Optimal Utility of H-Reflex RDD as a Biomarker of Spinal Disinhibition in Painful and Painless Diabetic Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Recruitment
2.2. Participants and Demographics
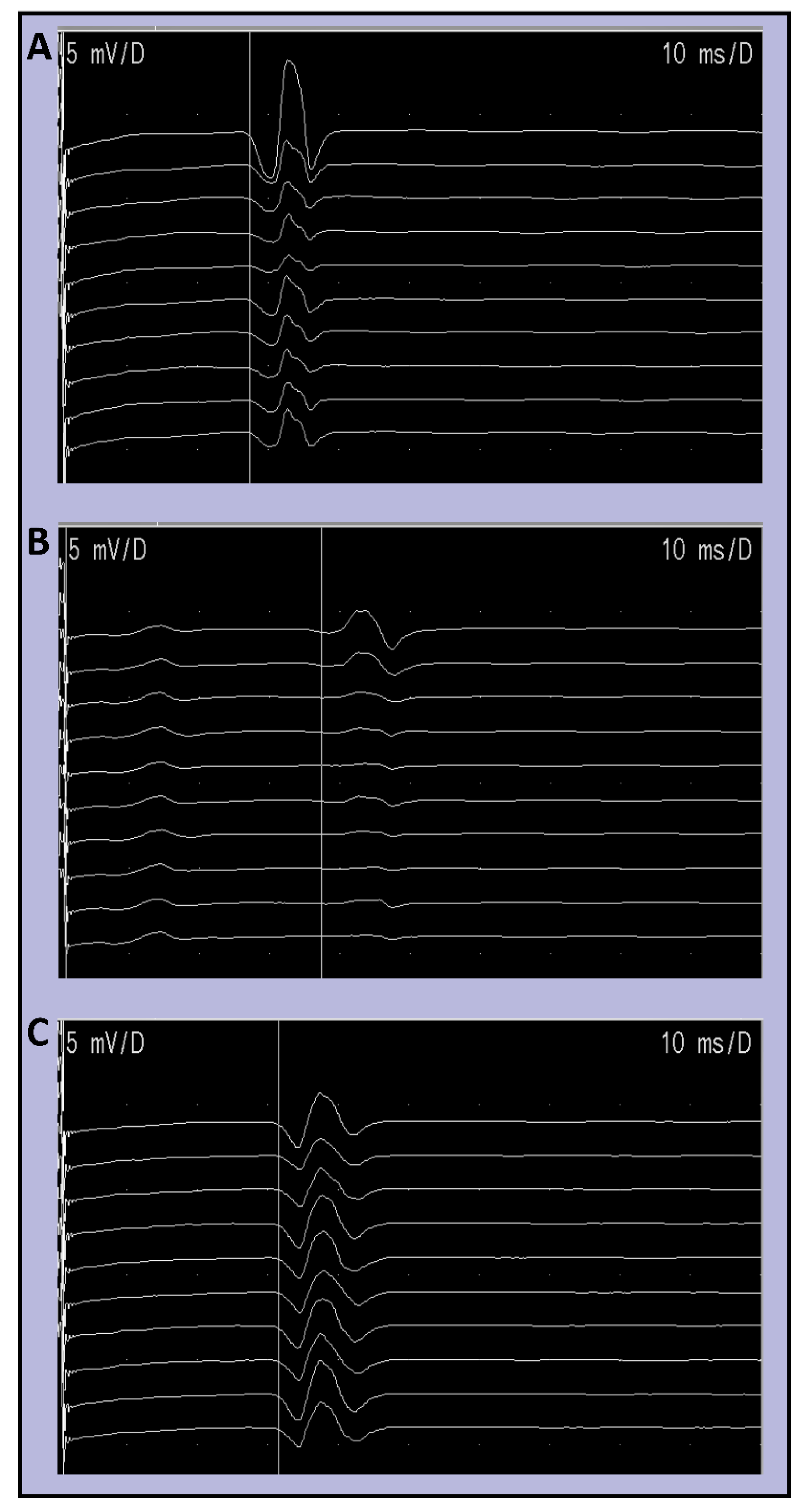
2.3. Nerve Conduction and H-Reflex Studies
2.4. Corneal Confocal Microscopy
2.5. Statistical Methods
3. Results
3.1. Clinical, Demographic and Neuropathy Measures
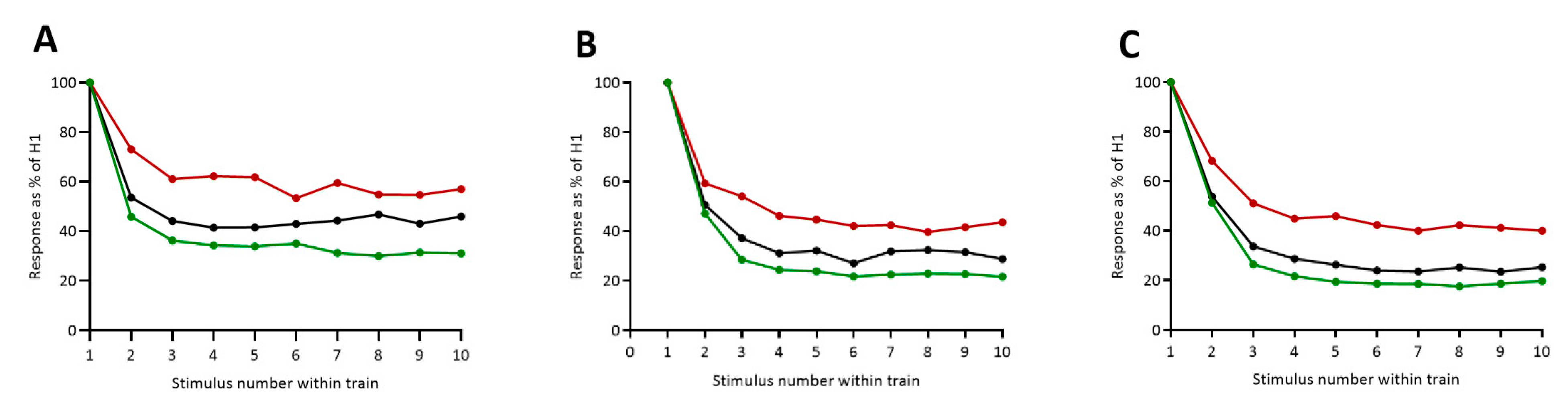
3.2. HRDD Time-Course
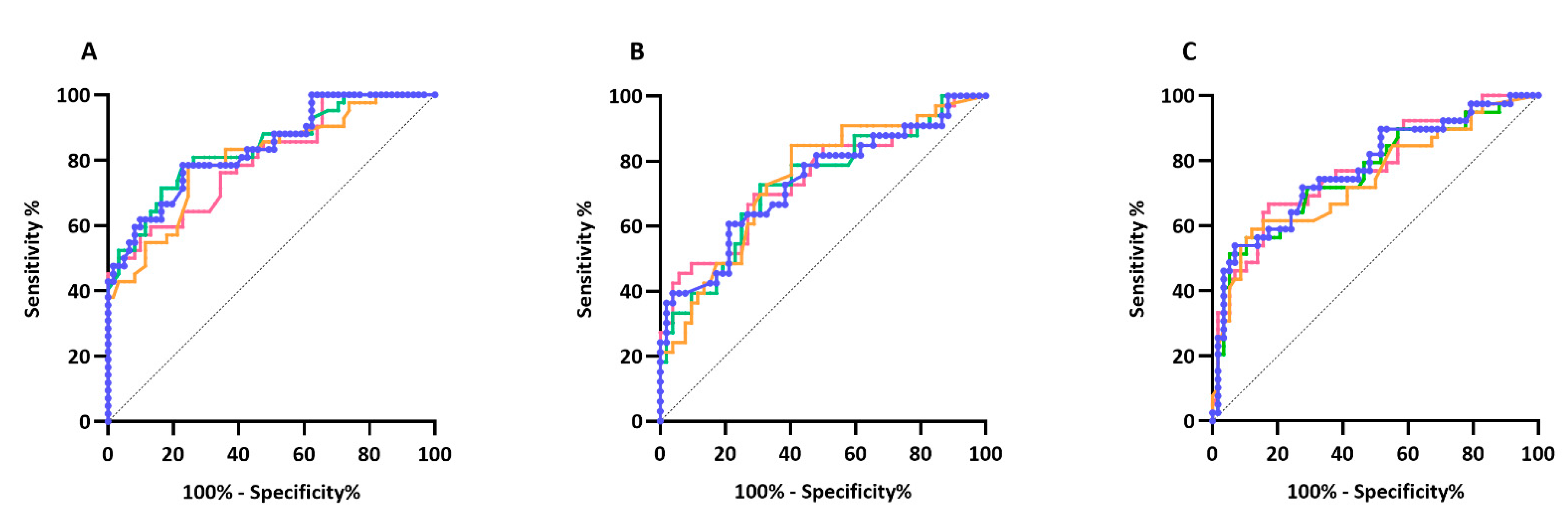
3.3. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, P.R.; Yaksh, T.L. Baclofen is antinociceptive in the spinal intrathecal space of animals. Eur. J. Pharmacol. 1978, 51, 323–330. [Google Scholar] [CrossRef]
- Sivilotti, L.; Woolf, C.J. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J. Neurophysiol. 1994, 72, 169–179. [Google Scholar] [CrossRef]
- Roberts, L.A.; Beyer, C.; Komisaruk, B.R. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986, 39, 1667–1674. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Lee, C.A.; Ramos, K.M.; Calcutt, N.A. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain 2008, 140, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmberg, A.B.; O’Connor, W.T.; Glennon, J.C.; Ceseña, R.; Calcutt, N.A. Impaired formalin-evoked changes of spinal amino acid levels in diabetic rats. Brain Res. 2006, 1115, 48–53. [Google Scholar] [CrossRef]
- Coull, J.A.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sik, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Lee-Kubli, C.A.; Calcutt, N.A. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain 2014, 155, 250–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, A.G.; Lee-Kubli, C.; Azmi, S.; Zhang, M.; Ferdousi, M.; Mixcoatl-Zecuatl, T.; Petropoulos, I.N.; Ponirakis, G.; Fineman, M.S.; Fadavi, H.; et al. Spinal Disinhibition in Experimental and Clinical Painful Diabetic Neuropathy. Diabetes 2017, 66, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Misiaszek, J.E. The H-reflex as a tool in neurophysiology: Its limitations and uses in understanding nervous system function. Muscle Nerve 2003, 28, 144–160. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ott, K.; Porter, R.W.; Stuart, D. Low frequency depression of the H wave in normal and spinal man. Exp. Neurol. 1966, 15, 140–156. [Google Scholar] [CrossRef]
- Chang, Y.J.; Liu, Y.C.; Hsu, M.J.; Fang, C.Y.; Wong, A.M.; DeJong, S.L.; Shields, R.K. Novel human models for elucidating mechanisms of rate-sensitive H-reflex depression. Biomed. J. 2020, 43, 44–52. [Google Scholar] [CrossRef]
- Hultborn, H.; Illert, M.; Nielsen, J.; Paul, A.; Ballegaard, M.; Wiese, H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996, 108, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Gandevia, S.C.; McKeon, B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J. Neurophysiol. 1984, 52, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Kakinohana, O.; Hefferan, M.P.; Nakamura, S.; Kakinohana, M.; Galik, J.; Tomori, Z.; Marsala, J.; Yaksh, T.L.; Marsala, M. Development of GABA-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: A qualitative and quantitative electrophysiological and histopathological study. Neuroscience 2006, 141, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Worthington, A.; Kalteniece, A.; Ferdousi, M.; Donofrio, L.; Dhage, S.; Azmi, S.; Adamson, C.; Hamdy, S.; Malik, R.; Calcutt, N.; et al. Spinal inhibitory dysfunction in patients with painful or painless diabetic neuropathy. Diabetes Care 2021. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Malik, R.A. Corneal confocal microscopy: A novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J. Vis. Exp. 2011, 47, e2194. [Google Scholar] [CrossRef] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S.; Sturdivant, R. Applied Logistic Regression, 3rd ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Palmieri, R.M.; Ingersoll, C.D.; Hoffman, M.A. The hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J. Athl. Train. 2004, 39, 268–277. [Google Scholar]
- Ferdousi, M.; Azmi, S.; Kalteniece, A.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Boulton, A.J.M.; Efron, N.; et al. Greater small nerve fibre damage in the skin and cornea of type 1 diabetic patients with painful compared to painless diabetic neuropathy. Eur. J. Neurol. 2021, 28, 1745–1751. [Google Scholar] [CrossRef]
- Karlsson, P.; Provitera, V.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Borreca, I.; Manganelli, F.; Santoro, L.; Jensen, T.S.; Nolano, M. Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain 2021, 162, 778–786. [Google Scholar] [CrossRef]
- Mixcoatl-Zecuatl, T.; Jolivalt, C.G. A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br. J. Pharmacol. 2011, 164, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.J.; Lu, Y.; Detke, M.J.; Lee, T.C.; Iyengar, S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005, 116, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Raskin, J.; Pritchett, Y.L.; Wang, F.; D’Souza, D.N.; Waninger, A.L.; Iyengar, S.; Wernicke, J.F. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005, 6, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Wilhelm, S.; Lledo, A.; Schacht, A.; Tolle, T.; Bouhassira, D.; Cruccu, G.; Skljarevski, V.; Freynhagen, R. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study”—A multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013, 154, 2616–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Themistocleous, A.C.; Ramirez, J.D.; Shillo, P.R.; Lees, J.G.; Selvarajah, D.; Orengo, C.; Tesfaye, S.; Rice, A.S.; Bennett, D.L. The Pain in Neuropathy Study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016, 157, 1132–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raputova, J.; Srotova, I.; Vlckova, E.; Sommer, C.; Üçeyler, N.; Birklein, F.; Rittner, H.L.; Rebhorn, C.; Adamova, B.; Kovalova, I.; et al. Sensory phenotype and risk factors for painful diabetic neuropathy: A cross-sectional observational study. Pain 2017, 158, 2340–2353. [Google Scholar] [CrossRef] [Green Version]
- Lee-Kubli, C.A.; Zhou, X.; Jolivalt, C.G.; Calcutt, N.A. Pharmacological Modulation of Rate-Dependent Depression of the Spinal H-Reflex Predicts Therapeutic Efficacy against Painful Diabetic Neuropathy. Diagnostics 2021, 11, 283. [Google Scholar] [CrossRef]
- Terunuma, M. Diversity of structure and function of GABA. Proc. Jpn. Acad. Ser. B 2018, 94, 390–411. [Google Scholar] [CrossRef] [Green Version]
| Diabetes with Neuropathic Pain (n = 42) | Diabetes without Neuropathic Pain (n = 62) | Controls (n = 34) | |
|---|---|---|---|
| Type of Diabetes (1/2) | 11/31 | 26/36 | |
| Gender (Female/Male) | 18/24 | 18/44 | 20/14 |
| Ethnicity (White/Asian/Black) | 32/8/2 | 46/13/3 | 25/7/2 |
| Median ± Interquartile Range | |||
| Age (years) | 61.5 (49.8–69.5) *** | 65.0 (51.5–71) **** | 46.5 (31–55) |
| Duration (years) | 12.5 (4.8–20.3) | 16.0 (10.0–23.3) | |
| HbA1c %(mmol/mol) | 7.0 (6.2–7.5) *** 53.0 (44.5–58) *** | 7.5 (6.8–8.4) **** 58.5 (51–68.25) **** | 5.4 (4.9–5.7) 35.0 (30.5–38.75) |
| BMI (kg/m²) | 29.2 (25.5–34.9) **** | 27.3 (24.3–31.9) ** | 23.8 (22.5–25.4) |
| SNAP (µV) | 7.7 (3.7–15) *** | 6.9 (4.3–11.5) **** | 17.0 (15–22) |
| SNCV (m/s) | 43.1 (40–46.7) *** | 42.4 (40–46.7) **** | 48.3 (45.2–51.9) |
| PMNAP (mV) | 3.8 (2.4–5.7) | 3.5 (2.4–5.9) * | 4.9 (3.4–7.5) |
| PMNCV (m/s) | 41.4 (38.1–43.7) **** | 41.2 (38.6–44) **** | 47.5 (43.4–50) |
| CDT (°C) | 27.8 (23.3–29.6) *** | 28.0 (24.1–29.9) ** | 29.8 (28.5–30.5) |
| WDT (°C) | 40.3 (36.4–46.3) ** | 40.0 (37.7–43.6) *** | 36.3 (34.8–39.3) |
| VAS Pain Current (0–100) | 20 (8–46.5) | ||
| VAS Pain Average past 24 h (0–100) | 35.5 (15.8–64.5) | ||
| VAS Pain Maximum past 24 h (0–100) | 56 (30–80) | ||
| Mean ± SE | |||
| CNFD (no.mm²) ^ | 23.84 ± 1.84 *** | 25.30 ± 1.31 *** | 32.36 ± 1.59 |
| CNFL (mm/mm²) ^ | 18.16 ± 1.563 *** | 18.43 ± 1.11 *** | 25.11 ± 1.35 |
| CNBD (no.mm²) ^ | 46.64 ± 5.18 | 47.61 ± 4.20 | 59.74 ± 6.85 |
| HRDD meanH2-10 @ 1 Hz ^ | 60.40 ± 2.74 *** | 34.92 ± 2.33 * +++ | 43.46 ± 3.31 |
| H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | Average of H2–4 | Average of H2–5 | Average of H2–10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 Hz | Pain v No Pain | 0.177 | 0.552 | 0.551 | 0.417 | 0.366 | 0.273 | 0.906 | 0.077 | 0.177 | 0.279 | 0.272 | 0.229 |
| Pain v Control | 0.868 | 0.673 | 0.955 | 0.940 | 0.692 | 0.960 | 0.906 | 0.786 | 0.941 | 0.793 | 0.944 | 0.979 | |
| No Pain v Control | 0.063 | 0.134 | 0.388 | 0.677 | 0.916 | 0.467 | 0.998 | 0.359 | 0.351 | 0.078 | 0.161 | 0.363 | |
| 0.5 Hz | Pain v No Pain | 0.006 | 0.004 | 0.621 | 0.001 | 0.016 | 0.498 | 0.382 | 0.053 | 0.248 | 0.021 | 0.003 | 0.018 |
| Pain v Control | 0.837 | 0.591 | 0.839 | 0.066 | 0.255 | 0.996 | 0.928 | 0.772 | 0.680 | 0.951 | 0.546 | 0.655 | |
| No Pain v Control | 0.037 | 0.074 | 0.262 | 0.356 | 0.541 | 0.434 | 0.191 | 0.245 | 0.763 | 0.051 | 0.075 | 0.175 | |
| 1 Hz | Pain v No Pain | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Pain v Control | <0.001 | <0.001 | <0.001 | <0.001 | 0.079 | 0.006 | 0.310 | 0.080 | 0.057 | <0.0001 | <0.0001 | <0.001 | |
| No Pain v Control | 0.236 | 0.147 | 0.256 | 0.233 | 0.183 | 0.012 | 0.004 | 0.055 | 0.003 | 0.125 | 0.117 | 0.018 | |
| 2 Hz | Pain v No Pain | 0.034 | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | <0.001 | <0.001 | <0.0001 | <0.0001 | <0.0001 |
| Pain v Control | 0.236 | 0.031 | 0.006 | 0.050 | 0.009 | 0.103 | 0.317 | 0.108 | 0.039 | 0.016 | 0.014 | 0.018 | |
| No Pain v Control | 0.753 | 0.320 | 0.275 | 0.185 | 0.462 | 0.107 | 0.093 | 0.125 | 0.386 | 0.327 | 0.254 | 0.175 | |
| 3 Hz | Pain v No Pain | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Pain v Control | 0.022 | 0.005 | 0.007 | <0.001 | 0.001 | 0.002 | 0.009 | 0.002 | 0.016 | 0.002 | <0.001 | <0.001 | |
| No Pain v Control | 0.867 | 0.320 | 0.304 | 0.259 | 0.627 | 0.482 | 0.306 | 0.573 | 0.482 | 0.393 | 0.342 | 0.397 |
| Optimal Cut Off | Area under Curve 95% CI | Sensitivity | Specificity | |
|---|---|---|---|---|
| 95% CI | 95% CI | |||
| 1 Hz H3 | 45.73 | 0.8 | 78.57 | 75.42 |
| 0.72–0.89 | 63.19–89.70 | 62.71–85.54 | ||
| 1 Hz mean H2–3 | 58.93 | 0.82 | 88.52 | 64.29 |
| 0.73–0.90 | 78.16–94.33 | 49.17–77.01 | ||
| 1 Hz mean H2–4 | 49.65 | 0.84 | 78.57 | 77.05 |
| 0.76–0.92 | 63.19–89.70 | 64.50–86.85 | ||
| 1 Hz mean H2–5 | 49.13 | 0.84 | 78.57 | 77.05 |
| 0.76–0.92 | 64.06–88.29 | 65.09–85.81 | ||
| 1 Hz mean H2–10 | 57.49 | 0.8 | 50.34 | 96.72 |
| 0.72–0.89 | 34.19–65.81 | 88.65–99.60 | ||
| 2 Hz H3 | 29.81 | 0.74 | 84.85 | 59.62 |
| 0.64–0.85 | 69.04–93.35 | 46.07–71.84 | ||
| 2 Hz mean H2–3 | 37.66 | 0.7 | 53.85 | 81.82 |
| 0.58–0.81 | 40.5–66.66 | 65.61–91.39 | ||
| 2 Hz mean H2–4 | 38.84 | 0.73 | 72.73 | 69.23 |
| 0.62–0.84 | 55.78–84.93 | 55.73–80.09 | ||
| 2 Hz mean H2–5 | 42.84 | 0.73 | 60.61 | 78.85 |
| 0.62–0.85 | 43.68–75.32 | 65.97–87.76 | ||
| 2 Hz mean H2–10 | 33.61 | 0.74 | 69.7 | 71.15 |
| 0.63–0.85 | 57.73–81.67 | 57.73–81.67 | ||
| 3 Hz H3 | 48.49 | 0.74 | 56.41 | 89.66 |
| 0.64–0.85 | 40.98–70.70 | 79.21–95.17 | ||
| 3 Hz mean H2–3 | 43.54 | 0.74 | 63.79 | 79.49 |
| 0.64–0.85 | 50.93–74.95 | 64.47–89.22 | ||
| 3 Hz mean H2–4 | 57.12 | 0.76 | 51.28 | 94.83 |
| 0.66–0.86 | 36.20–68.13 | 85.86–98.59 | ||
| 3 Hz mean H2–5 | 50.91 | 0.78 | 53.85 | 93.1 |
| 0.68–0.87 | 38.57–68.43 | 83.57–97.29 | ||
| 3 Hz mean H2–10 | F | 0.78 | 66.67 | 82.76 |
| 0.68–0.87 | 50.98–79.37 | 71.09–90.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worthington, A.; Kalteniece, A.; Ferdousi, M.; D’Onofrio, L.; Dhage, S.; Azmi, S.; Adamson, C.; Hamdy, S.; Malik, R.A.; Calcutt, N.A.; et al. Optimal Utility of H-Reflex RDD as a Biomarker of Spinal Disinhibition in Painful and Painless Diabetic Neuropathy. Diagnostics 2021, 11, 1247. https://doi.org/10.3390/diagnostics11071247
Worthington A, Kalteniece A, Ferdousi M, D’Onofrio L, Dhage S, Azmi S, Adamson C, Hamdy S, Malik RA, Calcutt NA, et al. Optimal Utility of H-Reflex RDD as a Biomarker of Spinal Disinhibition in Painful and Painless Diabetic Neuropathy. Diagnostics. 2021; 11(7):1247. https://doi.org/10.3390/diagnostics11071247
Chicago/Turabian StyleWorthington, Anne, Alise Kalteniece, Maryam Ferdousi, Luca D’Onofrio, Shaishav Dhage, Shazli Azmi, Clare Adamson, Shaheen Hamdy, Rayaz A. Malik, Nigel A. Calcutt, and et al. 2021. "Optimal Utility of H-Reflex RDD as a Biomarker of Spinal Disinhibition in Painful and Painless Diabetic Neuropathy" Diagnostics 11, no. 7: 1247. https://doi.org/10.3390/diagnostics11071247
APA StyleWorthington, A., Kalteniece, A., Ferdousi, M., D’Onofrio, L., Dhage, S., Azmi, S., Adamson, C., Hamdy, S., Malik, R. A., Calcutt, N. A., & Marshall, A. G. (2021). Optimal Utility of H-Reflex RDD as a Biomarker of Spinal Disinhibition in Painful and Painless Diabetic Neuropathy. Diagnostics, 11(7), 1247. https://doi.org/10.3390/diagnostics11071247






