Association of Excessive Daytime Sleepiness with the Zung Self-Rated Depression Subscales in Adults with Coronary Artery Disease and Obstructive Sleep Apnea
Abstract
:1. Introduction
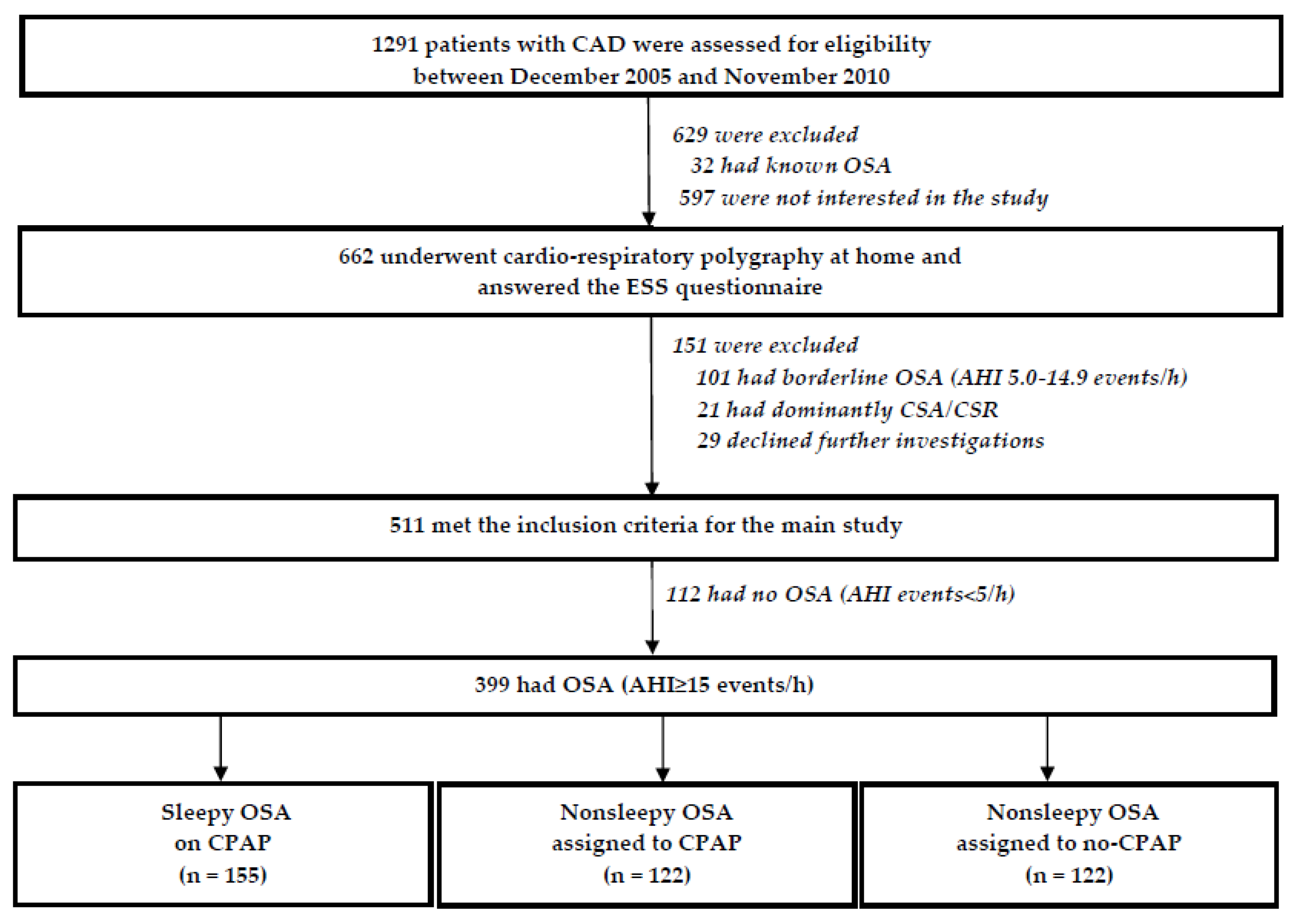
2. Materials and Methods
2.1. Study Population
2.2. Cardiorespiratory Polygraphy
2.3. Epworth Sleepiness Scale
2.4. Zung Self-Rating Depression Scale
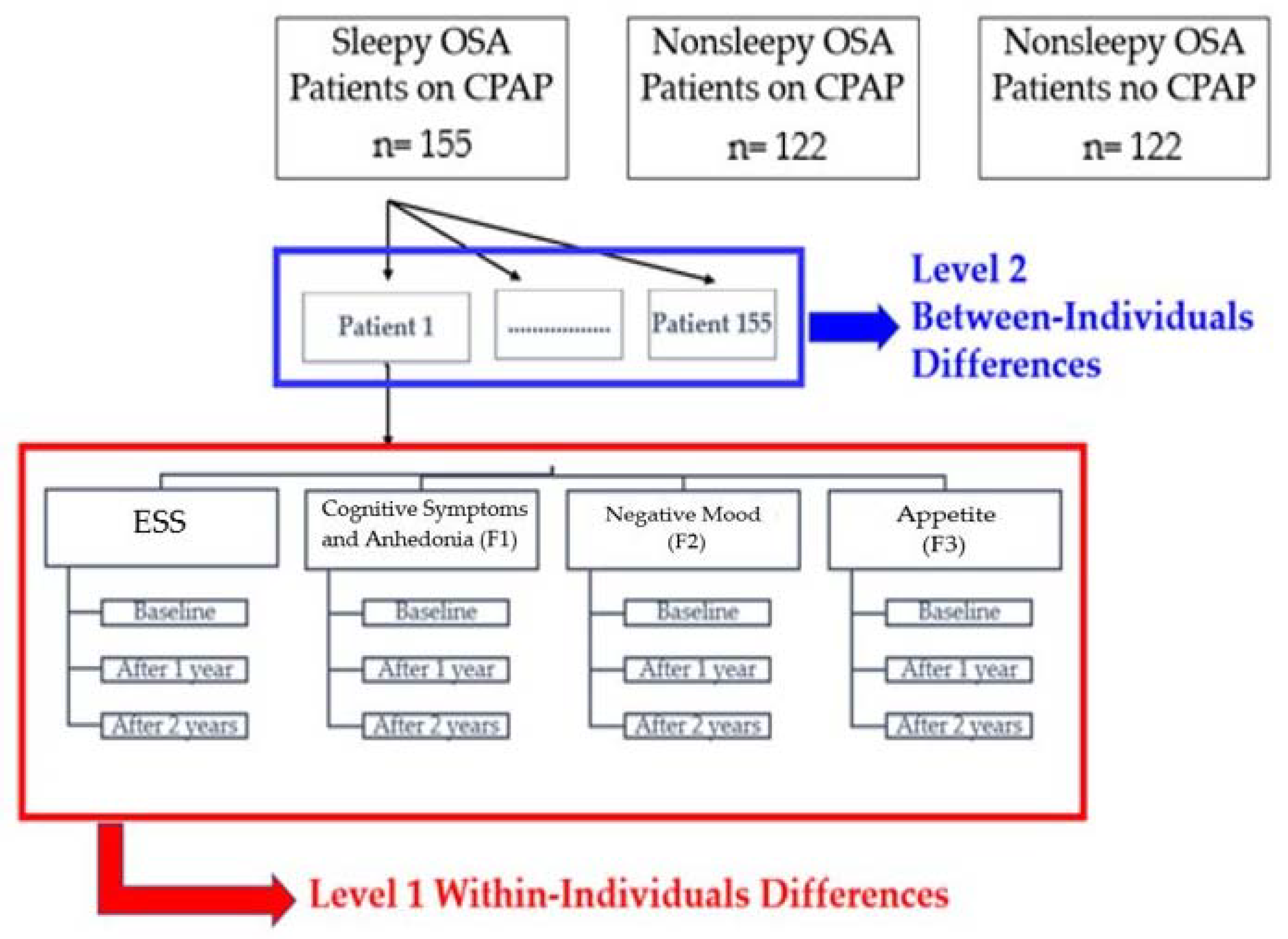
2.5. Group Assignment, Randomization, Interventions and Follow-Up
2.6. Adherence to CPAP
2.7. Statistical Analysis
3. Results
3.1. Principle Component Analysis
3.2. Baseline Characteristics of the Study Population
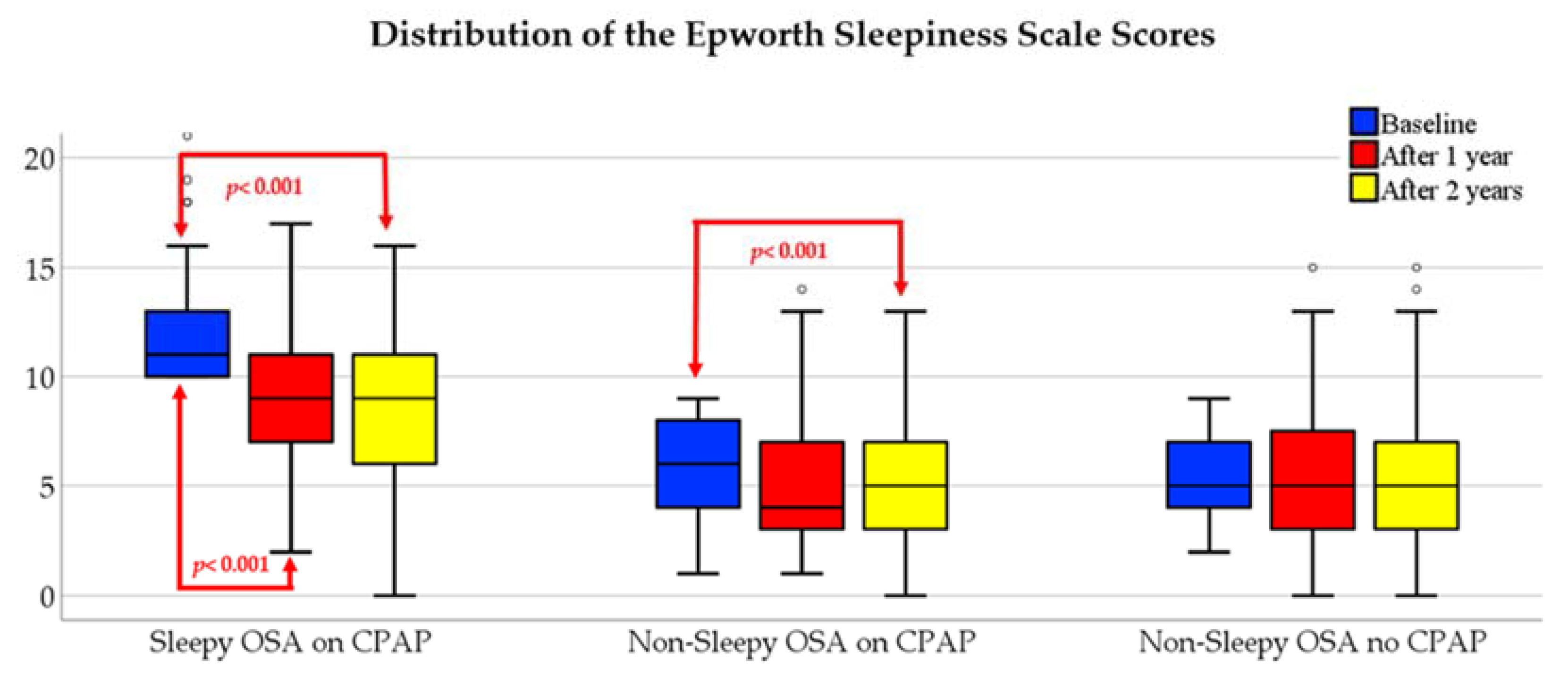
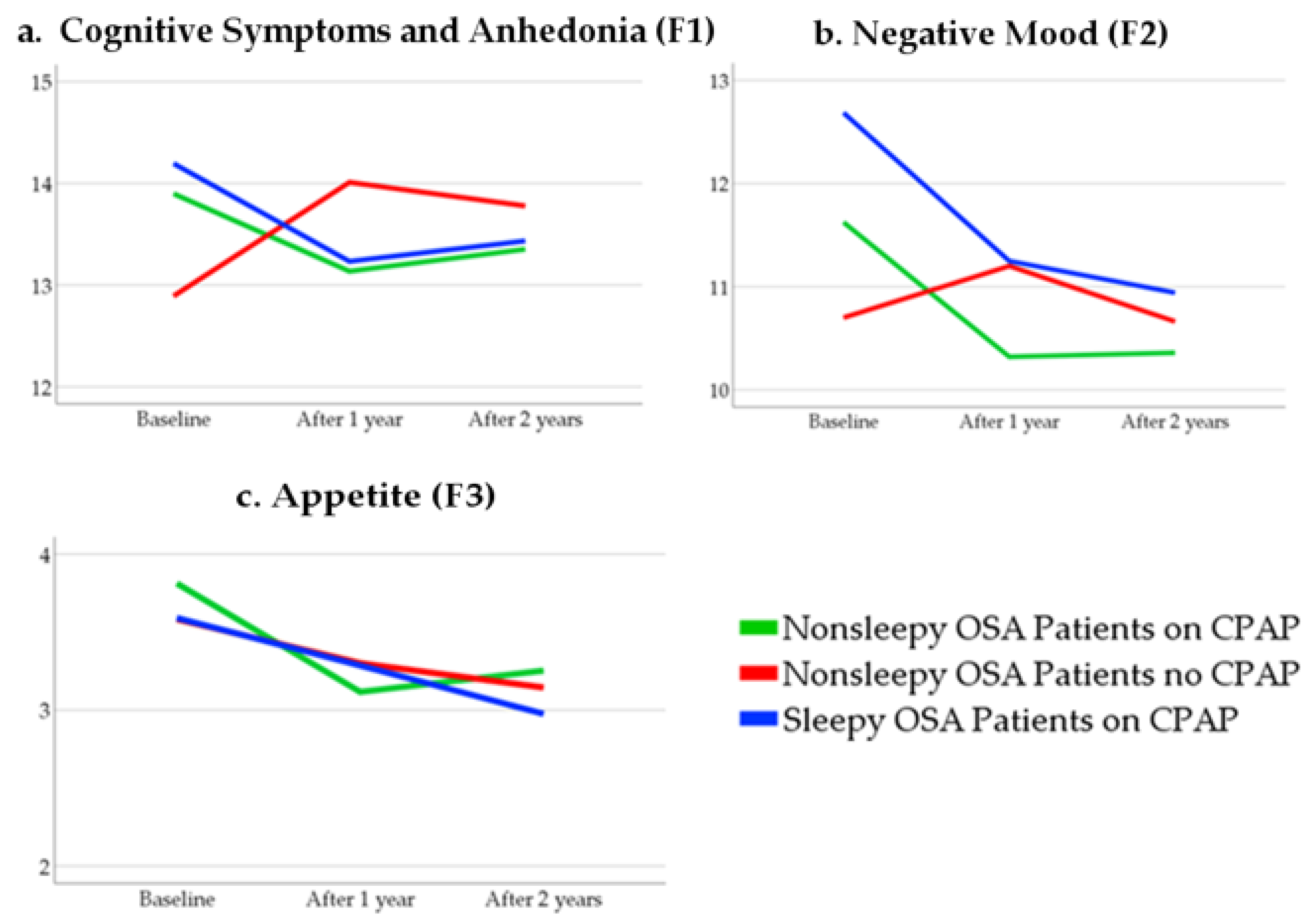
3.3. Within-Group Differences Over Time
3.4. The Linear Mix-Model
3.4.1. Null Model
3.4.2. The Shape of Growth Trajectory
3.4.3. Varying Time-Related Slope in the Sleepy OSA Patients on CPAP
3.4.4. Adding the Between-Subject Predictors
3.4.5. Final Mixed Models in Sleepy OSA Patients on CPAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovas-cular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Ronksley, P.E.; Hemmelgarn, B.R.; Heitman, S.J.; Ward, F.W.; Ghali, W.A.; Manns, B.; Tsai, W.H. Excessive daytime sleepiness is associated with increased health care utilization among pa-tients referred for assessment of OSA. Sleep 2011, 34, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Léger, D.; Stepnowsky, C. The economic and societal burden of excessive daytime sleepiness in patients with obstructive sleep apnea. Sleep Med. Rev. 2020, 51, 101275. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S. Update on Persistent Excessive Daytime Sleepiness in OSA. Chest 2020, 158, 776–786. [Google Scholar] [CrossRef]
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innov. Clin. Neurosci. 2011, 8, 17–25. [Google Scholar]
- Balcan, B.; Thunström, E.; Strollo, P.J.; Peker, Y. Continuous Positive Airway Pressure Treatment and Depression in Adults with Coronary Artery Disease and Nonsleepy Obstructive Sleep Apnea. A Secondary Analysis of the RICCADSA Trial. Ann. Am. Thorac. Soc. 2019, 16, 62–70. [Google Scholar] [CrossRef]
- Balcan, B.; Thunström, E.; Strollo, P.J., Jr.; Peker, Y. Determinants of depressive mood in coronary artery disease patients with obstructive sleep apnea and response to continuous positive airway pressure treatment in non-sleepy and sleepy phenotypes in the RICCADSA cohort. J. Sleep Res. 2019, 28, e12818. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.J.; Appleton, S.L.; Vakulin, A.; McEvoy, R.D.; Vincent, A.D.; Wittert, G.A.; Martin, S.A.; Grant, J.F.; Taylor, A.W.; Antic, N.; et al. Associations of Undiagnosed Obstructive Sleep Apnea and Excessive Daytime Sleepi-ness With Depression: An Australian Population Study. J. Clin. Sleep Med. 2017, 13, 575–582. [Google Scholar] [CrossRef]
- Ishman, S.L.; Cavey, R.M.; Mettel, T.L.; Gourin, C.G. Depression, sleepiness, and disease severity in patients with obstruc-tive sleep apnea. Laryngoscope 2010, 120, 2331–2335. [Google Scholar] [CrossRef]
- Budhiraja, R.; Kushida, C.A.; Nichols, D.A.; Walsh, J.K.; Simon, R.D.; Gottlieb, D.J.; Quan, S.F. Predictors of sleepiness in ob-structive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur. Respir. J. 2017, 50, 1700348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peker, Y.; Glantz, H.; Thunstrom, E.; Kallryd, A.; Herlitz, J.; Ejdeback, J. Rationale and design of the Randomized Interven-tion with CPAP in Coronary Artery Disease and Sleep Apnoea--RICCADSA trial. Scand. Cardiovasc. J. 2009, 43, 24–31. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunstrom, E. Effect of Positive Airway Pressure on Car-diovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RIC-CADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef]
- Dingli, K.; Coleman, E.; Vennelle, M.; Finch, S.; Wraith, P.; Mackay, T.; Douglas, N. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur. Respir. J. 2003, 21, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, S.F.; Gillin, J.C.; Littner, M.R.; Shepard, J.W. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. editorials. Sleep 1999, 22, 662–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zung, W.W.K.; Richards, C.B.; Short, M.J. Self-Rating Depression Scale in an Outpatient Clinic. Arch. Gen. Psychiatry 1965, 13, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.H.; Thomas, S.L.; Tabata, L.N. Multilevel and Longitudinal Modeling with IBM SPSS; Routledge: New York, NY, USA, 2014. [Google Scholar]
- Smith, S.; Rossdale, J.; Serry, Y.; Sekaran, A.; Drakatos, P.; Steier, J. AB004. Multiple dimensions of excessive daytime sleepiness. J. Thorac. Dis. 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecotic, R.; Dodig, I.P.; Valić, M.; Galic, T.; Kalcina, L.L.; Ivkovic, N.; Dogas, Z. Effects of CPAP therapy on cognitive and psychomotor performances in patients with severe obstructive sleep apnea: A prospective 1-year study. Sleep Breath. 2018, 23, 41–48. [Google Scholar] [CrossRef]
- Chen, B.; Drager, L.F.; Peker, Y.; Vgontzas, A.N.; Phillips, C.L.; Hoyos, C.M.; Salles, G.F.; Guo, M.; Li, Y. Effect of CPAP on Weight and Local Adiposity in Adults with Obstructive Sleep Apnea: A Meta-Analysis. Ann. Am. Thorac. Soc. 2021. [Google Scholar] [CrossRef]
- van Melle, J.P.; de Jonge, P.; Spijkerman, T.A.; Tijssen, J.G.; Ormel, J.; van Veldhuisen, D.J.; van den Brink, R.H.; van den Berg, M.P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: A meta-analysis. Psychosom. Med. 2004, 66, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, T.; Reis, V.A.; Linke, S.E.; Greenberg, B.H.; Mills, P.J. Depression in heart failure a meta-analytic review of preva-lence, intervention effects, and associations with clinical outcomes. J. Am. Coll. Cardiol. 2006, 48, 1527–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, K.W.; Kupfer, D.J.; Bigger, J.T.; Califf, R.M.; Carney, R.M.; Coyne, J.C.; Czajkowski, S.M.; Frank, E.; Frasure-Smith, N.; Freedland, K.E.; et al. Assessment and treatment of depression in patients with cardio-vascular disease: National Heart, Lung, and Blood Institute working group report. Annals of behavioral medicine: A publication of the Society of Behavioral Medicine. Ann. Behav. Med. 2006, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Perez Adel, P.; Fernandez, I.I.; Ojeda, F.B.; Gonzalez, R.D.; Gaos Meizoso, M.T. Zung scale factor invariance in male coro-nary patients and healthy people. Span. J. Psychol. 2013, 16, E72. [Google Scholar] [CrossRef]
- Koivula, M.; Halme, N.; Åstedt-Kurki, P. Predictors of depressive symptoms among coronary heart disease patients: A cross-sectional study nine years after coronary artery bypass grafting. Hear. Lung 2010, 39, 421–431. [Google Scholar] [CrossRef]
- Shumway, M.; Sentell, T.; Unick, G.; Bamberg, W. Cognitive complexity of self-administered depression measures. J. Affect. Disord. 2004, 83, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Parissis, J.T.; Nikolaou, M.; Farmakis, D.; Bistola, V.; Paraskevaidis, I.A.; Adamopoulos, S.; Filippatos, G.; Kremastinos, D.T. Clinical and prognostic implications of self-rating depression scales and plasma B-type natriuretic peptide in hos-pitalised patients with chronic heart failure. Heart 2008, 94, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Ahto, M.; Isoaho, R.; Puolijoki, H.; Vahlberg, T.; Kivelä, S.-L. Stronger symptoms of depression predict high coronary heart disease mortality in older men and women. Int. J. Geriatr. Psychiatry 2007, 22, 757–763. [Google Scholar] [CrossRef]
| Symptoms | F1 | F2 | F3 |
|---|---|---|---|
| 1. I feel down-hearted and blue | 0.75 | ||
| 2. Morning is when I feel the best | 0.31 | ||
| 3. I have crying spells or feel like it | 0.53 | ||
| 4. I have trouble sleeping at night | |||
| 5. I eat as much as I used to | 0.66 | ||
| 6. I still enjoy sex | 0.48 | ||
| 7. I notice that I am losing weight | 0.76 | ||
| 8. I have trouble with constipation | 0.31 | ||
| 9. My heart beats faster than usual | 0.49 | ||
| 10. I get tired for no reason | 0.55 | ||
| 11. My mind is as clear as it used to be | 0.70 | ||
| 12. I find it easy to do the things I used to | 0.70 | ||
| 13. I am restless and can’t keep still | 0.60 | ||
| 14. I feel hopeful about the future | 0.61 | ||
| 15. I am more irritable than usual | 0.63 | ||
| 16. I find it easy to make decisions | 0.66 | ||
| 17. I feel that I am useful and needed | 0.64 | ||
| 18. My life is pretty full | 0.59 | ||
| 19. I feel that others would be better off if I were dead | 0.53 | ||
| 20. I still enjoy the things I used to do | 0.60 |
| Sleepy OSA on CPAP n = 155 | Nonsleepy OSA on CPAP n = 122 | Nonsleepy OSA no CPAP n = 122 | |
|---|---|---|---|
| Age, yrs | 61.9 (46–78) | 66.1 (40–84) | 67.3 (43–81) |
| Male sex, % | 89.0 | 82.0 | 86.1 |
| BMI, kg/m2 | 29.0 (22–45) | 27.9 (20–42) | 28.6(20–39) |
| Obesity *, % | 41.3 | 27.9 | 27.9 |
| PCI, % | 74.2 | 73.0 | 73.0 |
| Current smoking, % | 17.4 | 18.0 | 13.9 |
| Diabetes, % | 25.2 | 27.9 | 20.5 |
| Stroke, % | 3.9 | 9.0 | 10.7 |
| Lung Disease, % | 9.7 | 3.3 | 9.8 |
| ESS score * | 11 (10–23) | 6 (1–9) | 5 (2–9) |
| AHI, events/h * | 27.7 (12–96) | 23.7 (15–69) | 24.8 (15–87) |
| ODI, events/h * | 14.7 (0–89) | 13.4 (0–64) | 11.7 (0–55) |
| Zung SDS score | 43.8 (26–75) | 41.3 (25–89) | 40.0 (25–66) |
| Estimates of Fixed Effects | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Std. Error | 95% Confidence Interval | p | |
| Lower Bound | Upper Bound | ||||
| Intercept | |||||
| Sleepy OSA on CPAP | 10.20 | 0.19 | 9.82 | 10.59 | <0.001 |
| Nonsleepy OSA on CPAP | 5.21 | 0.20 | 4.79 | 5.62 | <0.001 |
| Nonsleepy OSA no CPAP | 5.45 | 0.21 | 5.04 | 5.87 | <0.001 |
| Estimates of Covariance Parameters | |||||
| Repeated Measures | |||||
| Sleepy OSA on CPAP | 9.91 | 0.88 | 8.32 | 11.81 | <0.001 |
| Nonsleepy OSA on CPAP | 3.34 | 0.33 | 2.75 | 4.06 | <0.001 |
| Nonsleepy OSA no CPAP | 3.25 | 0.32 | 2.68 | 3.93 | <0.001 |
| Intercept (Subject = ID) | |||||
| Sleepy OSA on CPAP | 2.06 | 0.76 | 0.99 | 4.26 | 0.007 |
| Nonsleepy OSA on CPAP | 3.88 | 0.68 | 2.75 | 5.49 | <0.001 |
| Nonsleepy OSA no CPAP | 4.13 | 0.69 | 2.97 | 5.75 | <0.001 |
| Estimates of Fixed Effects | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Std. Error | 95% Confidence Interval | p | |
| Lower Bound | Upper Bound | ||||
| Sleepy OSA on CPAP | |||||
| Intercept | 12.22 | 0.244 | 11.74 | 12.70 | <0.001 |
| Linear Trend | −3.96 | 0.53 | −5.01 | −2.9 | <0.001 |
| Quadratic Trend | 1.05 | 0.26 | 0.53 | 1.56 | <0.001 |
| Nonsleepy OSA on CPAP | |||||
| Intercept | 5.46 | 0.24 | 4.98 | 5.94 | <0.001 |
| Linear Trend | −0.65 | 0.45 | −1.55 | 0.23 | 0.15 |
| Quadratic Trend | 0.21 | 0.22 | −0.21 | 0.65 | 0.32 |
| Nonsleepy OSA no CPAP | |||||
| Intercept | 5.45 | 0.24 | 4.97 | 5.94 | <0.001 |
| Linear Trend | 0.05 | 0.43 | −0.81 | 0.91 | 0.91 |
| Quadratic Trend | −0.03 | 0.21 | −0.45 | 0.39 | 0.88 |
| Estimates of Covariance Parameters | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Std. Error | 95% Confidence Interval | p | |
| Lower Bound | Upper Bound | ||||
| Repeated Measures (Variance) | 4.90 | 0.64 | 3.76 | 6.28 | <0.001 |
| Intercept + Linear Trend | |||||
| UN (1,1) | 2.06 | 0.91 | 0.86 | 4.92 | 0.024 |
| UN (2,1) | 0.36 | 0.56 | −0.74 | 1.48 | 0.516 |
| UN (2,2) | 1.16 | 0.58 | 0.43 | 3.10 | 0.046 |
| Estimates of Covariance Parameters | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Std. Error | 95% Confidence Interval | p | |
| Lower Bound | Upper Bound | ||||
| Intercept | 6.21 | 2.85 | 0.58 | 11.84 | 0.03 |
| F1 | −0.06 | 0.05 | −0.15 | 0.03 | 0.19 |
| F2 | 0.27 | 0.07 | 0.15 | 0.41 | <0.001 |
| F3 | 0.19 | 0.11 | −0.01 | 0.41 | 0.06 |
| CPAP adj/hrs | −0.12 | 0.10 | −0.29 | 0.06 | 0.19 |
| Male | 0.73 | 0.64 | −0.53 | 2.00 | 0.25 |
| BMI | −0.06 | 0.05 | −0.16 | 0.03 | 0.17 |
| Age | 0.10 | 0.03 | 0.00 | 0.12 | 0.04 |
| AHI | −0.01 | 0.02 | −0.05 | 0.04 | 0.81 |
| ODI | −0.01 | 0.02 | −0.05 | 0.03 | 0.66 |
| Smoking | 0.05 | 0.51 | −0.96 | 1.06 | 0.92 |
| Hypertension | −0.03 | 0.40 | −0.82 | 0.75 | 0.93 |
| History of AF | 0.48 | 0.55 | −0.62 | 1.58 | 0.39 |
| Diabetes | 0.72 | 0.44 | −0.17 | 1.60 | 0.11 |
| Stroke | −0.11 | 0.93 | −1.95 | 1.73 | 0.90 |
| Lung disease | −0.20 | 0.63 | −1.46 | 1.10 | 0.74 |
| PCI | 0.30 | 0.47 | −0.63 | 1.22 | 0.52 |
| Linear | −2.68 | 0.70 | −4.07 | −1.29 | <0.001 |
| Quadratic | 0.59 | 0.29 | 0.00 | 1.17 | 0.05 |
| Estimates of Fixed Effects | |||||
|---|---|---|---|---|---|
| Parameter | Estimate | Std. Error | 95% Confidence Interval | p | |
| Lower Bound | Upper Bound | ||||
| Intercept | 8.98 | 0.71 | 7.58 | 10.38 | <0.001 |
| F2 | 0.26 | 0.05 | 0.15 | 0.36 | 0.001 |
| Linear | −3.41 | 0.49 | −4.40 | −2.41 | <0.001 |
| Quadratic | 0.87 | 0.24 | 0.40 | 1.36 | <0.001 |
| Estimates of Covariance Parameters | |||||
| Repeated Measures | 4.67 | 0.62 | 3.60 | 6.10 | <0.001 |
| Intercept (Subject = ID) UN (1,1) | 2.16 | 0.91 | 0.95 | 4.94 | 0.01 |
| UN (2,1) | 0.47 | 0.55 | −0.60 | 1.55 | 0.38 |
| UN (2,2) | 0.87 | 0.55 | 0.26 | 2.97 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, Y.; Yapici-Eser, H.; Balcan, B.; Peker, Y. Association of Excessive Daytime Sleepiness with the Zung Self-Rated Depression Subscales in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. Diagnostics 2021, 11, 1176. https://doi.org/10.3390/diagnostics11071176
Celik Y, Yapici-Eser H, Balcan B, Peker Y. Association of Excessive Daytime Sleepiness with the Zung Self-Rated Depression Subscales in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. Diagnostics. 2021; 11(7):1176. https://doi.org/10.3390/diagnostics11071176
Chicago/Turabian StyleCelik, Yeliz, Hale Yapici-Eser, Baran Balcan, and Yüksel Peker. 2021. "Association of Excessive Daytime Sleepiness with the Zung Self-Rated Depression Subscales in Adults with Coronary Artery Disease and Obstructive Sleep Apnea" Diagnostics 11, no. 7: 1176. https://doi.org/10.3390/diagnostics11071176
APA StyleCelik, Y., Yapici-Eser, H., Balcan, B., & Peker, Y. (2021). Association of Excessive Daytime Sleepiness with the Zung Self-Rated Depression Subscales in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. Diagnostics, 11(7), 1176. https://doi.org/10.3390/diagnostics11071176







