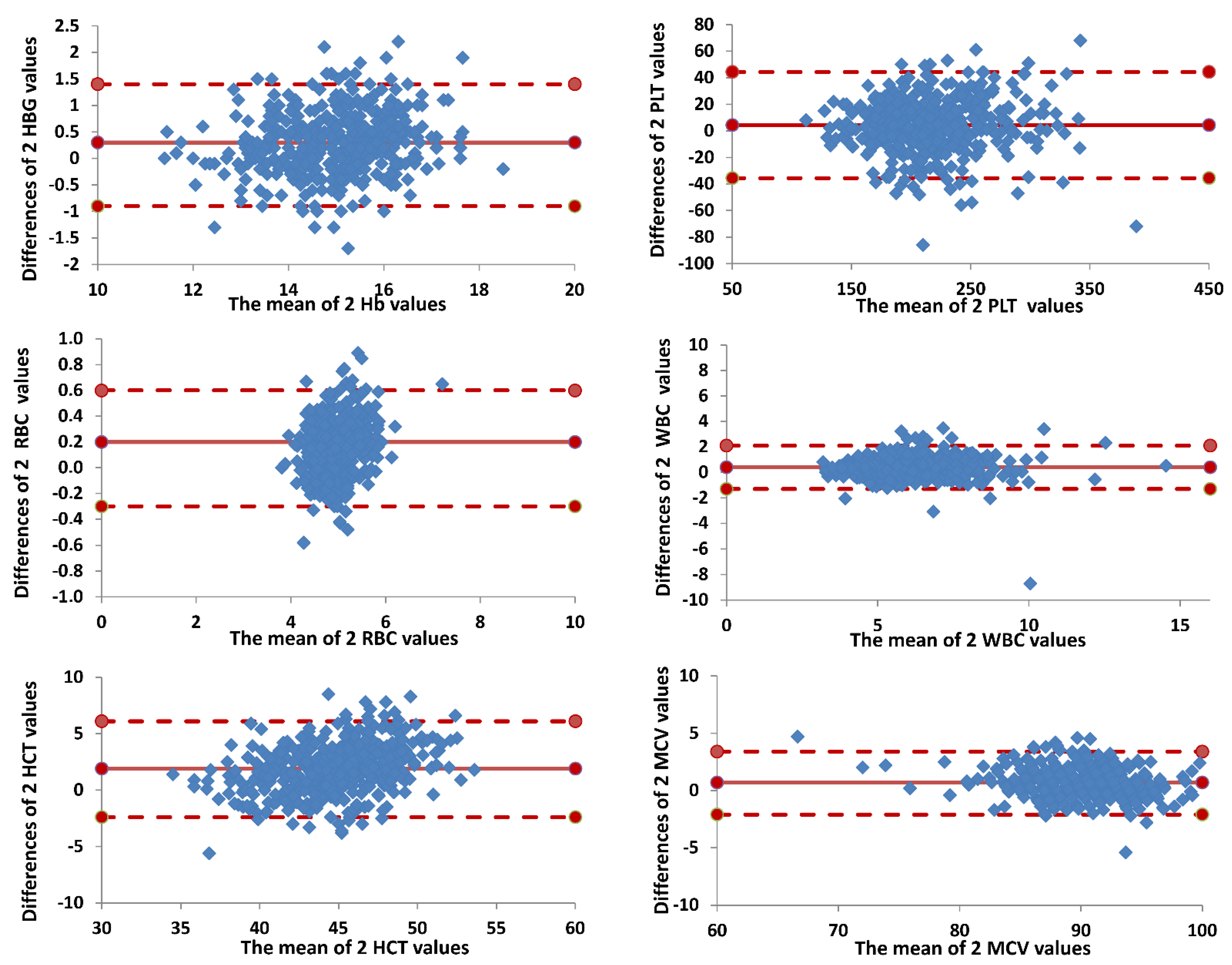
3.1. Comparison between Horiba Micros ES 60 and Beckman Coulter DXH 800
3.2. Intra-Assay Agreement Horiba Micros ES 60
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Angelantonio, E.; Thompson, S.G.; Kaptoge, S.; Moore, C.; Walker, M.; Armitage, J.; Ouwehand, W.H.; Roberts, D.J.; Danesh, J.; INTERVAL Trial Group. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): A randomized trial of 45,000 donors. Lancet 2017, 390, 2360–2371. [Google Scholar] [CrossRef]
- EDQM. European Directorate for the Quality of Medicines & HealthCare. In Guide to the Preparation, Use and Quality Assurance of Blood Components, 20th ed.; EDQM: Strasbourg, France, 2020. [Google Scholar]
- Decreto del Ministero Della Salute 2 Novembre 2015. Disposizioni Relative ai Requisiti di Qualità e Sicurezza del Sangue e Degli Emocomponenti. Gazzetta Ufficiale n. 300—Suppl. Ordinario n. 69, 28 Dicembre 2015. Available online: https://www.gazzettaufficiale.it/eli/id/2015/12/28/15A09709/sg (accessed on 20 January 2022). (In Italian).
- Chaudhary, R.; Dubey, A.; Sonker, A. Techniques used for the screening of hemoglobin levels in blood donors: Current insights and future directions. Rev. J. Blood Med. 2017, 8, 75–88. [Google Scholar] [CrossRef] [PubMed]
- User manual ABX Micros ES60 P/n:RAB237AEN HORIBA Group, HAN706A, 2007 Horiba ABX. Available online: https://toolkits.horiba-abx.com/documentation/download.php?id=35078 (accessed on 14 September 2022).
- NCCLS. Evaluation of Precision Performance of Quantitative Measurement Methods. In Approved Guideline, 2nd ed.; NCCLS document EP5-A2; NCCLS: Wayne, PE, USA, 2004; ISBN 1-56238-542-9. [Google Scholar]
- Taouinet, R.; Mougin, G.; Brun, C.; Darodes de Tailly, P. Évaluation d’une trousse de réactifs consommables compatibles SFRI® sur l’analyseur de numeration sanguine Micros 60® Statistical comparison of the results of the blood cells count obtained with a Micros 60 ABX analyzer using the genuine reagents and generics reagents kits. Ann. Biol. Clin. 2007, 65, 671–676. [Google Scholar]
- Hollis, V.S.; Holloway, J.A.; Harris, S.; Spencer, D.; van Berkel, C.; Morgan, H. Comparison of Venous and Capillary Differential Leukocyte Counts Using a Standard Hematology Analyzer and a Novel Microfluidic Impedance Cytometer. PLoS ONE 2012, 7, e43702. [Google Scholar] [CrossRef] [PubMed]
- McNair, E. Performance Evaluation of the Plateletworks® in the Measurement of Blood Cell Counts as compared to the Beckman Coulter Unicel. DXH 800. J. Extra Corpor. Technol. 2015, 47, 113–118. [Google Scholar] [PubMed]
- Alan, A.E.; Murphy, E. The price of blood is measured in iron. Lancet 2017, 390, 2331–2333. [Google Scholar]
- Armstrong, K.L. Blood donation and anemia. Can. Fam. Physician 2016, 62, 730–731. [Google Scholar] [PubMed]
- Cable, R.G.; Glynn, S.A.; Kiss, J.E.; Mast, A.E.; Steele, W.R.; Murphy, E.L.; Wright, D.J.; Sacher, R.A.; Gottschall, J.L.; Tobler, L.H.; et al. Iron deficiency in blood donors: The REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2012, 52, 702–711. [Google Scholar] [CrossRef]
- Gil-Betacur, A.; Mantilla-Gutiérrez, C.Y.; Cardona-Arias, J.A. Effect of plateletpheresis on hematocrit, hemoglobin and erythrocyte count: Meta-analysis 1980–2018. Sci. Rep. 2019, 9, 19770. [Google Scholar] [CrossRef]
- Spencer, B. Blood donor iron status: Are we bleeding them dry? Curr. Opin. Hematol. 2013, 20, 533–539. [Google Scholar] [CrossRef]
- Cable, R.G.; Steele, W.R.; Melmed, R.S.; Johnson, B.; Mast, A.E.; Carey, P.M.; Kiss, J.E.; Kleinman, S.H.; Wright, D.J.; NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II). The difference between fingerstick and venous hemoglobin and hematocrit varies by sex and iron stores. Transfusion 2012, 52, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.J.; Hopkins, J.A.; Arceo, S.M.; Leitman, S.F. Evaluation of low red blood cell mean corpuscular volume in an apheresis donor population. Transfusion 2009, 49, 1971–1976. [Google Scholar] [CrossRef]
- Das, S.S.; Chaudhary, R.; Verma, S.K.; Ojha, S.; Khetan, D. Pre- and post- donation haematological values in healthy donors undergoing plateletpheresis with five different systems. Blood Transfus. 2009, 7, 188–192. [Google Scholar] [PubMed]
- Feng, Q.; Zhu, F.; Li, C.; Guo, B.; Ye, J.; Chen, J. Effect of Frequency of Platelet Apheresis on Coagulation Function in Donors: A Prospective Cohort Study. Indian J. Hematol. Blood Transfus. 2019, 35, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Butkiewicz, A.M.; Kemona, H.; Dymicka-Piekarska, V.; Matowicka-Karna, J.; Radziwon, P.; Lipska, A. Platelet count, mean platelet volume and thrombocytopoietic indices in healthy women and men. Thromb. Res. 2006, 118, 199–204. [Google Scholar] [CrossRef]
- Gallerani, G.; Reverberi, R.; Salmi, R.; Smolensky, M.H.; Manfredini, R. Seasonal variation of platelets in a cohort of Italian blood donors: A preliminary report. Eur. J. Med. Res. 2013, 18, 31. [Google Scholar] [CrossRef] [PubMed]


| Horiba Micros ES60 | Beckman Coulter DXH 800 | ρ-Pearson’s Coefficient | p-Value F-Test (*) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | |||
| HGB (g/dL) | 15.14 | 1.23 | 0.08 | 14.84 | 1.13 | 0.08 | 0.88 | 0.041 |
| PLT (109/L) | 220.23 | 44.37 | 0.20 | 215.86 | 42.97 | 0.20 | 0.89 | 0.251 |
| RBC (106/mm3) | 5.07 | 0.46 | 0.09 | 4.90 | 0.41 | 0.08 | 0.88 | 0.004 |
| WBC (103/mm3) | 6.19 | 1.58 | 0.26 | 5.81 | 1.55 | 0.27 | 0.84 | 0.356 |
| HCT (%) | 45.72 | 3.82 | 0.08 | 43.86 | 3.09 | 0.07 | 0.82 | 0.000 |
| MCV (fl) | 90.38 | 3.93 | 0.04 | 89.72 | 4.20 | 0.05 | 0.94 | 0.082 |
| HGB (g/dL) | PLT (109/L) | RBC (106/mm3) | WBC (103/mm3) | HCT (%) | MCV (fl) | |
|---|---|---|---|---|---|---|
| threshold | <1.5% | <5% | <2% | <2.5% | <2% | <1.5% |
| mean | 0.8% | 4.4% | 1.1% | 1.7% | 1.2% | 0.6% |
| lower limit (95% CIs) | 0.7% | 4.0% | 1.0% | 1.6% | 1.1% | 0.5% |
| upper limit (95% CIs) | 0.9% | 4.7% | 1.2% | 1.9% | 1.3% | 0.6% |
| median | 0.7% | 4.1% | 1.0% | 1.6% | 1.1% | 0.5% |
| SD | 0.6% | 1.8% | 0.6% | 0.7% | 0.6% | 0.4% |
| min | 0.2% | 2.0% | 0.4% | 0.7% | 0.5% | 0.0% |
| max | 3.8% | 11.3% | 4.3% | 6.5% | 4.4% | 3.5% |
| IQ | 0.4% | 2.0% | 0.4% | 0.7% | 0.4% | 0.1% |
| Kruskal–Wallis test (*) | 20.46 (0.025) | 32.95 (0.000) | 13.80 (0.182) | 12.27 (0.269) | 14.27 (0.161) | 10.63 (0.387) |
| Median test (*) | 18.95 (0.041) | 20.58 (0.024) | 13.26 (0.210) | 7.65 (0.663) | 18.94 (0.041) | 8.77 (0.554) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillati, S.; Pati, I.; Delle Donne, M.; Meneghel, A.; Londero, D.; De Angelis, V. Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study. Diagnostics 2022, 12, 2586. https://doi.org/10.3390/diagnostics12112586
Tillati S, Pati I, Delle Donne M, Meneghel A, Londero D, De Angelis V. Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study. Diagnostics. 2022; 12(11):2586. https://doi.org/10.3390/diagnostics12112586
Chicago/Turabian StyleTillati, Silvia, Ilaria Pati, Michela Delle Donne, Alessandra Meneghel, Donatella Londero, and Vincenzo De Angelis. 2022. "Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study" Diagnostics 12, no. 11: 2586. https://doi.org/10.3390/diagnostics12112586
APA StyleTillati, S., Pati, I., Delle Donne, M., Meneghel, A., Londero, D., & De Angelis, V. (2022). Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study. Diagnostics, 12(11), 2586. https://doi.org/10.3390/diagnostics12112586








