The Combination of SOFA Score and Urinary NGAL May Be an Effective Predictor for Ventilator Dependence among Critically Ill Surgical Patients: A Pilot Study
Abstract
1. Background
2. Material and Methods
2.1. Data Source and Patient Population
2.2. Blood Sampling and Assays
2.3. Statistical Analysis
3. Result
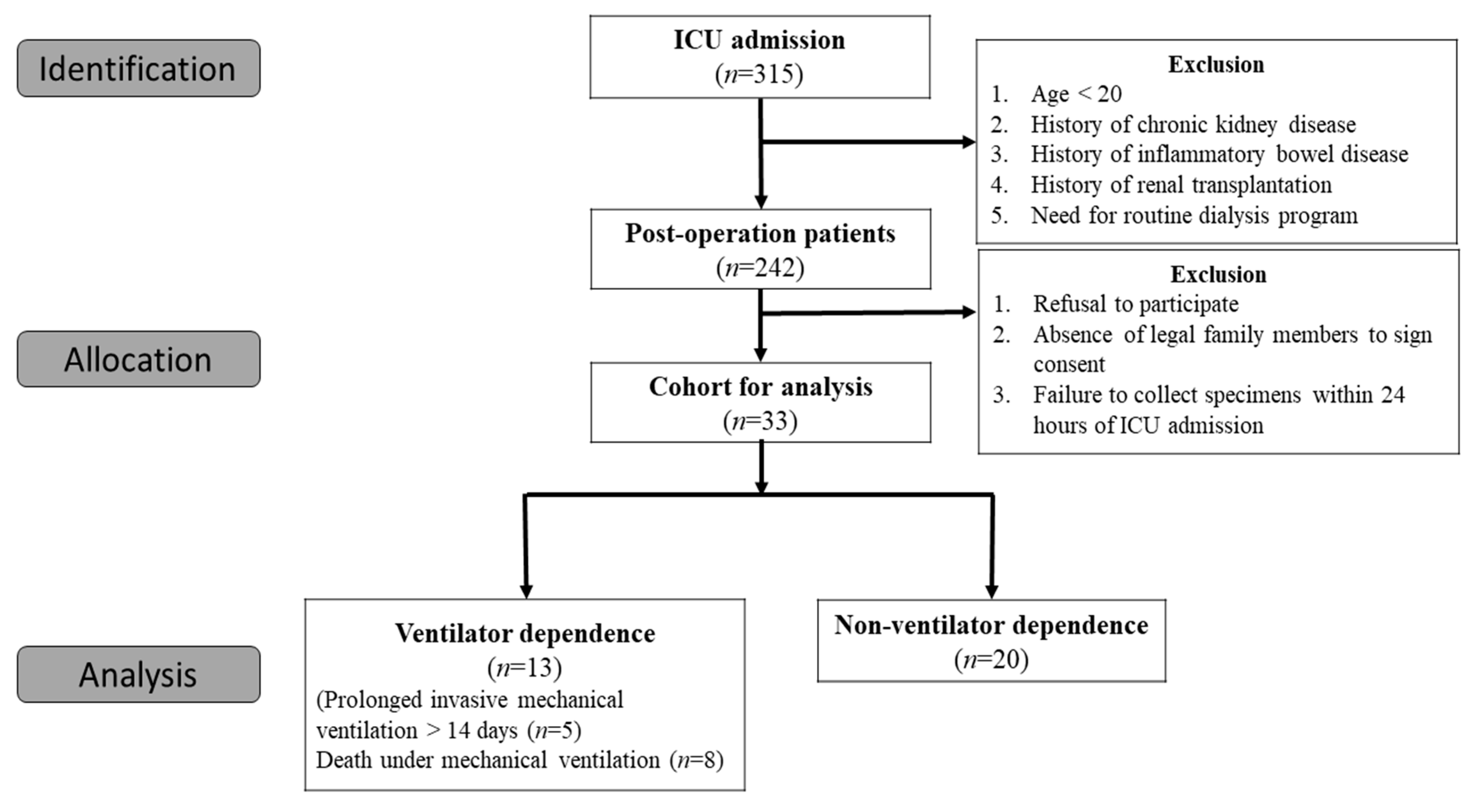
3.1. Study Population
3.2. Patient Characteristics
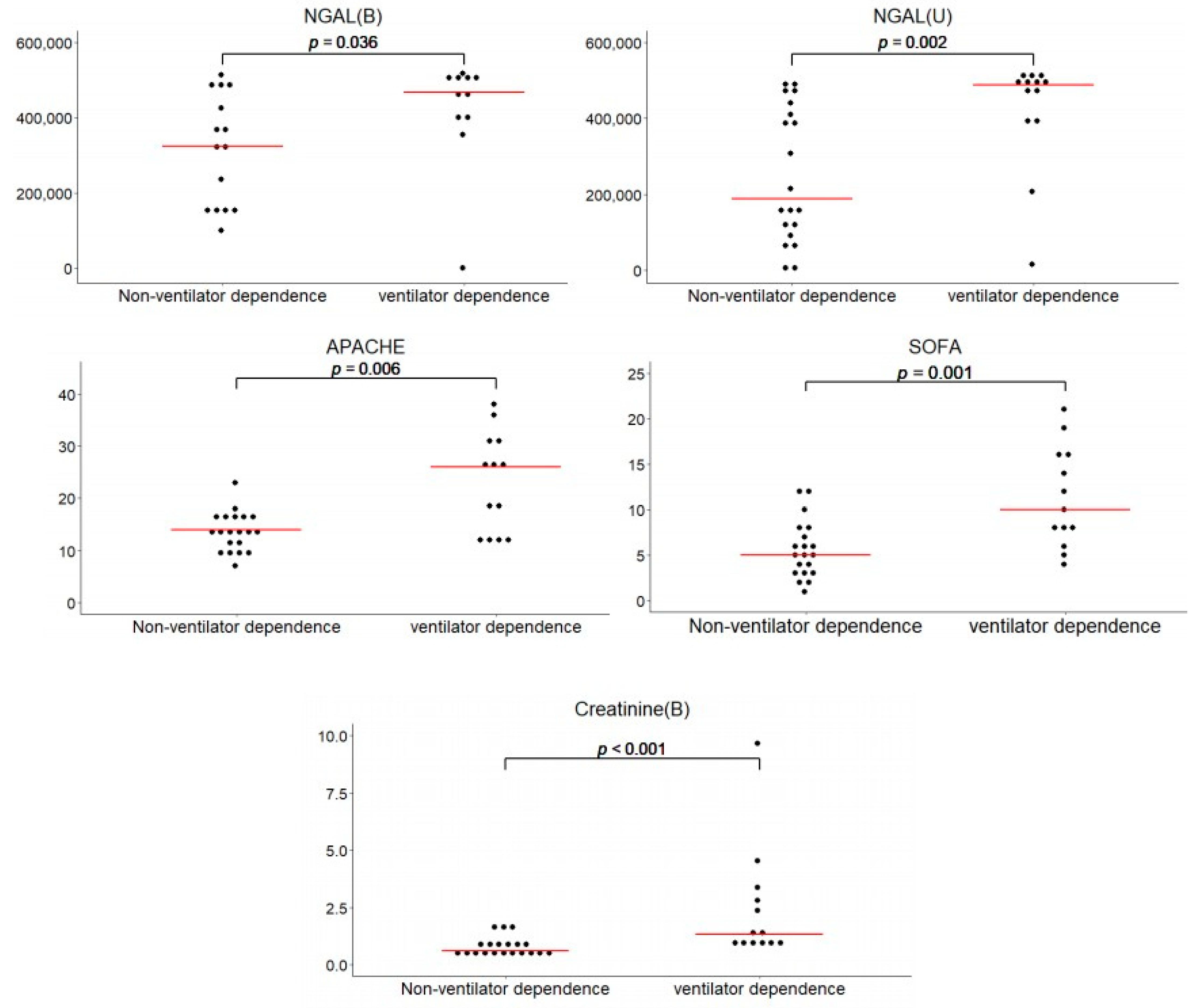
3.3. Analysis of Biomarkers Regarding Ventilator Dependence
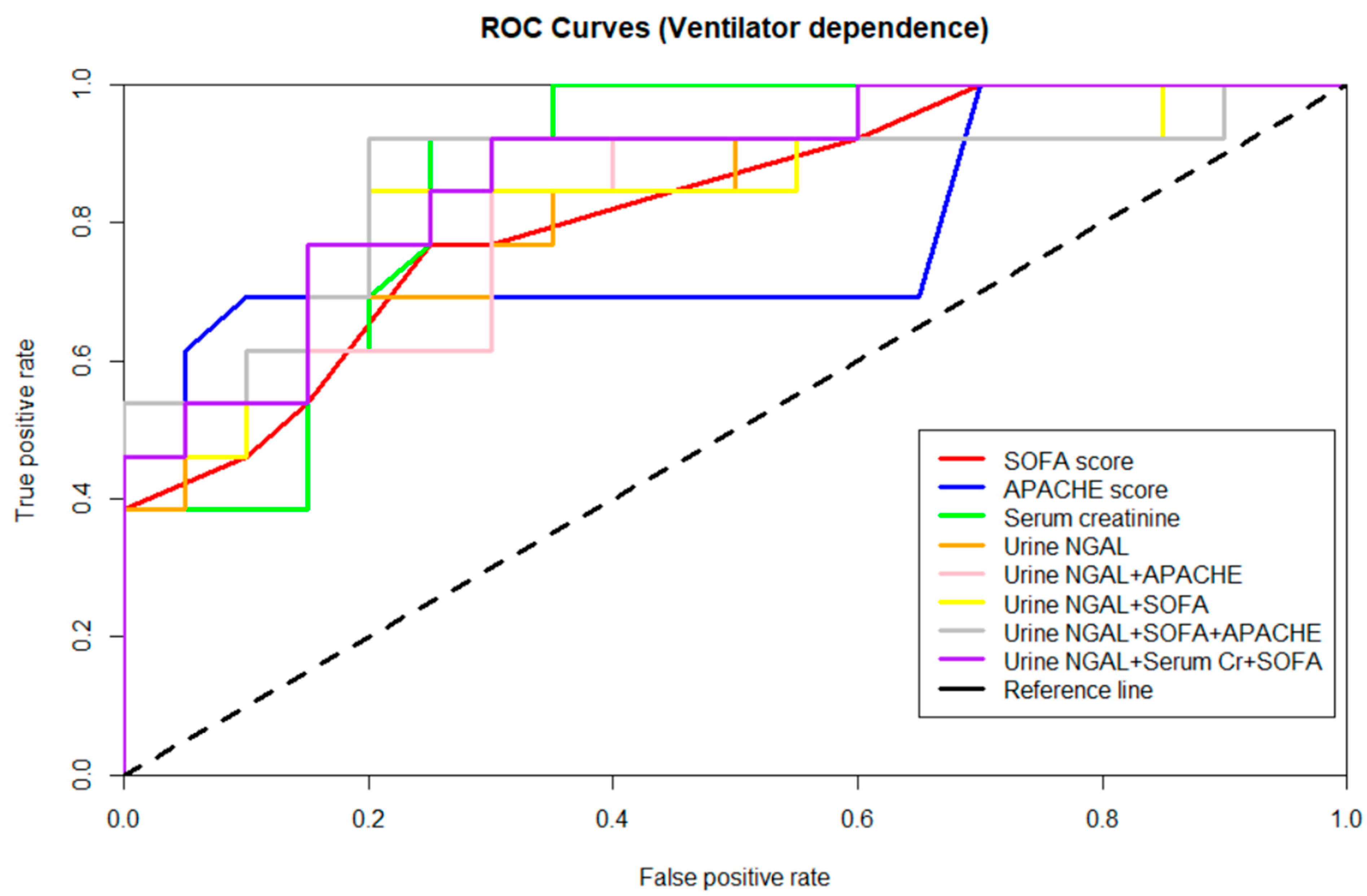
3.4. Performance of Biomarkers in Predicting Ventilator Dependence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| AUROC | area under the ROC curve |
| CGMH | Chang Gung Memorial Hospital |
| eGFR | estimated glomerular filtration rate |
| ELISA | enzyme-linked immunosorbent assay |
| FiO2 | fraction of inspired oxygen |
| GCS | Glasgow coma scale |
| GDF-15 | growth differentiation factor 15 |
| ICU | intensive care unit |
| IRB | institutional review board |
| KIM-1 | kidneyinjury molecule-1 |
| MAP | mean arterial blood pressure |
| MV | mechanical ventilation |
| NGAL | neutrophil gelatinase-associated lipocalin |
| nVD | non-ventilator dependence |
| PaO2 | partial pressure of oxygen |
| ROC curve | receiver operating characteristic curve |
| SOFA | Sequential Organ Failure Assessment |
| VD | ventilator dependence |
References
- Shander, A.; Fleisher, L.A.; Barie, P.S.; Bigatello, L.M.; Sladen, R.N.; Watson, C.B. Clinical and economic burden of postoperative pulmonary complications: Patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit. Care Med. 2011, 39, 2163–2172. [Google Scholar] [CrossRef]
- Simonneau, G.; Vivien, A.; Sartene, R.; Kunstlinger, F.; Samii, K.; Noviant, Y.; Duroux, P. Diaphragm dysfunction induced by upper abdominal surgery. Role of postoperative pain. Am. Rev. Respir. Dis. 1983, 128, 899–903. [Google Scholar] [CrossRef]
- MacIntyre, N.R.; Epstein, S.K.; Carson, S.; Scheinhorn, D.; Christopher, K.; Muldoon, S. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest 2005, 128, 3937–3954. [Google Scholar] [CrossRef]
- Sahetya, S.; Allgood, S.; Gay, P.C.; Lechtzin, N. Long-Term Mechanical Ventilation. Clin. Chest Med. 2016, 37, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Damuth, E.; Mitchell, J.A.; Bartock, J.L.; Roberts, B.W.; Trzeciak, S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 544–553. [Google Scholar] [CrossRef]
- Van den Akker, J.P.; Egal, M.; Groeneveld, A.B. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: A systematic review and meta-analysis. Crit. Care 2013, 17, R98. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, P.; Oeyen, S.; De Smet, S.; De Raedt, S.; Benoit, D.; Decruyenaere, J.; Derom, E. Long-term outcome and health-related quality of life in difficult-to-wean patients with and without ventilator dependency at ICU discharge: A retrospective cohort study. BMC Pulm. Med. 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rose, L. Strategies for weaning from mechanical ventilation: A state of the art review. Intensive Crit. Care Nurs. 2015, 31, 189–195. [Google Scholar] [CrossRef]
- Savi, A.; Teixeira, C.; Silva, J.M.; Borges, L.G.; Pereira, P.A.; Pinto, K.B.; Gehm, F.; Moreira, F.C.; Wickert, R.; Trevisan, C.B.; et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J. Crit. Care 2012, 27, 221.e1–221.e8. [Google Scholar] [CrossRef]
- Ghauri, S.K.; Javaeed, A.; Mustafa, K.J.; Khan, A.S. Predictors of prolonged mechanical ventilation in patients admitted to intensive care units: A systematic review. Int. J. Health Sci. 2019, 13, 31–38. [Google Scholar]
- Joannidis, M.; Forni, L.G.; Klein, S.J.; Honore, P.M.; Kashani, K.; Ostermann, M.; Prowle, J.; Bagshaw, S.M.; Cantaluppi, V.; Darmon, M.; et al. Lung-kidney interactions in critically ill patients: Consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020, 46, 654–672. [Google Scholar] [CrossRef]
- Hepokoski, M.; Englert, J.A.; Baron, R.M.; Crotty-Alexander, L.E.; Fuster, M.M.; Beitler, J.R.; Malhotra, A.; Singh, P. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am. J. Physiol. Ren. Physiol. 2017, 312, F654–F660. [Google Scholar] [CrossRef]
- Singbartl, K.; Miller, L.; Ruiz-Velasco, V.; Kellum, J.A. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2016, 44, e492–e501. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.J.; Lehner, G.F.; Forni, L.G.; Joannidis, M. Oliguria in critically ill patients: A narrative review. J. Nephrol. 2018, 31, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Haase, M.; Albert, A.; Zapf, A.; Braun-Dullaeus, R.C.; Haase-Fielitz, A. Biomarker-Guided Risk Assessment for Acute Kidney Injury: Time for Clinical Implementation? Ann. Lab. Med. 2021, 41, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Kashani, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 2016, 8, E305–E311. [Google Scholar] [CrossRef]
- Er, R.E.; Ulusal Okyay, G.; Aygencel, B.K.G.; Türko Lu, M.; Erten, Y. Comparison Between RIFLE, AKIN, and KDIGO: Acute Kidney Injury Definition Criteria for Prediction of In-hospital Mortality in Critically Ill Patients. Iran. J. Kidney Dis. 2020, 14, 365–372. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Göcze, I.; Schlitt, H.J.; Bergler, T. Biomarker-guided Intervention to Prevent AKI or KDIGO Care Bundle to Prevent AKI in High-risk Patients Undergoing Major Surgery? Ann. Surg. 2018, 268, e68–e69. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hepokoski, M.L.; Malhotra, A.; Singh, P.; Crotty Alexander, L.E. Ventilator-Induced Kidney Injury: Are Novel Biomarkers the Key to Prevention? Nephron 2018, 140, 90–93. [Google Scholar] [CrossRef] [PubMed]
- van Duijl, T.T.; Ruhaak, L.R.; de Fijter, J.W.; Cobbaert, C.M. Kidney Injury Biomarkers in an Academic Hospital Setting: Where Are We Now? Clin. Biochem. Rev. 2019, 40, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Foley, R.J.; Wu, R.; Grady, J.; Scalise, P. Renal Function, Weaning, and Survival in Patients With Ventilator-Dependent Respiratory Failure. J. Intensive Care Med. 2019, 34, 212–217. [Google Scholar] [CrossRef]
- Datta, D.; Foley, R.; Wu, R.; Grady, J.; Scalise, P. Can Creatinine Height Index Predict Weaning and Survival Outcomes in Patients on Prolonged Mechanical Ventilation After Critical Illness? J. Intensive Care Med. 2018, 33, 104–110. [Google Scholar] [CrossRef]
- Kou, H.W.; Yeh, C.H.; Tsai, H.I.; Hsu, C.C.; Hsieh, Y.C.; Chen, W.T.; Cheng, H.T.; Yu, M.C.; Lee, C.W. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS ONE 2019, 14, e0220699. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Jia, L.; Sheng, X.; Zamperetti, A.; Xie, Y.; Corradi, V.; Chandel, S.; De Cal, M.; Montin, D.P.; Caprara, C.; Ronco, C. Combination of biomarker with clinical risk factors for prediction of severe acute kidney injury in critically ill patients. BMC Nephrol. 2020, 21, 540. [Google Scholar] [CrossRef]
- Küllmar, M.; Massoth, C.; Ostermann, M.; Campos, S.; Grau Novellas, N.; Thomson, G.; Haffner, M.; Arndt, C.; Wulf, H.; Irqsusi, M.; et al. Biomarker-guided implementation of the KDIGO guidelines to reduce the occurrence of acute kidney injury in patients after cardiac surgery (PrevAKI-multicentre): Protocol for a multicentre, observational study followed by randomised controlled feasibility trial. BMJ Open 2020, 10, e034201. [Google Scholar] [CrossRef]
- Nickolas, T.L.; O’Rourke, M.J.; Yang, J.; Sise, M.E.; Canetta, P.A.; Barasch, N.; Buchen, C.; Khan, F.; Mori, K.; Giglio, J.; et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 2008, 148, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Md Ralib, A.; Mat Nor, M.B.; Pickering, J.W. Plasma Neutrophil Gelatinase-Associated Lipocalin diagnosed acute kidney injury in patients with systemic inflammatory disease and sepsis. Nephrology 2017, 22, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Tangri, N.; Komenda, P.; Kaushal, A.; Sood, M.; Brar, R.; Gill, K.; Walker, S.; MacDonald, K.; Hiebert, B.M.; et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am. J. Kidney Dis. 2015, 66, 993–1005. [Google Scholar] [CrossRef]
- Yoneyama, F.; Okamura, T.; Takigiku, K.; Yasukouchi, S. Novel Urinary Biomarkers for Acute Kidney Injury and Prediction of Clinical Outcomes After Pediatric Cardiac Surgery. Pediatric Cardiol. 2020, 41, 695–702. [Google Scholar] [CrossRef]
| Categorical Variables | No. | % | |
|---|---|---|---|
| Gender | Male | 20 | 61 |
| Female | 13 | 39 | |
| Comorbidity | Chronic lung disease | 3 | 9.1 |
| Hypertension | 10 | 30.3 | |
| Liver cirrhosis | 4 | 12.1 | |
| Cardiovascular disease | 5 | 15.2 | |
| Diabetes mellitus | 5 | 15.1 | |
| Cerebrovascular disease | 3 | 9.1 | |
| Malignancy | 18 | 54.5 | |
| Type of surgery | Hepatobiliary surgery | 6 | 18.2 |
| Gastrointestinal surgery | 21 | 63.6 | |
| Others | 2 | 6.1 | |
| Without surgery | 4 | 12.1 | |
| Ventilator dependence | Yes | 13 | 39.4 |
| No | 20 | 60.6 | |
| Dialysis | Yes | 2 | 6.06 |
| No | 31 | 93.94 | |
| In-hospital mortality | Yes | 15 | 45.45 |
| No | 18 | 54.55 | |
| 3 months mortality | Yes | 15 | 45.45 |
| No | 18 | 54.55 | |
| 6 months mortality | Yes | 16 | 48.48 |
| No | 17 | 51.52 | |
| 12 months mortality | Yes | 16 | 50 |
| No | 16 | 50 | |
| Continuous Variables | Mean | SE a | |
| Age (years) | 66.33 | 2.56 | |
| BMI (kg/m2) | 23.58 | 0.62 | |
| SOFA score b | 7.848 | 0.874 | |
| APACHE II score c | 17.364 | 1.401 | |
| MV d (days) | 8.85 | 3.53 | |
| ICU e stay (days) | 12.64 | 4.41 | |
| Hospital stay (days) | 39.09 | 4.38 | |
| Baseline Egfr f (mL/min) | 107.1842 | 6.52 | |
| Creatinine (mg/dL) | 1.426 | 0.303 | |
| eGFR (mL/min) | 80.38424 | 8.92 | |
| Urine output(mL/day) | 1548.788 | 190.871 | |
| Albumin (g/dL) | 2.733 | 0.100 | |
| White blood cell count (1000/μL) | 14.352 | 1.545 | |
| CRP g (mg/L) | 123.329 | 18.414 | |
| Procalcitonin (ng/mL) | 33.761 | 12.234 |
| Variables | Ventilator Dependence (n = 13) | Non-Ventilator Dependence (n = 20) | p-Value |
|---|---|---|---|
| Mean ± SEM a | Mean ± SEM a | ||
| Age (years) | 63.23 ± 4.54 | 68.35 ± 3.03 | 0.353 |
| BMI (kg/m2) | 22.45 ± 0.933 | 24.31 ± 0.798 | 0.169 |
| APACHE II b score | 23.154 ± 2.641 | 13.600 ± 0.835 | 0.006 |
| SOFA c score | 11.308 ± 1.521 | 5.600 ± 0.705 | 0.001 |
| MV d (days) | 19.31 ± 8.295 | 2.05 ± 0.478 | 0.014 |
| ICU e stays (days) | 27.62 ± 10.005 | 2.90 ± 0.447 | 0.036 |
| Hospital stays (days) | 52.31 ± 8.860 | 30.50 ± 3.335 | 0.024 |
| Baseline eGFR f (mL/min) | 103.4254 ± 9.731097 | 109.63 ± 8.86 | 0.65 |
| Creatinine (mg/dL) | 2.412 ± 0.684 | 0.786 ± 0.095 | <0.001 |
| eGFR upon ICU admission f (mL/min) | 49.875 ± 8.073 | 100.215 ± 11.911 | 0.001 |
| Urine output (mL/day) | 1198.308 ± 288.885 | 1776.600 ± 245.046 | 0.235 |
| White blood cell count (1000/μL) | 18.654 ± 3.026 | 11.555 ± 1.354 | 0.027 |
| CRP g (mg/L) | 146.519 ± 23.598 | 101.796 ± 27.505 | 0.054 |
| Procalcitonin (ng/mL) | 40.640 ± 17.401 | 24.818 ± 17.347 | 0.410 |
| No. (%) | No. (%) | p-Value | |
| Sepsis | 0.002 | ||
| Yes | 13 (100) | 9 (45.0) | |
| No | 0 (0) | 11 (55.0) | |
| In-hospital mortality | <0.001 | ||
| Yes | 11 (84.62) | 4 (20.0) | |
| No | 2 (15.38) | 16 (80.0) | |
| Dialysis | 0.148 | ||
| Yes | 2 (15.38) | 0 (0) | |
| No | 11 (84.62) | 20 (100) |
| Variables | Ventilator Dependence (n = 13) | Non-Ventilator Dependence (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Mean | SEM a | Mean | SEM a | ||
| Serum | |||||
| NGAL (ng/mL) | 420.25 | 45.18 | 314.68 | 38.12 | 0.036 |
| Calprotectin (pg/mL) | 1026.90 | 386.21 | 407.06 | 185.17 | 0.097 |
| KIM-1 (pg/mL) | 984.08 | 516.69 | 572.57 | 253.75 | 0.760 |
| Cystatin C (pg/mL) | 1323.27 | 285.35 | 838.96 | 118.19 | 0.069 |
| GDF-15 (ng/mL) | 6.32 | 2.61 | 11.09 | 2.71 | 0.097 |
| Urine | |||||
| NGAL (ng/mL) | 420.87 | 41.08 | 250.84 | 39.45 | 0.002 |
| Calprotectin (pg/mL) | 970.74 | 489.55 | 775.46 | 295.20 | 0.221 |
| KIM-1 (pg/mL) | 8433.73 | 2276.40 | 7689.04 | 1802.37 | 0.758 |
| Cystatin C (ng/mL) | 378.18 | 141.11 | 147.96 | 45.50 | 0.477 |
| GDF-15 (ng/mL) | 24.56 | 4.68 | 30.30 | 2.85 | 0.281 |
| Variables a | Prediction of Ventilator Dependence | p Value | ||
|---|---|---|---|---|
| AUROC | Cut-Off Value | Sensitivity/Specificity (%) | ||
| Serum | ||||
| NGAL (ng/mL) | 0.789 | 398.4 | 81.82/66.67 | 0.036 |
| Calprotectin (pg/mL) | 0.694 | 150.8 | 81.82/66.67 | 0.097 |
| KIM-1 (pg/mL) | 0.522 | 0.87 | ||
| Cystatin C (pg/mL) | 0.715 | 1008.29 | 81.82/73.33 | 0.065 |
| GDF-15 (ng/mL) | 0.389 | 0.414 | ||
| Creatinine (mg/dL) | 0.867 | 0.94 | 92.31/71.4 | 0.001 |
| CRP (mg/L) | 0.720 | 89.03 | 84.62/64.29 | 0.052 |
| Urine | ||||
| NGAL (ng/mL) | 0.808 | 465.2 | 69.23/85 | 0.003 |
| Calprotectin (pg/mL) | 0.392 | 0.312 | ||
| KIM-1 (pg/mL) | 0.579 | 0.46 | ||
| Cystatin C (ng/mL) | 0.579 | 0.46 | ||
| GDF-15 (ng/mL) | 0.417 | 0.436 | ||
| APACHE II score | 0.783 | 18 | 69.23/90 | 0.007 |
| SOFA score | 0.821 | 8 | 76.92/75 | 0.002 |
| Urine NGAL + APACHE score | 0.827 | 0.002 | ||
| Urine NGAL + SOFA score | 0.835 | 0.001 | ||
| Urine NGAL + APACHE score + SOFA score | 0.865 | <0.001 | ||
| Urine NGAL + Serum Cr + SOFA score | 0.873 | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-I.; Lu, Y.-C.; Kou, H.-W.; Hsu, H.-Y.; Huang, S.-F.; Huang, C.-W.; Lee, C.-W. The Combination of SOFA Score and Urinary NGAL May Be an Effective Predictor for Ventilator Dependence among Critically Ill Surgical Patients: A Pilot Study. Diagnostics 2021, 11, 1186. https://doi.org/10.3390/diagnostics11071186
Tsai H-I, Lu Y-C, Kou H-W, Hsu H-Y, Huang S-F, Huang C-W, Lee C-W. The Combination of SOFA Score and Urinary NGAL May Be an Effective Predictor for Ventilator Dependence among Critically Ill Surgical Patients: A Pilot Study. Diagnostics. 2021; 11(7):1186. https://doi.org/10.3390/diagnostics11071186
Chicago/Turabian StyleTsai, Hsin-I, Yu-Chieh Lu, Hao-Wei Kou, Heng-Yuan Hsu, Song-Fong Huang, Chun-Wei Huang, and Chao-Wei Lee. 2021. "The Combination of SOFA Score and Urinary NGAL May Be an Effective Predictor for Ventilator Dependence among Critically Ill Surgical Patients: A Pilot Study" Diagnostics 11, no. 7: 1186. https://doi.org/10.3390/diagnostics11071186
APA StyleTsai, H.-I., Lu, Y.-C., Kou, H.-W., Hsu, H.-Y., Huang, S.-F., Huang, C.-W., & Lee, C.-W. (2021). The Combination of SOFA Score and Urinary NGAL May Be an Effective Predictor for Ventilator Dependence among Critically Ill Surgical Patients: A Pilot Study. Diagnostics, 11(7), 1186. https://doi.org/10.3390/diagnostics11071186






