Digital Biomarkers for Neuromuscular Disorders: A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
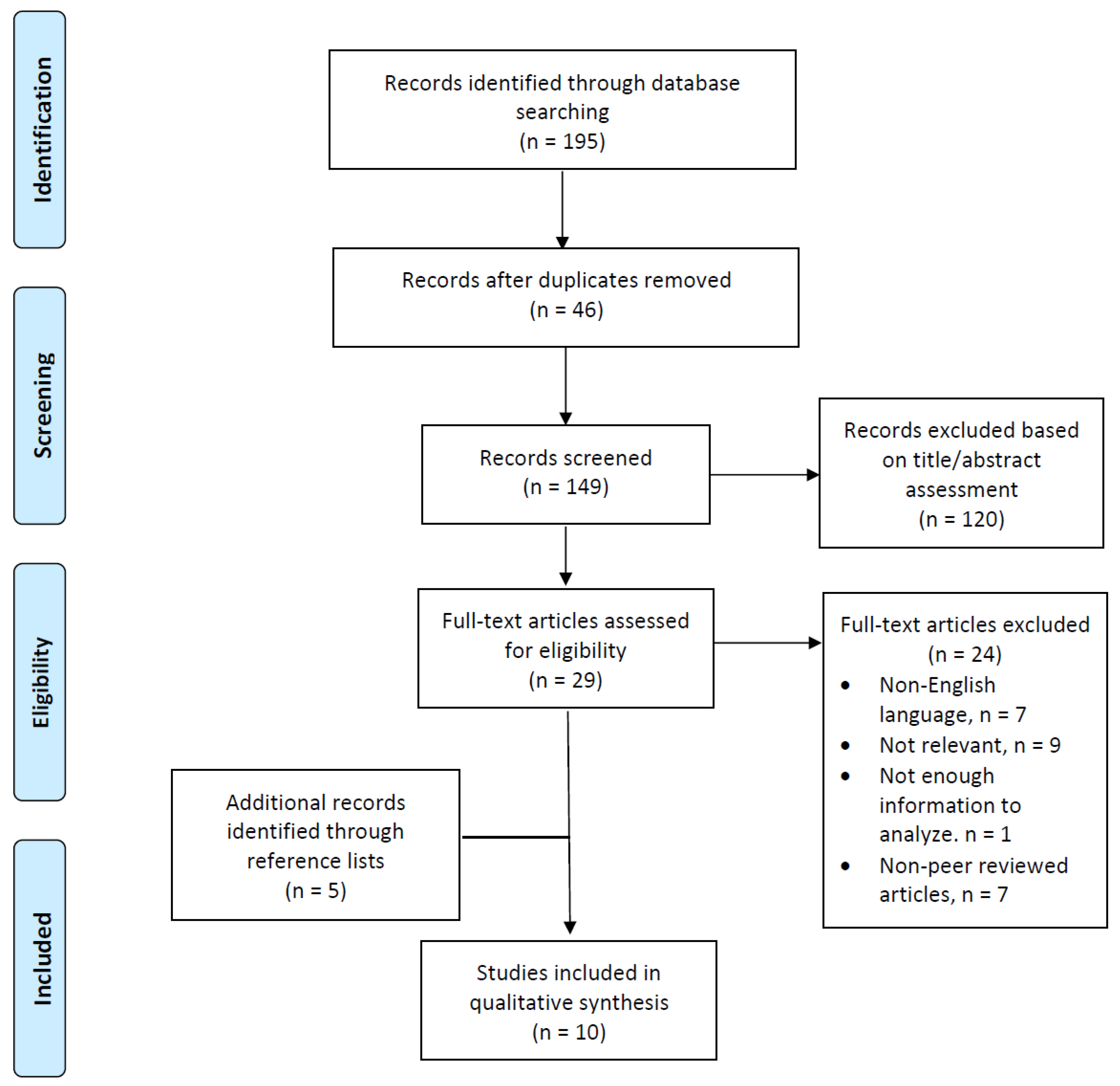
2.1. Data Sources and Searching
2.2. Eligibility Criteria
2.3. Study Selection Process and Data Extraction
3. Results
3.1. Overview of Observational Studies
3.2. Overview of Included Animal Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
Appendix B
References
- McDonald, C.M. Clinical approach to the diagnostic evaluation of hereditary and acquired neuromuscular diseases. Phys. Med. Rehabili. Clin. N. Am. 2012, 23, 496–563. [Google Scholar] [CrossRef] [PubMed]
- Scotton, C.; Passarelli, C.; Neri, M.; Ferlini, A. Biomarkers in rare neuromuscular diseases. Exp. Cell. Res. 2013, 325, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Papapetropoulos, S.; Xiong, M.; Kieburtz, K. The first frontier: Digital biomarkers for neurodegenerative disorders. Digit. Biomark. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Market Research Blog-The Digital Biomarkers Market: Key Drivers and Challenges: 11 March 2020. Available online: https://blog.marketresearch.com/the-digital-biomarkers-market-key-drivers-and-challenges (accessed on 28 May 2021).
- Dodge, H.H.; Mattek, N.C.; Austin, D.; Hayes, T.L.; Kaye, J.A. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology 2012, 78, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Seelye, A.; Mattek, N.; Sharma, N.; Riley, T.; Austin, J.; Wild, K.; Dodge, H.H.; Lore, E.; Kaye, J. Weekly observations of online survey metadata obtained through home computer use allow for detection of changes in everyday cognition before transition to mild cognitive impairment. Alzheimers Dement. 2018, 14, 187–194. [Google Scholar] [CrossRef]
- Godinho, C.; Domingos, J.; Cunha, G.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Goncalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabil. 2016, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/wearable devices opportunity. NPJ Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Thielen, F.W.; Van Mastrigt, G.; Burgers, L.T.; Bramer, W.M.; Majoie, H.J.M.; Evers, S.; Kleijinen, J. How to prepare a systematic review of economic evaluations for clinical practice guidelines: Database selection and search strategy development (part 2/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 705–721. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS peer review of electronic search strategies: 2015 guideline statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Garcia-Gancedo, L.; Kelley, M.L.; Lavrow, A.; Parr, J.; Hart, R.; Marsden, R.; Turner, M.R.; Talbot, K.; Chiwera, T.; Shaw, C.E.; et al. Objectively monitoring amyotrophic lateral sclerosis patient symptoms during clinical trials with sensors: Observational study. JMIR Mhealth Uhealth 2019, 7, e13433. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Lavrow, A.; Garcia-Gancedo, L.; Parr, J.; Hart, R.; Chiwera, T.; Shaw, C.E.; Al-Chalabi, A.; Marsden, R.; Turner, M.R.; et al. The use of biotelemetry to explore disease progression markers in amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2020, 21, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, G.M.; Hahn, S.; Liss, J.; Shefner, J.; Rutkove, S.B.; Kawabata, K.; Bhandari, S.; Shelton, K.; Duncan, C.J.; Berisha, V. Repeatability of commonly used speech and language features for clinical applications. Digit. Biomark. 2020, 4, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, G.M.; Hahn, S.; Liss, J.; Shefner, J.; Rutkove, S.; Shelton, K.; Duncan, C.J.; Berisha, V. Early detection and tracking of bulbar changes in ALS via frequent and remote speech analysis. NPJ. Digit. Med. 2020, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Herberer, K.; Fowler, E.; Staudt, L.; Sienko, S.; Buckon, C.E.; Bagley, A.; Sison-Williamson, M.; McDonald, C.M.; Sussman, M.D. Hip kinetics during gait are clinically meaningful outcomes in young boys with Duchenne muscular dystrophy. Gait Posture. 2016, 48, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Le Moing, A.-G.; Seferian, A.M.; Moraux, A.; Annoussamy, M.; Dorveaux, E.; Gasnier, E.; Hogrel, J.-Y.; Voit, T.; Vissiere, D.; Servais, L. A movement monitor based on magneto-inertial sensors for non-ambulant patients with Duchenne muscular dystrophy: A pilot study in controlled environment. PLoS ONE 2016, 11, e0156696. [Google Scholar] [CrossRef] [PubMed]
- Lilien, C.; Gasnier, E.; Gidaro, T.; Seferia, A.; Grelet, M.; Vissiere, D.; Servais, L. Home-based monitor for gait and activity analysis. J. Vis. Exp. 2019, 150, e69668. [Google Scholar] [CrossRef]
- Chen, X.; Siebourg-Polster, J.; Wolf, D.; Czech, C.; Bonati, U.; Fischer, D.; Khwaja, O.; Strahm, M. Feasibility of using Microsoft Kinect to assess upper limb movement in type III spinal muscular atrophy patients. PLoS ONE 2017, 12, e0170472. [Google Scholar] [CrossRef]
- Chabanon, A.; Seferian, A.M.; Daron, A.; Pereon, Y.; Cances, C.; Vuillerot, C.; De Waele, L.; Cuisset, J.-M.; Laugel, V.; Schara, U.; et al. Prospective and longitudinal natural history study of patients with Type 2 and 3 spinal muscular atrophy: Baseline data NatHis-SMA study. PLoS ONE 2018, 13, e0201004. [Google Scholar] [CrossRef]
- Golini, E.; Rigamonti, M.; Iannello, F.; De Rosa, C.; Scavizzi, F.; Raspa, M.; Mandillo, S. A non-invasive digital biomarker for the detection of rest disturbances in the SOD1G93A mouse model of ALS. Front. Neurosci. 2020, 14, 896. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.L.S.; Andersen, H. Outcome measures in clinical trials of patients with myasthenia gravis. Front. Neurol. 2020, 11, 596382. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, D.H.; Choi, B.K.; Han, I.H. Internet of things, digital biomarker, and artificial intelligence in spine: Current and future perspectives. Neurospine 2019, 16, 705–711. [Google Scholar] [CrossRef]
- Haghi, M.; Thurow, K.; Stoll, R. Wearable devices in medical Internet of Things: Scientific research and commercially available devices. Health Inform. Res. 2017, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, G.; Povitz, M. Respiratory management of patients with neuromuscular disease: Current perspectives. Degener Neurol Neuromuscul. Dis. 2016, 6, 111–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coravos, A.; Khozin, S.; Mandl, K.D. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digit. Med. 2019, 2, 14. [Google Scholar] [CrossRef]
- Pinto, S.; Quintarelli, S.; Silani, V. New technologies and amyotrophic lateral sclerosis—Which step forward rushed by the COVID-19 pandemic? J. Neurol. Sci. 2020, 418, 117081. [Google Scholar] [CrossRef] [PubMed]
- De Canniere, H.; Smeets, C.J.P.; Schoutteten, M.; Varon, C.; Van Hoof, C.; Van Huffel, S.; Groenendaal, W.; Vandervoort, P. Using biosensors and digital biomarkers to assess response to cardiac rehabilitation: Observational study. J. Med. Internet Res. 2020, 22, e17326. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Design | Study Setting | Sensor | Biomarkers | Main Results | Outcome |
|---|---|---|---|---|---|---|
| Garcia-Gancedo 2019 [13] | Prospective Longitudinal, Cohort Study (variable length pilot study and 48-week core study phase) | ALS patients diagnosed within 18 months of symptom onset (n = 25, mean = 53.1 ± 9.93 years) | Home Monitoring Sensor | Physical activity, HRV, digital speech characteristics | A reduction in the patients’ ability to perform activities of daily living over time was observed across all end points. Obtained HRV data were lower than expected. No obvious pattern of speech change over time was observed. There were no serious side effects. | The novel monitoring platform tested in study was successful in collecting ALS patient data, which may be useful in identifying digital markers of disease progression. |
| Kelly 2020 [14] | Prospective Longitudinal Cohort Study (variable strength pilot study and 48-week core study phase) | ALS patients (n = 25, mean age = 53.1 ± 9.93) | Mega Faros 180 accelerometer, 2-lead ECG sensor, bespoke digital speech capture, comparing with ALSFRS-R score | Physical activity (average daytime active, percentage of daytime active, total daytime activity score, total 24 h activity score), HRV, and speech (jitter, shimmer, or speaking rate) | Four physical endpoints showed moderate or strong between patient correlation with ALSFRS-R total and gross motor domain scores. | Four physical activity endpoints showed potential for use as clinical measures of ALS disease progression, using direct, objective, and real-life assessment of physical function. |
| Stegmann 2020 [15] | Prospective Observational Comparative Study | ALS patients (n = 65, mean age = 61 ± 10.2 years) and healthy controls (n = 21, mean age = 55 ± 12.5 years) | Mobile application | Articulatory precision (AP), speaking rate (SR) | AP and SR decline was detected earlier than declines on the ALSFRS-R bulbar subscale. AP had significantly decreased as ALS progressed. In the bulbar-onset ALS group, SR showed significant decline. | This study demonstrated that it is possible to remotely detect early speech changes and track speech progression in ALS via automated algorithmic assessment of speech collected digitally. |
| Stegmann 2020 [16] | Prospective Observational Comparative Study | Sample 1 and 2; ALS patients (n = 72, mean age = 59.8 ± 10.4 years) and healthy controls (n = 22, mean age = 50.1 ± 14.7 years); sample 3; ALS patients (n = 24, mean age = 67.4 ± 11.3 years) | Open-source tool kits (openSMILE, Talk2me, and Praat) | 6 acoustic features; energy, frequency, MFCC, pitch, spectral, temporal; 4 language features; lexical, pragmatic, semantic, syntactic | This study evaluated repeatability measures (within-subjects coefficient of variation and intra-class correlation) of acoustic and language features. The repeatability of speech features extracted using open-source tool kits was low. | Researchers should exercise caution when developing digital health models with open-source speech features. |
| Heberer 2016 [17] | Prospective, Longitudinal Case-Control Study (Baseline and Post-treatment) | DMD patients—steroid group (n = 12, mean age = 5.7 ± 1.3) vs. naïve group (n = 9, mean age = 5.1 ± 1.1) | Three-dimensional gait analysis | Peak hip extensor moment during stance, duration of the hip extensor moment through stance, peak hip power generation during hip extension | Significant between-group differences favoring the Steroid group were found for peak hip extensor moment, duration of the hip extensor moment, peak hip power generation, and peak ankle power generation. | Hip joint kinetics are early markers of proximal weakness that are responsive to change with corticosteroid intervention, suggesting quantitative gait analysis could play a larger role in the assessment of the efficacy of novel therapeutics. |
| Le Moing 2016 [18] | Prospective Observational Cohort Study | Non-ambulatory DMD patients (n = 7, mean age 18.5 ± 5.5 years | Magneto-Inertial Sensors (ActiMyo®) | Angular velocity of the wrist, ratio of the vertical component of the acceleration to the overall acceleration, model-based computed power, elevation rate | The norm of the angular velocity, a model-based computed power, and the elevation rate were significantly correlated with the Minnesota scores and with the writing task. | The mean of the rotation rate and mean of the elevation rate appeared promising since these variables had the best reliability scores and correlations with task scores, suggesting they are good candidates as potential outcome measures in non-ambulant DMD patients |
| Lilien 2019 [19] | Prospective Observational Cohort Study | DMD patients (n = 23, age >5 years) | Wearable Magneto-Inertial Sensor (WMIS) | 7 walking parameters and 7 upper limb parameters | The validated 6 min walk test and the North Star Ambulatory Assessment were correlated with their device’s variables and were sensitive to change in the DMD population over a 6-month period. | This study suggests the WMIS can record a set of digital biomarkers and can be used to evaluate even the most severely impaired patients and provides objective and reliable data. |
| Chen 2017 [20] | Prospective Longitudinal Observational Comparative Study (at baseline, week 12, week 24, week 48) | SMA Type 3 patients (n = 18, mean age = 32.3 ± 12.7 years) vs. healthy controls (n = 19, mean age = 33.2 ± 13.9 years) | Microsoft Kinect Sensor | Upper limb movement; elbow angle, arm lifting angle, hand velocity | Elbow angle and arm-lifting angle did not show any difference between SMA type 3 patients and controls, hand velocity was faster in SMA patients. | This study suggests that the Microsoft Kinect sensor provides reproducible, objective, and detailed information of body point motion, so has the potential of being developed into a complimentary output measure for SMA. |
| Chabanon 2018 [21] | Prospective Longitudinal Cohort Study | Type 2 and 3 SMA patients (age 2–30 years); (1) non-sitter SMA Type 2 (n = 19), (2) sitter SMA Type (n = 34), (3) non-ambulatory SMA Type 3 (n = 9), (4) ambulatory SMA Type 3 (n = 19) 2 (n = 34), (3) non-ambulatory SMA Type 3 (n = 9), (4) ambulatory SMA Type 3 (n = 19) | Magneto-Inertial Sensors (ActiMyo®) | Wrist angular velocity, wrist acceleration, wrist vertical acceleration against gravity, the power, the percentage of activity time | The strongest correlations in this study were observed with the wrist vertical acceleration, and the median wrist angular velocity was decreased in the sitter patients with SMA Type 2 when compared with the non-sitter individuals. | The pending two-year study results will evaluate the sensitivity of the studied outcomes and biomarkers to disease progression. |
| Golini 2020 [22] | Prospective Observational Comparative Study (Animal Study) | Male and female wild-type (WT) vs. transgenic (SOD1G93A) mice; (1) Males, WT (n = 18), (2) Males, TG (n = 18), (3) Females, WT (n = 22), (4) Females, TG (n = 18) | Home cage activity monitoring: Digital ventilated cage (DVC) system; comparing with BW and neuromuscular function | Regularity Disruption Index (RDI) | The rise of RDI in TG mice was remarkable. When computed during daytime. The increase of irregularity in day activity pattern in TG mice could reflect disturbances in their rest/sleep behavior; RDI rose during the early symptomatic stage parallels grid hanging, and BW was declined. | This study suggests that the RDI metric is able to capture potential rest/sleep disturbances in ALS models. Thus, it could be used as a digital biomarker to detect disease-related phenotypes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, B.-Y.; Ko, Y.; Moon, S.; Lee, J.; Ko, S.-G.; Kim, J.-Y. Digital Biomarkers for Neuromuscular Disorders: A Systematic Scoping Review. Diagnostics 2021, 11, 1275. https://doi.org/10.3390/diagnostics11071275
Youn B-Y, Ko Y, Moon S, Lee J, Ko S-G, Kim J-Y. Digital Biomarkers for Neuromuscular Disorders: A Systematic Scoping Review. Diagnostics. 2021; 11(7):1275. https://doi.org/10.3390/diagnostics11071275
Chicago/Turabian StyleYoun, Bo-Young, Youme Ko, Seunghwan Moon, Jinhee Lee, Seung-Gyu Ko, and Jee-Young Kim. 2021. "Digital Biomarkers for Neuromuscular Disorders: A Systematic Scoping Review" Diagnostics 11, no. 7: 1275. https://doi.org/10.3390/diagnostics11071275
APA StyleYoun, B.-Y., Ko, Y., Moon, S., Lee, J., Ko, S.-G., & Kim, J.-Y. (2021). Digital Biomarkers for Neuromuscular Disorders: A Systematic Scoping Review. Diagnostics, 11(7), 1275. https://doi.org/10.3390/diagnostics11071275







