Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Setting, and Data Collected
2.2. Assessments of Vitamin D Status
2.3. Statistical Analysis
3. Results
3.1. Demographic, Clinic, and Catheterization Characteristics of Our Study Population
3.2. Incidence of SMI
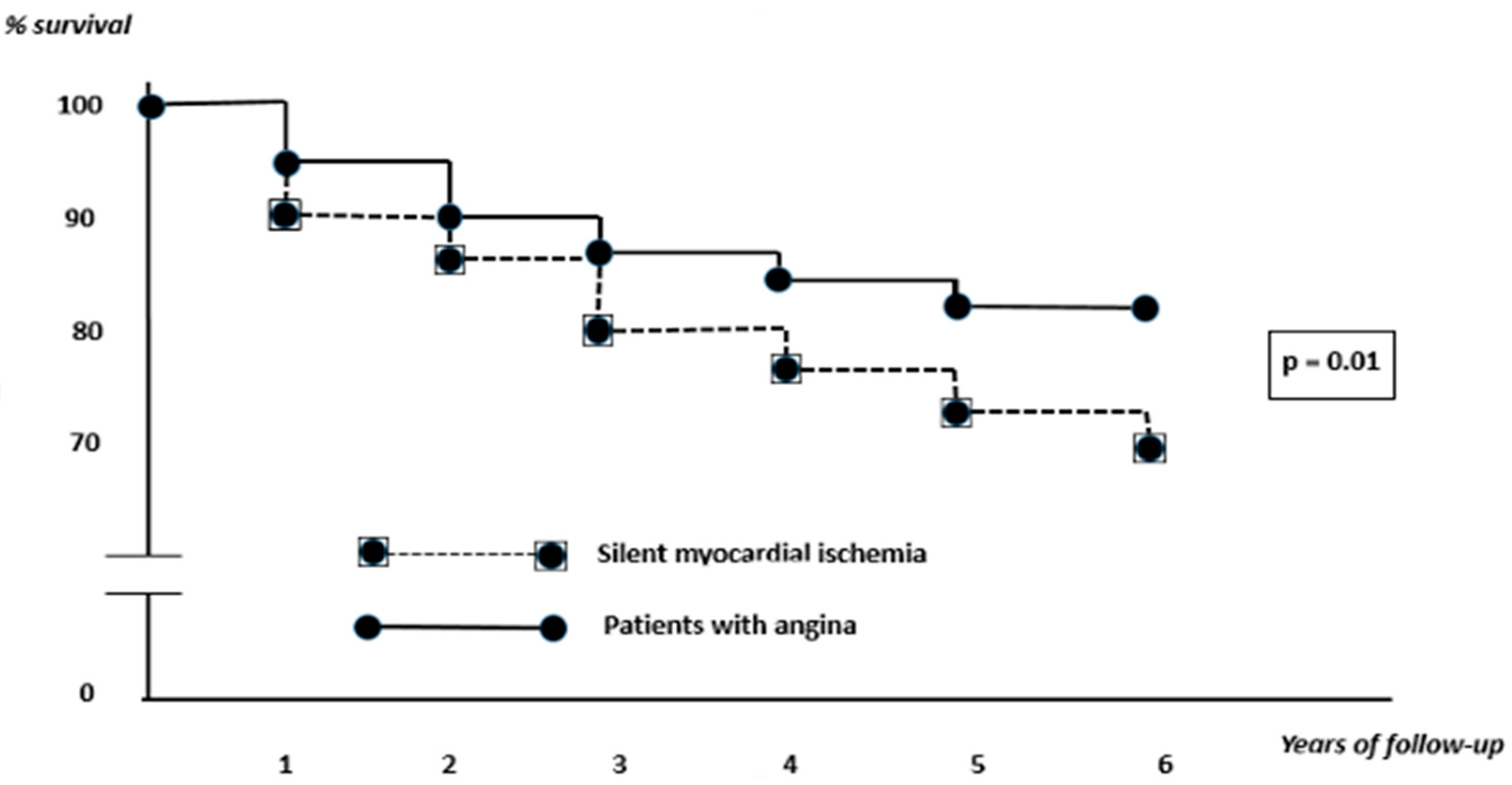
3.3. Therapeutic Options and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Kift, R.; Durkin, M.; O’Brien, S.; Vail, A.; Berry, J.; Rhodes, L. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br. J. Dermatol. 2010, 163, 1050–1055. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J.; et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Erba, P.; Saggese, G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital. J. Pediatr. 2014, 40, 54. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Muscogiuri, G.; Rubino, M.; Vuolo, L.; Pivonello, C.; Sabatino, P.; Pizzo, M.; Campanile, G.; Fittipaldi, R.; Lombardi, G.; et al. Hypovitaminosis D in adolescents living in the land of sun is correlated with incorrect life style: A survey study in Campania region. Endocrine 2015, 49, 521–527. [Google Scholar] [CrossRef]
- Beska, B.; Chan, D.; Gu, S.; Qiu, W.; Mossop, H.; Neely, D.; Kunadian, V. The association between vitamin D status and clinical events in high-risk older patients with non-ST elevation acute coronary syndrome undergoing invasive management. PLoS ONE 2019, 14, e0217476. [Google Scholar] [CrossRef]
- Qi, L.; Ma, W.; Heianza, Y.; Zheng, Y.; Wang, T.; Sun, D.; Rimm, E.B.; Hu, F.B.; Giovannucci, E.; Albert, C.; et al. Independent and Synergistic Associations of Biomarkers of Vitamin D Status with Risk of Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2204–2212. [Google Scholar] [CrossRef]
- Liu, L.; Chen, M.; Hankins, S.R.; Nùñez, A.E.; Watson, R.A.; Weinstock, P.J.; Newschaffer, C.J.; Eisen, H.J.; Drexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group. Serum 25-Hydroxyvitamin D Concentration and Mortality from Heart Failure and Cardiovascular Disease, and Premature Mortality from All-Cause in United States Adults. Am. J. Cardiol. 2012, 110, 834–839. [Google Scholar] [CrossRef]
- Zhu, K.; Knuiman, M.; Divitini, M.; Hung, J.; Lim, E.M.; Cooke, B.R.; Walsh, J.P. Serum 25-hydroxyvitamin D as a predictor of mortality and cardiovascular events: A 20-year study of a community-based cohort. Clin. Endocrinol. 2018, 88, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, H.; Yang, L.; Zhu, H.; Chen, S.; Lai, S. Age and Gender Differences in the Association between Serum 25-Hydroxyvitamin D and Stroke in the General US Population: The National Health and Nutrition Examination Survey, 2001–2006. J. Stroke Cerebrovasc. Dis. 2017, 26, 2510–2518. [Google Scholar] [CrossRef]
- Oz, F.; Cizgici, A.Y.; Oflaz, H.; Elitok, A.; Karaayvaz, E.B.; Mercanoglu, F.; Bugra, Z.; Omer, B.; Adalet, K.; Oncul, A. Impact of vitamin D insufficiency on the epicardial coronary flow velocity and endothelial function. Coron. Artery Dis. 2013, 24, 392–397. [Google Scholar] [CrossRef]
- Karohl, C.; Vaccarino, V.; Veledar, E.; Goldberg, J.; Tangpricha, V.; Bellasi, A.; Raggi, P. Vitamin D Status and Coronary Flow Reserve Measured by Positron Emission Tomography: A Co-Twin Control Study. J. Clin. Endocrinol. Metab. 2013, 98, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Safaie, N.; Rezaee, H.; Seif Dvati, B.; Entezari-Maleki, T. Vitamin D deficiency predicts the ST elevation type of myocardial infarction in patients with acute coronary syndrome. Iran. J. Pharm. Res. 2018, 17, 73–78. [Google Scholar] [PubMed]
- BARI Investigators. Seven-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) by treatment and diabetic status. J. Am. Coll. Cardiol. 2000, 35, 1122–1129. [Google Scholar] [CrossRef]
- Jouven, X.; Lemaître, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.-P.; Siscovick, D.S. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef]
- Eranti, A.; Kerola, T.; Aro, A.L.; Tikkanen, J.T.; Rissanen, H.A.; Anttonen, O.; Junttila, M.J.; Knekt, P.; Huikuri, H.V. Diabetes, glucose tolerance, and the risk of sudden cardiac death. BMC Cardiovasc. Disord. 2016, 16, 51. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Karppi, J.; Kurl, S. Impaired Fasting Plasma Glucose and Type 2 Diabetes Are Related to the Risk of Out-of-Hospital Sudden Cardiac Death and All-Cause Mortality. Diabetes Care 2013, 36, 1166–1171. [Google Scholar] [CrossRef]
- Cohn, P.F. Silent myocardial ischemia in patients with a defective anginal warning system. Am. J. Cardiol. 1980, 45, 697–702. [Google Scholar] [CrossRef]
- Marchant, B.; Umachandran, V.; Stevenson, R.; Kopelman, P.G.; Timmis, A.D. Silent myocardial ischemia: Role of subclinical neuropathy in patients with and without diabetes. J. Am. Coll. Cardiol. 1993, 22, 1433–1437. [Google Scholar] [CrossRef]
- Bosone, D.; Fogari, R.; Ramusino, M.C.; Ghiotto, N.; Guaschino, E.; Zoppi, A.; D’Angelo, A.; Costa, A. Ambulatory 24-h ECG monitoring and cardiovascular autonomic assessment for the screening of silent myocardial ischemia in elderly type 2 diabetic hypertensive patients. Heart Vessel. 2017, 32, 507–513. [Google Scholar] [CrossRef]
- Baltzis, D.; Roustit, M.; Grammatikopoulou, M.G.; Katsaboukas, D.; Athanasiou, V.; Iakovou, I.; Veves, A.; Manes, C.; Trakatelli, M.-C. Diabetic Peripheral Neuropathy as a Predictor of Asymptomatic Myocardial Ischemia in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Adv. Ther. 2016, 33, 1840–1847. [Google Scholar] [CrossRef]
- Filipović, N.; Ferhatović, L.; Marelja, I.; Puljak, L.; Grković, I. Increased vitamin D receptor expression in dorsal root ganglia neurons of diabetic rats. Neurosci. Lett. 2013, 549, 140–145. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Chevli, P.A.; Li, Y.; Soliman, E.Z. Vitamin D deficiency and electrocardiographic subclinical myocardial injury: Results from National Health and Nutrition Examination Survey-III. Clin. Cardiol. 2018, 41, 1468–1473. [Google Scholar] [CrossRef]
- Lv, W.S.; Zhao, W.J.; Gong, S.L.; Fang, D.D.; Wang, B.; Fu, Z.J.; Yan, S.L.; Wang, Y.G. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: A systematic review and meta-analysis. J. Endocrinol. Investig. 2015, 38, 513–518. [Google Scholar] [CrossRef]
- Zhou, Y.-K.; Liang, Z.; Guo, Y.; Zhang, H.-T.; Wang, K.-H. High glucose upregulates CYP24A1 expression which attenuates the ability of 1,25(OH)2D3 to increase NGF secretion in a rat Schwann cell line RSC96. Mol. Cell. Endocrinol. 2015, 404, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, R.; Fard, M.S.; Rahmani, F.; Moloudi, K.; Kalani, B.S.; Azarnezhad, A. Predisposing role of vitamin D receptor (VDR) polymorphisms in the development of multiple sclerosis: A case-control study. J. Neurol. Sci. 2016, 367, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Sismanlar, T.; Aslan, A.T.; Gulbahar, O.; Ozkan, S. The effect of vitamin D on lower respiratory tract infections in children. Turk. Arch. Pediatr. 2016, 51, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, M.; Sheng, W.; Liu, Q.; Yu, D.; Dong, Q.; Li, Q.; Wang, J. Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology 2015, 67, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Abreu, D.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S265–S277. [Google Scholar] [CrossRef]
- Prüfer, K.; Veenstra, T.D.; Jirikowski, G.F.; Kumar, R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J. Chem. Neuroanat. 1999, 16, 135–145. [Google Scholar] [CrossRef]
- Tague, S.E.; Smith, P.G. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: Modulation by ovarian hormones. J. Chem. Neuroanat. 2011, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Terenghi, G.; Warner, G.; Kopelman, P.; Williams-Chestnut, R.; Sinicropi, D. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat. Med. 1996, 2, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Ozuguz, U.; Oruc, S.; Ulu, M.S.; Demirbas, H.; Acay, A.; Coker, B.; Beyazıt, B.; Yaman, M.; Koken, T. Does vitamin D have any role in the improvement of diabetic peripheral neuropathy in type 1 diabetic patients? J. Endocrinol. Investig. 2016, 39, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Assey, M.E.; Walters, G.L.; Hendrix, G.H.; Carabello, B.A.; Usher, B.W.; Spann, J.F. Incidence of acute myocardial infarction in patients with exercise-induced silent myocardial ischemia. Am. J. Cardiol. 1987, 59, 497–500. [Google Scholar] [CrossRef]
- Gottlieb, S.O.; Achuff, S.C.; Baumgardner, R.; Mellits, E.D.; Weisfeldt, M.L.; Gerstenblith, G. Silent ischemia on Holter monitoring predicts mortality in high-risk postinfarction patients. JAMA 1988, 259, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Egstrup, K. Asymptomatic myocardial ischemia as a predictor of cardiac events after coronary artery bypass grafting for stable angina pectoris. Am. J. Cardiol. 1988, 61, 248–252. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, J.M.; Park, I.; Kim, J.; Rhee, T.-M.; Hwang, D.; Park, J.; Park, T.K.; Yang, J.H.; Bin Song, Y.; et al. Comparison of long-term clinical outcomes between revascularization versus medical treatment in patients with silent myocardial ischemia. Int. J. Cardiol. 2018, 277, 47–53. [Google Scholar] [CrossRef]
- Chiariello, M.; Indolfi, C. Silent Myocardial Ischemia in Patients with Diabetes Mellitus. Circulation 1996, 93, 2089–2091. [Google Scholar] [CrossRef]
- Ditchburn, C.J.; Hall, J.; De Belder, M.; Davies, A.G.; Kelly, W.F.; Bilous, R.W. Silent myocardial ischaemia in patients with proved coronary artery disease: A comparison of diabetic and non-diabetic patients. Postgrad. Med. J. 2001, 77, 395–398. [Google Scholar] [CrossRef]
- Fredenrich, A.; Castillo-Ros, S.; Hieronimus, S.; Baudouy, M.; Harter, M.; Canivet, B. Screening for silent myocardial ischemia in diabetic patients. Diabetes Care 2000, 23, 563–564. [Google Scholar] [CrossRef][Green Version]
| Parameter | Healthy Volunteers | No Myocardial Ischemia | Symptomatic Myocardial Ischemia | Silent Myocardial Ischemia | Post Hoc p-Value, Comparing Silent and Symptomatic Ischemia |
|---|---|---|---|---|---|
| n | 50 | 50 | 125 | 78 | |
| Demographic and anthropometric parameters | |||||
| Age, years | 62.0 ± 9.0 | 55.4 ± 8.2 | 61.9 ± 9.4 | 64.1 ± 10.3 | 0.03 |
| Male gender, % | 72.0 (n = 36) | 80.0 (n = 40) | 72.0 (n = 90) | 71.8 (n = 56) | 0.7 |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | |
| Coronary risk factors | |||||
| BMI, kg/m2 | 26.1 ± 4.6 | 30.1 ± 6.7 | 31.1 ± 5.5 | 33.8 ± 6.6 | 0.04 |
| Diabetes duration, years | 6.5 ± 6.0 | 13.5 ± 7.0 | 15.5 ± 9.0 | 0.03 | |
| HbA1c, mmol/mol | 62 ± 16 | 62 ± 15 | 65 ± 17 | 0.2 | |
| HbA1c, % | 7.9 ± 1.9 | 7.9 ± 1.6 | 8.1 ± 1.6 | ||
| Total cholesterol, mmol/L | 4.1 ± 1.4 | 4.2 ± 1.5 | 4.0 ± 1.6 | 0.6 | |
| MAP, mmHg | 91.1 ± 12.8 | 101.5 ± 11.4 | 100.9 ± 11.0 | 102.0 ± 12.7 | 0.1 |
| eGFR, ml/min/1.73 m2 | 90 ± 25 | 82 ± 21 | 67 ± 23 | 65 ± 24 | 0.5 |
| Urine ACR, mg/mmol | 0.6 ± 0.9 | 4.1 ± 7.2 | 3.3 ± 30.1 | 0.1 | |
| 25(OH)D, mmol/L (±SEM) | 62.1 (6.7) | 53.1 (6.2) | 49.6 (6.1) | 34.9 (5.8) | 0.01 |
| Summer (from July to September) determination of 25(OH)D, % (n) | 28.0 (n = 14) | 30.0 (n = 15) | 28.8 (n = 36) | 29.5 (n = 23) | 0.6 |
| Left ventricular ejection fraction, median (iqr), % | 65 (60–70) | 60 (50–70) | 58 (50–66) | 59 (52–66) | 0.1 |
| Exercise test characteristics | |||||
| Exercise time (iqr), min | 8.5 (7.8–9.2) | 4.5 (3.9–5.1) | 4.9 (3.9–5.9) | 0.1 | |
| Exercise heart rate, median (iqr), bpm | 128 (112–138) | 112 (105–119) | 118 (109–127) | 0.3 | |
| ST depression, median (iqr), mm | 0.17 (0.15–0.20) | 0.20 (0.12–0.28) | 0.2 | ||
| Catheterization characteristics | |||||
| 1-vessel CAD, % (n) | 20.0 (n = 25) | 19.3 (n = 15) | 0.9 | ||
| 2-vessel CAD, % (n) | 29.6 (n = 37) | 26.9 (n = 21) | 0.4 | ||
| 3-vessel CAD, % (n) | 47.2 (n = 59) | 50.0 (n = 39) | 0.5 | ||
| Left main, % (n) | 3.2 (n = 4) | 3.8 (n = 3) | 0.8 | ||
| Parameter | B | SE | Wald | p | Odds Ratio | 95% CI for Odds Ratio | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.10 | 0.06 | 2.8 | 0.09 | 1.11 | 0.98 | 1.25 |
| Body mass index | 0.02 | 0.06 | 1.3 | 0.22 | 1.02 | 0.92 | 1.20 |
| Diabetes duration | 0.08 | 0.05 | 2.5 | 0.11 | 1.08 | 0.97 | 1.19 |
| Male gender | 0.27 | 0.40 | 0.9 | 0.8 | 1.25 | 0.51 | 2.87 |
| Summer determination of 25(OH)D | 0.34 | 0.20 | 1.0 | 0.6 | 1.50 | 0.74 | 2.12 |
| 25(OH)D | −0.06 | 0.03 | 5.9 | 0.01 | 1.11 | 1.06 | 1.17 |
| Constant | −11.28 | 6.9 | 2.2 | 0.10 | 0.00 | ||
| Therapeutic Options * | Patients with Symptomatic Myocardial Ischemia (n = 125) | Patients with Silent Myocardial Ischemia (n = 78) | p ** |
|---|---|---|---|
| Optimal medical treatment | 3.2% (n = 4) | 3.8% (n = 3) | 0.5 |
| Percutaneous revascularization | 68.8% (n = 86) | 68.0% (n = 53) | 0.3 |
| Surgical revascularization | 28.0% (n = 35) | 28.2% (n = 22) | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, R.; Talarico, M.; Pascale, A.; Pascale, V.; Minici, R.; Boriani, G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics 2022, 12, 2572. https://doi.org/10.3390/diagnostics12112572
Rossi R, Talarico M, Pascale A, Pascale V, Minici R, Boriani G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics. 2022; 12(11):2572. https://doi.org/10.3390/diagnostics12112572
Chicago/Turabian StyleRossi, Rosario, Marisa Talarico, Alessandra Pascale, Vittorio Pascale, Roberto Minici, and Giuseppe Boriani. 2022. "Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance" Diagnostics 12, no. 11: 2572. https://doi.org/10.3390/diagnostics12112572
APA StyleRossi, R., Talarico, M., Pascale, A., Pascale, V., Minici, R., & Boriani, G. (2022). Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics, 12(11), 2572. https://doi.org/10.3390/diagnostics12112572








