Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Measures and Outcomes
2.3. Data Analysis
2.4. Endpoints of the Study
2.5. Statistical Analysis
3. Results
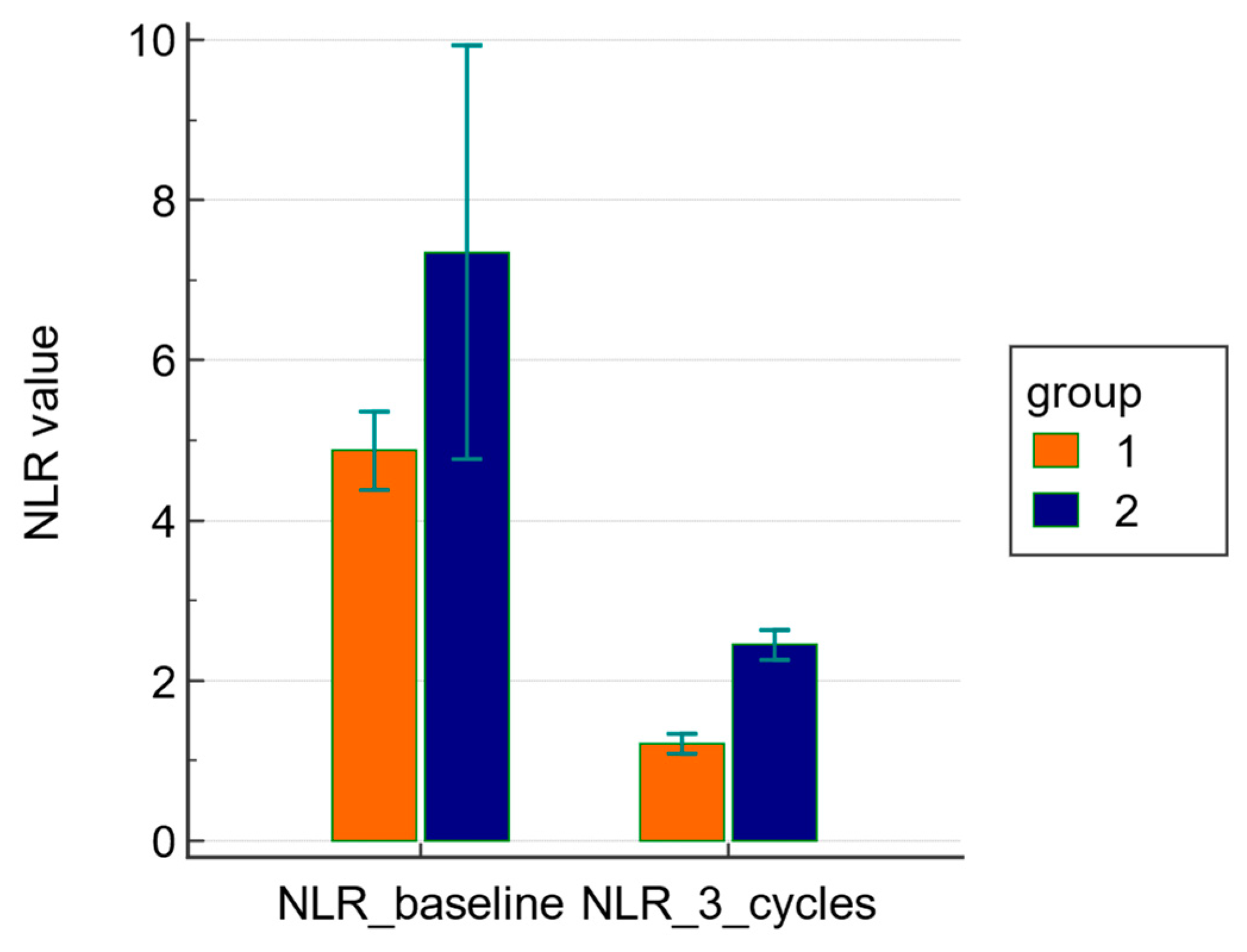
3.1. Primary Endpoints: Comparison of NLR at Baseline and after Three Cycles of NACT between Groups 1 and 2
3.2. Secondary Endpoints
3.2.1. Comparison of CRP, Fibrinogen, IL-6, Ferritin, Albumin, and mGPS Levels at Baseline and after Three Cycles of NACT between Groups 1 and 2
3.2.2. Correlation Analysis between NLR, CPR, Fibrinogen, IL-6, Albumin, mGPS, and Objective Tumor Response
3.2.3. Correlation Analysis between NLR and CRS Score
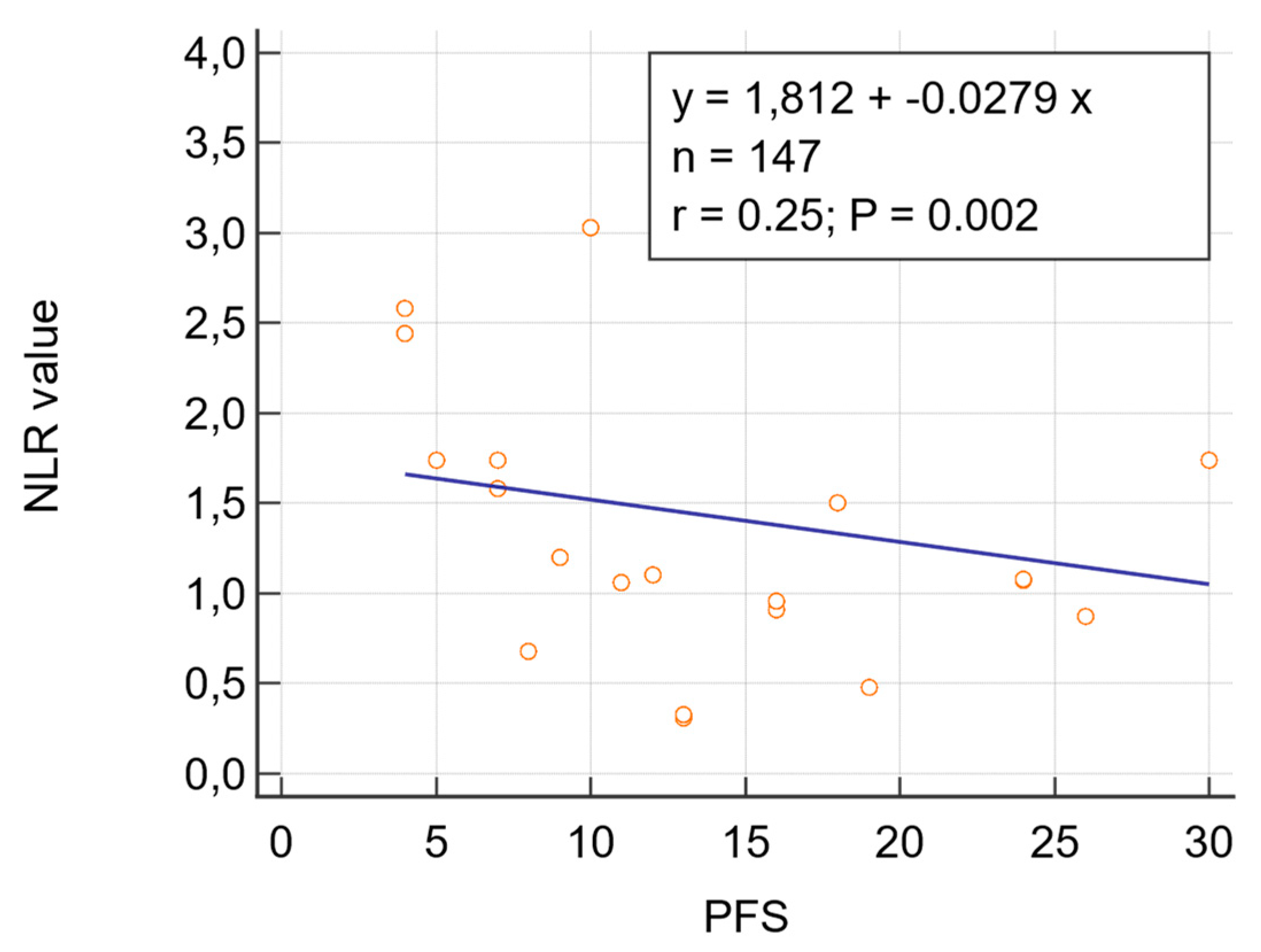
3.2.4. Correlation Analysis between NLR and PFS
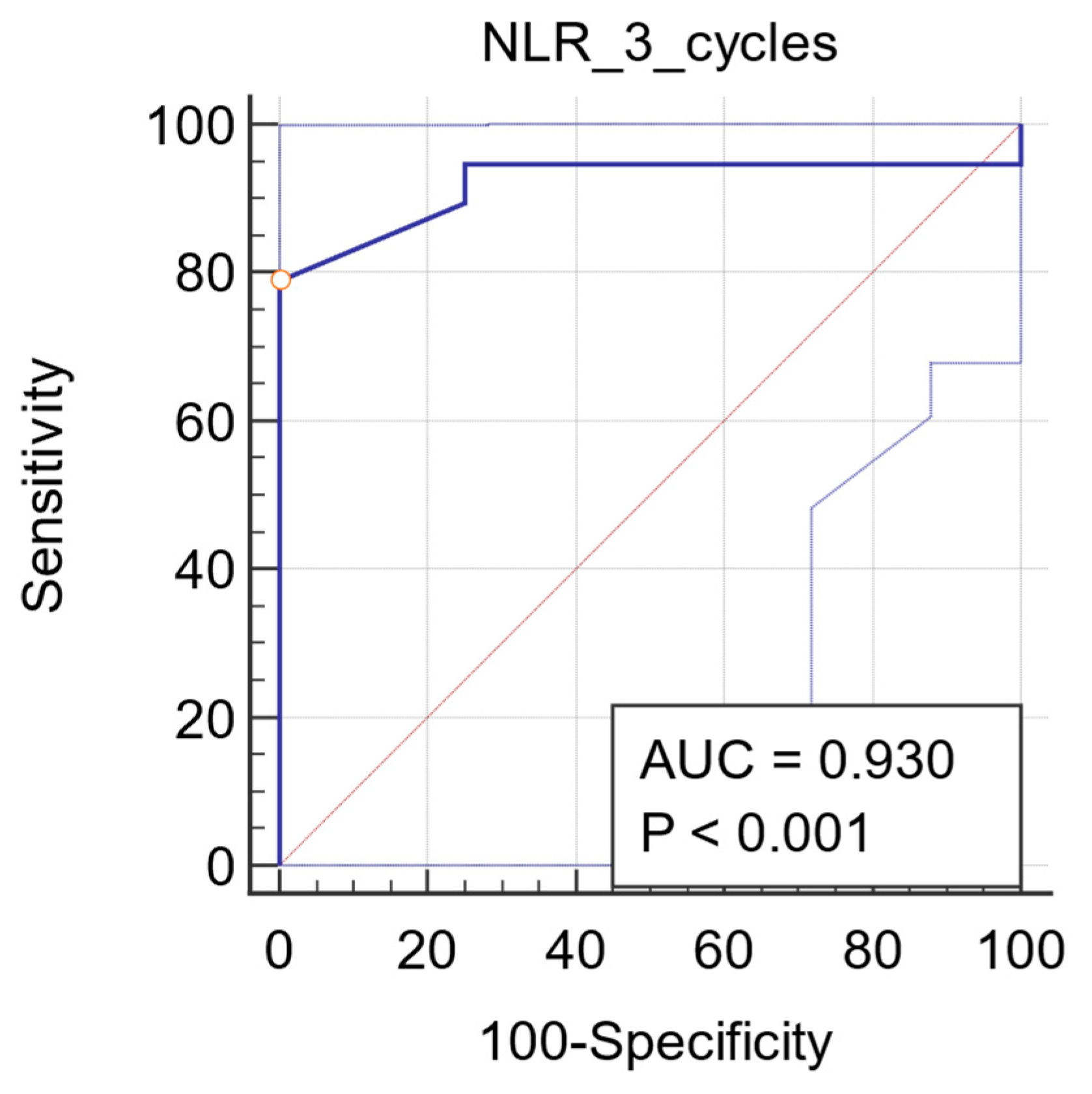
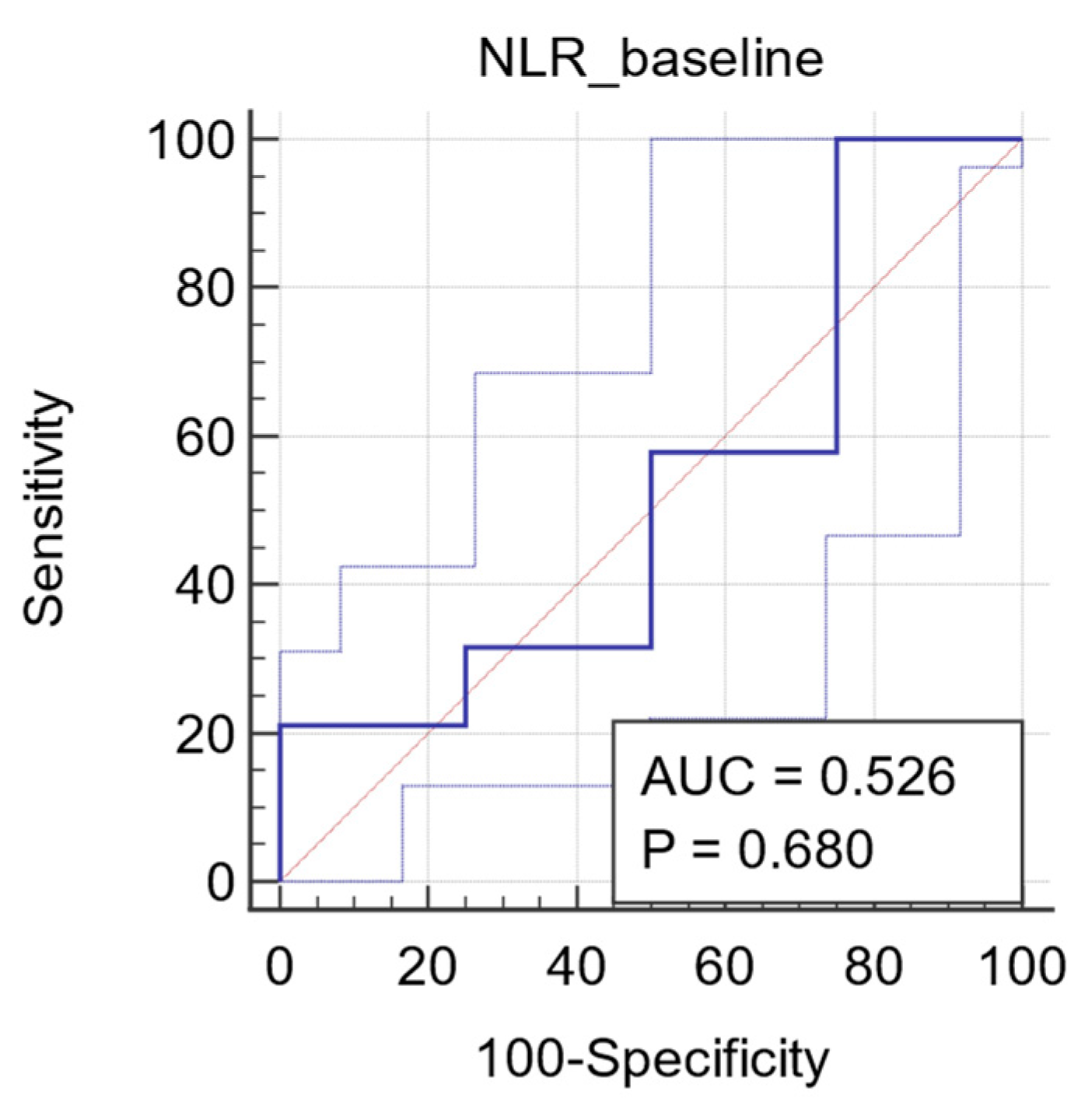
3.2.5. ROC Analysis
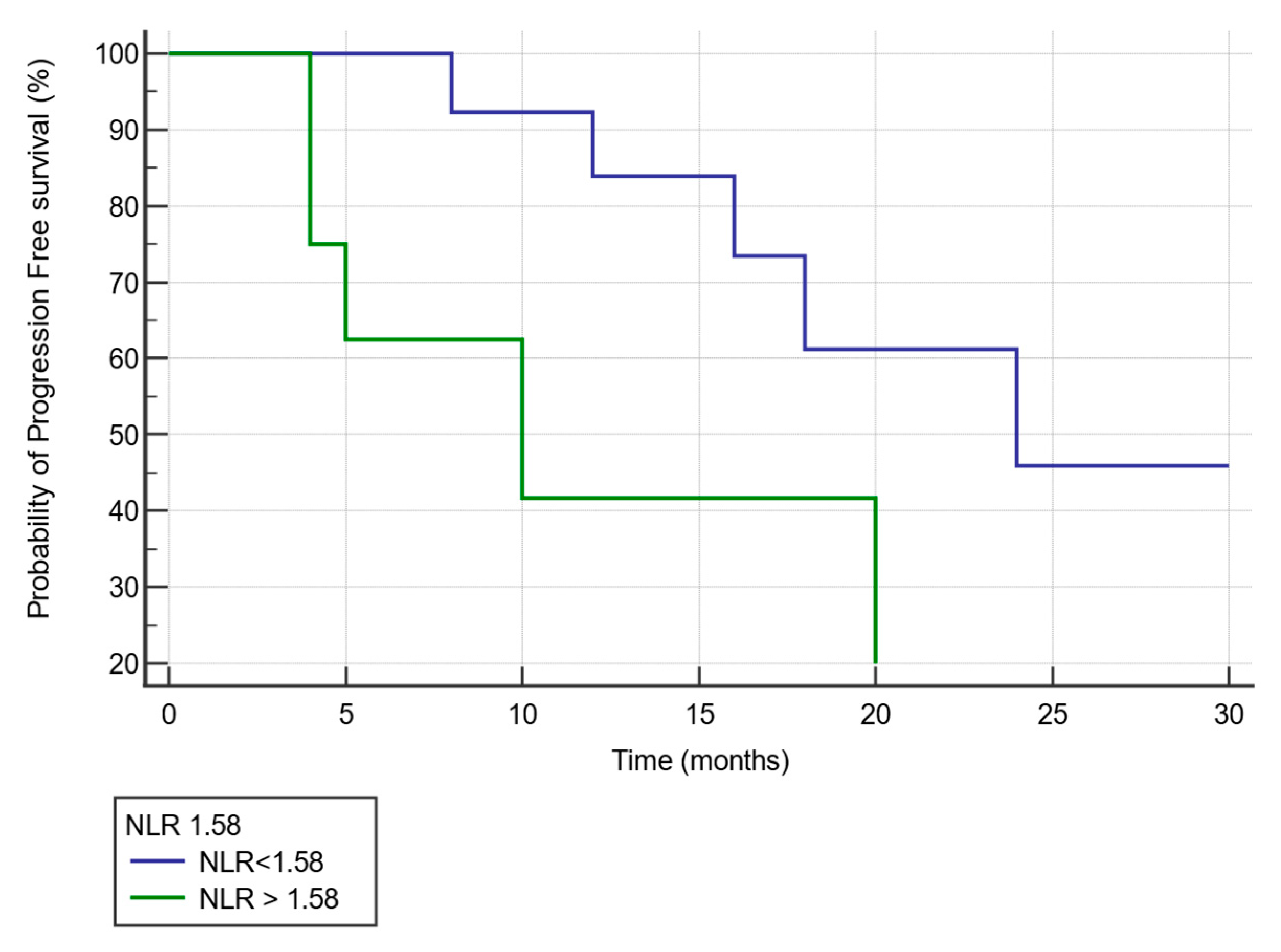
3.2.6. Kaplan–Meier Curve Analysis of Progression Free Survival by NLR Value after Three Cycles of NACT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A.J. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease tolerance as a defense strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef] [Green Version]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Macciò, A.; Gramignano, G.; Cherchi, M.C.; Tanca, L.; Melis, L.; Madeddu, C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci. Rep. 2020, 10, 6096. [Google Scholar] [CrossRef] [Green Version]
- Madeddu, C.; Gramignano, G.; Kotsonis, P.; Coghe, F.; Atzeni, V.; Scartozzi, M.; Macciò, A. Microenvironmental M1 tumor-associated macrophage polarization influences cancer-related anemia in advanced ovarian cancer: Key role of interleukin-6. Haematologica 2018, 103, e388–e391. [Google Scholar] [CrossRef] [Green Version]
- Macciò, A.; Lai, P.; Santona, M.C.; Pagliara, L.; Melis, G.B.; Mantovani, G. High serum levels of soluble IL-2 receptor, cytokines, and C reactive protein correlate with impairment of T cell response in patients with advanced epithelial ovarian cancer. Gynecol. Oncol. 1998, 69, 248–252. [Google Scholar] [CrossRef]
- Fox, P.; Hudson, M.; Brown, C.; Lord, S.; Gebski, V.; De Souza, P.; Lee, C.K. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br. J. Cancer 2013, 109, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, W.J.; Zhi, Q.; Shen, M.; Jiang, M.; Bian, X.; Gong, F.R.; Zhou, C.; Lian, L.; Wu, M.Y.; et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget 2017, 8, 88835–88844. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Yin, X.; Wu, L.; Yang, H.; Yang, H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine 2019, 98, e17475. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Labidi-Galy, S.I.; Terry, K.L.; Vitonis, A.F.; Welch, W.R.; Goodman, A.; Cramer, D.W. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol. Oncol. 2014, 132, 542–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Li, L.; Wang, X.; Wang, C.; Wang, D. Neutrophil-to-Lymphocyte Ratio and Platelet Count Predict Long-Term Outcome of Stage IIIC Epithelial Ovarian Cancer. Cell. Physiol. Biochem. 2018, 46, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Y.; Kucukzeybek, Y.; Alacacioglu, A.; Varol, U.; Yildiz, I.; Akyol, M.; Kabadayι, G.; Taskaynatan, H.; Salman, T.; Bayoglu, V.; et al. Prognostic value of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with epithelial ovarian cancer. Eur. J. Gynaecol. Oncol. 2017, 38, 444–448. [Google Scholar]
- Thavaramara, T.; Phaloprakarn, C.; Tangjitgamol, S.; Manusirivithaya, S. Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J. Med. Assoc. Thai 2011, 94, 871–877. [Google Scholar] [PubMed]
- Salman, L.; Sabah, G.; Jakobson-Setton, A.; Raban, O.; Yeoshoua, E.; Eitan, R. Neutrophil-to-lymphocyte ratio as a prognostic factor in advanced stage ovarian carcinoma treated with neoadjuvant chemotherapy. Int. J. Gynaecol. Obstet. 2020, 148, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Jeerakornpassawat, D.; Suprasert, P. Potential predictors for chemotherapeutic response and prognosis in epithelial ovarian, fallopian tube and primary peritoneal cancer patients treated with platinum-based chemotherapy. Obstet. Gynecol. Sci. 2020, 63, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Factor for High-Grade Serous Ovarian Cancer. PLoS ONE 2016, 11, e0156101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, P.; Xu, Y.; Zhang, W.; Tong, L.; Guo, Z.; Ni, H. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother. Pharmacol. 2015, 75, 255–262. [Google Scholar] [CrossRef]
- Li, Z.; Hong, N.; Robertson, M.; Wang, C.; Jiang, G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci. Rep. 2017, 7, 43001. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Hur, H.W.; Kim, S.W.; Kim, S.H.; Kim, J.H.; Kim, Y.T.; Lee, K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. 2009, 58, 15–23. [Google Scholar] [CrossRef]
- Miao, Y.; Yan, Q.; Li, S.; Li, B.; Feng, Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016, 17, 33–40. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, I.; Chung, Y.S.; Nam, E.; Kim, S.; Kim, S.W.; Lee, J.Y. Pretreatment neutrophil-to-lymphocyte ratio and its dynamic change during neoadjuvant chemotherapy as poor prognostic factors in advanced ovarian cancer. Obstet. Gynecol. Sci. 2018, 61, 227–234. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Cosentino, F.; Chiantera, V.; Legge, F.; Carbone, V.; Scambia, G.; Fagotti, A. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy Response Score: Development and Validation of a System to Quantify Histopathologic Response to Neoadjuvant Chemotherapy in Tubo-Ovarian High-Grade Serous Carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef]
- Zorzato, P.C.; Zannoni, G.F.; Tudisco, R.; Pasciuto, T.; Di Giorgio, A.; Franchi, M.; Scambia, G.; Fagotti, A. External validation of a “response score” after neoadjuvant chemotherapy in patients with high-grade serous ovarian carcinoma with complete clinical response. Int. J. Gynecol. Cancer 2020, 30, 67–73. [Google Scholar] [CrossRef]
- McMillan, D.C.; Crozier, J.E.M.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Tanca, L.; Cherchi, M.C.; Floris, C.; Omoto, I.; Barracca, A.; Ganz, T. The role of inflammation, iron, and nutritional status in cancer-related anemia: Results of a large, prospective, observational study. Haematologica 2015, 100, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Macciò, A.; Busquets, S.; Madeddu, C.; Argilés, J.M. Editorial: Biological Mechanism-Based and Patient-Centered Management of Cancer-Related Symptoms and Syndromes. Front. Physiol. 2018, 9, 1819. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Lewis, C.; Collins, H.H.; Shabto, J.M.; Akce, M.; Kissick, H.T.; Carthon, B.C.; Shaib, W.L.; et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 2019, 125, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, B.; Wang, L.; Wang, R.; Yang, X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine 2019, 98, e13788. [Google Scholar] [CrossRef] [PubMed]
- Huszno, J.; Kolosza, Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol. Lett. 2019, 18, 6275–6283. [Google Scholar] [CrossRef]
- Gong, J.; Jiang, H.; Shu, C.; Hu, M.Q.; Huang, Y.; Liu, Q.; Li, R.F. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: A meta-analysis. J. Ovarian Res. 2019, 12, 51. [Google Scholar] [CrossRef]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, W.; Wu, Y.; Luo, Y.; Wu, B.; Cheng, J.; Chen, J.; Liu, D.; Li, C. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 15. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, Y.S.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Pretreatment lymphocytopenia is an adverse prognostic biomarker in advanced-stage ovarian cancer. Cancer Med. 2019, 8, 564–571. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, S.; Scott, S.; Glanc, P.; Eiriksson, L.; Jang, J.H.; Sebastianelli, A.; Dean, E. Guideline No. 403: Initial Investigation and Management of Adnexal Masses. J. Obstet. Gynaecol. Can. 2020, 42, 1021–1029.e3. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Blackman, A.; Miller, M.C.; Robison, K.; DiSilvestro, P.A.; Eklund, E.E.; Strongin, R.; Messerlian, G. Multiple biomarker algorithms to predict epithelial ovarian cancer in women with a pelvic mass: Can additional makers improve performance? Gynecol. Oncol. 2019, 154, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Sanna, E.; Neri, M.; Oppi, S.; Madeddu, C. Cachexia as Evidence of the Mechanisms of Resistance and Tolerance during the Evolution of Cancer Disease. Int. J. Mol. Sci. 2021, 22, 2890. [Google Scholar] [CrossRef]
| Characteristics | Mean (Range) |
|---|---|
| Age (yr) | 57 (39–86) |
| BMI | 27 (16–32) |
| Histology | Mean (%) |
| HGSC | 161 (100.0) |
| Non-HGSC | 0 (0.0) |
| FIGO stage | Mean (%) |
| IIIC | 47 (29.2) |
| IVA | 76 (47.2) |
| IVB | 38 (23.6) |
| Performance status | |
| 0–1 | 30% |
| 2 | 60% |
| 3 | 10% |
| Regimen of NACT | Mean (%) |
| Taxane + carboplatin | 161 (100.0) |
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| N = 132 | N = 29 | ||
| NLR | 4.8 (1.3–13.7) | 7.3 (2.7–18.6) | 0.6688 |
| CRP (mg/dL) | 5.6 (0.1–21) | 5.9 (0.4–14.6) | 0.0984 |
| Fibrinogen (mg/dL) | 556 (318–1052) | 598 (299–774) | 0.1983 |
| IL-6 (pg/mL) | 22.3 ± 8.9 | 21 ± 1.3 | 0.2381 |
| Ferritin (ng/mL) | 421 ± 147 | 430 ± 168 | 0.3876 |
| Albumin (g/dL) | 3.1 ± 0.8 | 3.0 ± 0.7 | 0.5670 |
| GPS | 2 ± 1.5 | 2 ± 1.8 | 0.7652 |
| Baseline | After Three Cycles | |||||
|---|---|---|---|---|---|---|
| R | p Value | CI 95% | r | p Value | CI 95% | |
| CRP (mg/L) | 0.469 | <0.0001 * | 0.336 to 0.584 | 0.422 | <0.0001 * | 0.438 to 0.541 |
| Fibrinogen | 0.089 | 0.069 | −0.335 to 0.484 | 0.033 | 0.881 | −0.384 to 0.439 |
| Ferritin (ng/mL) | 0.329 | 0.036 * | 0.0765 to 0.370 | 0.554 | 0.006 * | 0.237 to 0.653 |
| IL-6 (pg/mL) | −0.652 | 0.001 * | 0.595 to 0.744 | 0.600 | 0.003 * | 0.337 to 0.561 |
| Albumin | −0.197 | 0.083 | −0.122 to 0.187 | 0.235 | 0.067 | −0.0517 to 0.254 |
| GPS | −0.490 | 0.021 * | 0.012 to 0.711 | 0.390 | 0.029 * | 0.065 to 0.752 |
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| N = 132 | N = 29 | ||
| NLR | 1.2 ± 0.8 | 2.4 ± 0.5 | <0.0001 |
| CRP (mg/dL) | 0.4 ± 0.3 | 1.3 ± 1.8 | 0.0038 |
| Fibrinogen (mg/dL) | 373 ± 88 | 400 ± 114 | 0.1894 |
| IL-6 (pg/mL) | 12.9 ± 10.5 | 21.3 ± 13.1 | 0.0068 |
| Ferritin (ng/mL) | 351 ± 167 | 500 ± 268 | 0.0166 |
| Albumin (g/dL) | 3.4 ± 1.1 | 3.1 ± 0.8 | 0.0423 |
| GPS | 1 ± 0.8 | 2 ± 1.5 | 0.0382 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, E.; Tanca, L.; Cherchi, C.; Gramignano, G.; Oppi, S.; Chiai, M.G.; Macciò, A.; Madeddu, C. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics 2021, 11, 1298. https://doi.org/10.3390/diagnostics11071298
Sanna E, Tanca L, Cherchi C, Gramignano G, Oppi S, Chiai MG, Macciò A, Madeddu C. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics. 2021; 11(7):1298. https://doi.org/10.3390/diagnostics11071298
Chicago/Turabian StyleSanna, Elisabetta, Luciana Tanca, Cristina Cherchi, Giulia Gramignano, Sara Oppi, Maria Gloria Chiai, Antonio Macciò, and Clelia Madeddu. 2021. "Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer" Diagnostics 11, no. 7: 1298. https://doi.org/10.3390/diagnostics11071298
APA StyleSanna, E., Tanca, L., Cherchi, C., Gramignano, G., Oppi, S., Chiai, M. G., Macciò, A., & Madeddu, C. (2021). Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics, 11(7), 1298. https://doi.org/10.3390/diagnostics11071298







