Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice
Abstract
1. Introduction
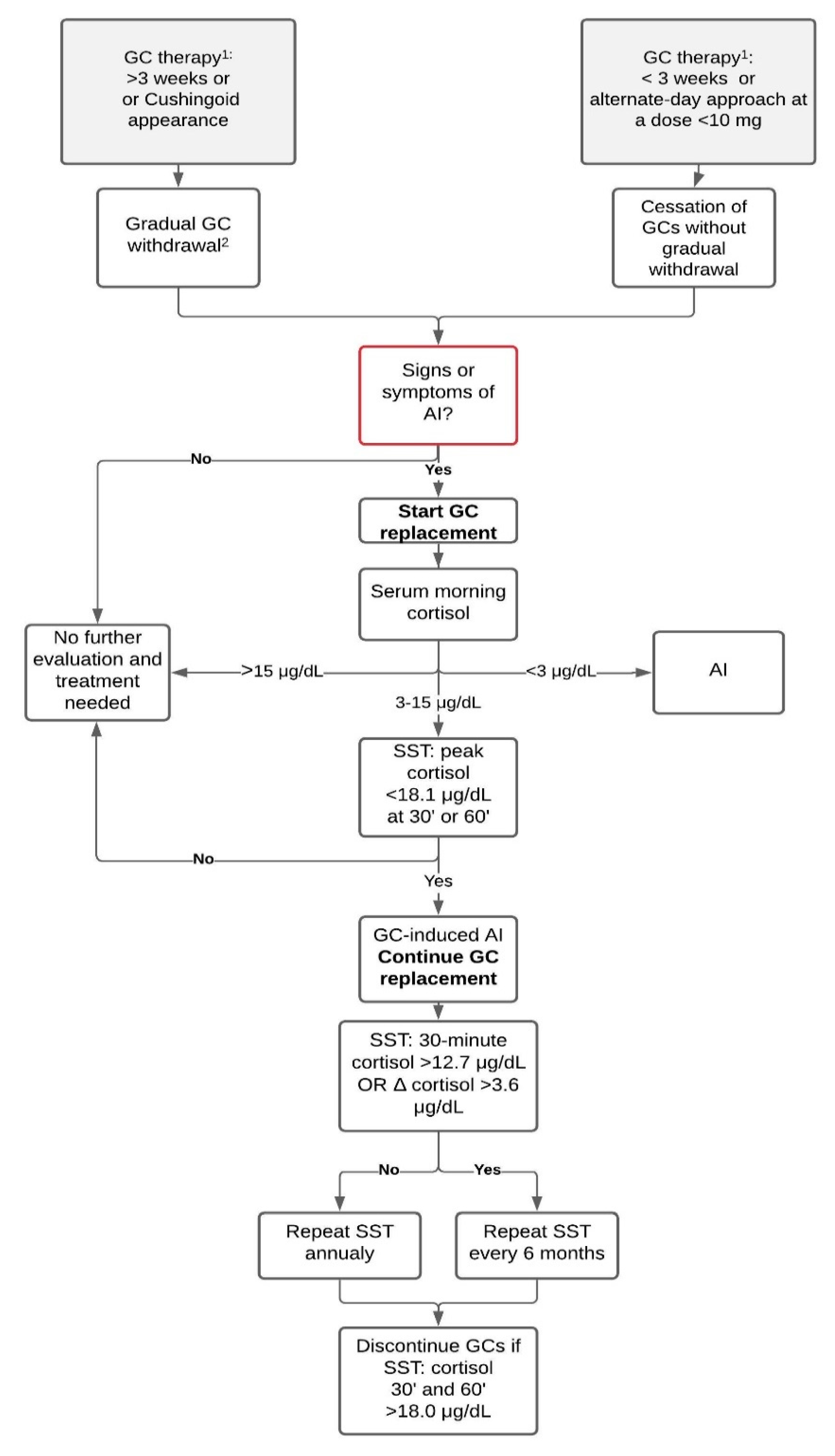
2. Diagnosing GC-Induced AI
2.1. Symptoms of AI
2.2. Baseline Measurements
2.3. Dynamic Tests
2.4. MRI of the Hypothalamic-Pituitary Area
3. GC Withdrawal
- 5 to 10 mg/day every one to two weeks from an initial dose >40 mg/day of prednisone (or equivalent);
- 5 mg/day every one to two weeks at prednisone doses ranging from 40 to 20 mg/day;
- 2.5 mg/day every two to three weeks at prednisone doses ranging from 20 to 10 mg/day;
- 1 mg/day every two to four weeks at prednisone doses ranging from 10 to 5 mg/day;
- 0.5 mg/day every two to four weeks at prednisone doses <5 mg/day.
4. Recovery of the HPA Axis
5. Treatment
Prevention of Adrenal Crisis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reitman, M.L. Hormone-Replacement Therapy for Melanocyte-Stimulating Hormone Deficiency. N. Engl. J. Med. 2016, 375, 278–279. [Google Scholar] [CrossRef]
- Charmandari, E.; Nicolaides, N.C.; Chrousos, G.P. Adrenal insufficiency. Lancet 2014, 383, 2152–2167. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Begaud, B.; Zhang, B.; Cooper, C. Use of oral corticosteroids in the United Kingdom. QJM Mon. J. Assoc. Physicians 2000, 93, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, K.; Lunde Jørgensen, J.O.; Petersen, I.; Sørensen, H.T. Fifteen-year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur. J. Endocrinol. 2019, 181, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Melby, J.C. Systemic corticosteroid therapy: Pharmacology and endocrinologic considerations. Ann. Intern. Med. 1974, 81, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.B.; Aron, D.C.; Findling, J.W.; Tyrrell, J.B. Glucocorticoids and Adrenal Androgens. In Greenspan’s Basic & Clinical Endocrinology, 10th ed.; Gardner, D.G., Shoback, D., Eds.; McGraw-Hill Education: New York, NY, USA, 2017; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2178§ionid=166249274 (accessed on 19 April 2021).
- Broersen, L.H.A.; Pereira, A.M.; Jørgensen, J.O.L.; Dekkers, O.M. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef]
- Joseph, R.M.; Hunter, A.L.; Ray, D.W.; Dixon, W.G. Systemic glucocorticoid therapy and adrenal insufficiency in adults: A systematic review. Semin. Arthritis Rheum. 2016, 46, 133–141. [Google Scholar] [CrossRef]
- Sacre, K.; Dehoux, M.; Chauveheid, M.P.; Chauchard, M.; Lidove, O.; Roussel, R.; Papo, T. Pituitary-Adrenal Function After Prolonged Glucocorticoid Therapy for Systemic Inflammatory Disorders: An Observational Study. J. Clin. Endocrinol. Metab. 2013, 98, 3199–3205. [Google Scholar] [CrossRef]
- Karangizi, A.H.K.; Al-Shaghana, M.; Logan, S.; Criseno, S.; Webster, R.; Boelaert, K.; Hewins, P.; Harper, L. Glucocorticoid induced adrenal insufficiency is common in steroid treated glomerular diseases—Proposed strategy for screening and management. BMC Nephrol. 2019, 20, 154. [Google Scholar] [CrossRef]
- Pokrzywa, A.; Ambroziak, U.; Foroncewicz, B.; Macech, M.; Pączek, L.; Florczak, M.; Mucha, K.; Bednarczuk, T. Detecting adrenal insufficiency in patients with immunoglobulin A nephropathy, lupus nephritis, and transplant recipients qualified for glucocorticoid withdrawal. Polish Arch. Intern. Med. 2019, 129, 874–882. [Google Scholar]
- Henzen, C.; Suter, A.; Lerch, E.; Urbinelli, R.; Schorno, X.H.; Briner, V.A. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet 2000, 355, 542–545. [Google Scholar] [CrossRef]
- Hahner, S.; Spinnler, C.; Fassnacht, M.; Burger-Stritt, S.; Lang, K.; Milovanovic, D.; Beuschlein, F.; Willenberg, H.S.; Quinkler, M.; Allolio, B. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: A prospective study. J. Clin. Endocrinol. Metab. 2015, 100, 407–416. [Google Scholar] [CrossRef]
- Hill, M.R.; Szefler, S.J.; Ball, B.D.; Bartoszek, M.; Brenner, A.M. Monitoring glucocorticoid therapy: A pharmacokinetic approach. Clin. Pharmacol. Ther. 1990, 48, 390–398. [Google Scholar] [CrossRef]
- Tornatore, K.M.; Logue, G.; Venuto, R.C.; Davis, P.J. Pharmacokinetics of Methylprednisolone in Elderly and Young Healthy Males. J. Am. Geriatr. Soc. 1994, 42, 1118–1122. [Google Scholar] [CrossRef]
- Chortis, V.; Taylor, A.E.; Schneider, P.; Tomlinson, J.W.; Hughes, B.A.; O’Neil, D.M.; Libé, R.; Allolio, B.; Bertagna, X.; Bertherat, J.; et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J. Clin. Endocrinol. Metab. 2013, 98, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Jabbour, A.; Artul, S.; Hakim, G. Intra-articular methylprednisolone acetate injection at the knee joint and the hypothalamic–pituitary–adrenal axis: A randomized controlled study. Clin. Rheumatol. 2014, 33, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Gazis, A.G.; Homer, J.J.; Henson, D.B.; Page, S.R.; Jones, N.S. The effect of six weeks topical nasal betamethasone drops on the hypothalamo-pituitary-adrenal axis and bone turnover in patients with nasal polyposis. Clin. Otolaryngol. Allied Sci. 1999, 24, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.J. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch. Intern. Med. 1999, 159, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A. Adverse effects of topical corticosteroid use. West. J. Med. 1995, 162, 123. [Google Scholar]
- Walsh, P.; Aeling, J.L.; Huff, L.; Weston, W.L. Hypothalamus-pituitary-adrenal axis suppression by superpotent topical steroids. J. Am. Acad. Dermatol. 1993, 29, 501–503. [Google Scholar] [CrossRef]
- White, M.; Crisalida, T.; Li, H.; Economides, A.; Kaliner, M. Effects of long-term inhaled corticosteroids on adrenal function in patients with asthma. Ann. Allergy Asthma Immunol. 2006, 96, 437–444. [Google Scholar] [CrossRef]
- Woods, C.P.; Argese, N.; Chapman, M.; Boot, C.; Webster, R.; Dabhi, V.; Grossman, A.B.; Toogood, A.A.; Arlt, W.; Stewart, P.M.; et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur. J. Endocrinol. 2015, 173, 633–642. [Google Scholar] [CrossRef]
- Ambroziak, U.; Bluszcz, G.; Bednarczuk, T.; Miśkiewicz, P. The influence of Graves’ orbitopathy treatment with intravenous glucocorticoids on adrenal function. Endokrynol. Pol. 2017, 68, 430–433. [Google Scholar] [CrossRef]
- Pelewicz, K.; Szewczyk, S.; Miśkiewicz, P. Treatment with Intravenous Methylprednisolone in Patients with Graves’ Orbitopathy Significantly Affects Adrenal Function: Assessment of Serum, Salivary Cortisol and Serum Dehydroepiandrosterone Sulfate. J. Clin. Med. 2020, 9, 3233. [Google Scholar] [CrossRef]
- Jespersen, S.; Nygaard, B.; Kristensen, L.Ø. Methylprednisolone Pulse Treatment of Graves’ Ophthalmopathy Is Not Associated with Secondary Adrenocortical Insufficiency. Eur. Thyroid J. 2015, 4, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Giotaki, Z.; Fountas, A.; Tsirouki, T.; Bargiota, A.; Tigas, S.; Tsatsoulis, A. Adrenal reserve following treatment of graves’ orbitopathy with intravenous glucocorticoids. Thyroid 2015, 25, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2021, 5, e10418. [Google Scholar] [CrossRef] [PubMed]
- Hagg, E.; Asplund, K.; Lithner, F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin. Endocrinol. 1987, 26, 221–226. [Google Scholar] [CrossRef]
- Erturk, E.; Jaffe, C.A.; Barkan, A.L. Evaluation of the integrity of the hypothalamic- pituitary-adrenal axis by insulin hypoglycemia test. J. Clin. Endocrinol. Metab. 1998, 83, 2350–2354. [Google Scholar] [CrossRef]
- Schlaghecke, R.; Kornely, E.; Santen, R.T.; Ridderskamp, P. The Effect of Long-Term Glucocorticoid Therapy on Pituitary–Adrenal Responses to Exogenous Corticotropin-Releasing Hormone. N. Engl. J. Med. 1992, 326, 226–230. [Google Scholar] [CrossRef]
- Sbardella, E.; Isidori, A.M.; Woods, C.P.; Argese, N.; Tomlinson, J.W.; Shine, B.; Jafar-Mohammadi, B.; Grossman, A.B. Baseline morning cortisol level as a predictor of pituitary–adrenal reserve: A comparison across three assays. Clin. Endocrinol. 2017, 86, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Patel, R.S.; Shaw, S.R.; McIntyre, H.E.; McGarry, G.W.; Wallace, A.M. Morning salivary cortisol versus short Synacthen test as a test of adrenal suppression. Ann. Clin. Biochem. 2004, 41, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Marcelli, G.; Martino, M.; Concettoni, C.; Brugia, M.; Trementino, L.; Michetti, G.; Arnaldi, G. The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: A real-life prospective study. J. Endocrinol. Invest. 2019, 42, 327–335. [Google Scholar] [CrossRef]
- Perogamvros, I.; Owen, L.J.; Keevil, B.G.; Brabant, G.; Trainer, P.J. Measurement of salivary cortisol with liquid chromatography-tandem mass spectrometry in patients undergoing dynamic endocrine testing. Clin. Endocrinol. 2010, 72, 17–21. [Google Scholar] [CrossRef]
- Deutschbein, T.; Broecker-Preuss, M.; Flitsch, J.; Jaeger, A.; Althoff, R.; Walz, M.K.; Mann, K.; Petersenn, S. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: Improved screening by an automatic immunoassay. Eur. J. Endocrinol. 2012, 166, 613–618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Gagnon, N.; Fréchette, I.; Mallet, P.L.; Dubé, J.; Houde, G.; Fink, G.D. Establishment of reference intervals for the salivary cortisol circadian cycle, by electrochemiluminescence (ECLIA), in healthy adults. Clin. Biochem. 2018, 54, 56–60. [Google Scholar] [CrossRef] [PubMed]
- George, G.S.; Jabbar, P.K.; Jayakumari, C.; John, M.; Mini, M.; Thekkumkara Surendran Nair, A.; Das, D.V.; Gomez, R.; Sreenath, R.; Prasad, N.; et al. Long-acting porcine ACTH stimulated salivary cortisol in the diagnosis of adrenal insufficiency. Clin. Endocrinol. 2020, 93, 652–660. [Google Scholar] [CrossRef]
- Oelkers, W.; Diederich, S.; Bähr, V. Diagnosis and therapy surveillance in Addison’s disease: Rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J. Clin. Endocrinol. Metab. 1992, 75, 259–264. [Google Scholar]
- Al-Aridi, R.; Abdelmannan, D.; Arafah, B.M. Biochemical diagnosis of adrenal insufficiency: The added value of dehydroepiandrosterone sulfate measurements. Endocr. Pract. 2011, 17, 261–270. [Google Scholar] [CrossRef]
- Charoensri, S.; Chailurkit, L.; Muntham, D.; Bunnag, P. Serum dehydroepiandrosterone sulfate in assessing the integrity of the hypothalamic-pituitary-adrenal axis. J. Clin. Transl. Endocrinol. 2017, 7, 42–46. [Google Scholar] [CrossRef]
- Bancos, I.; Hahner, S.; Tomlinson, J.; Arlt, W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015, 3, 216–226. [Google Scholar] [CrossRef]
- Furst, D.E.; Saag, K.G. Glucocorticoid Withdrawal. Available online: https://www.uptodate.com/contents/glucocorticoid-withdrawal (accessed on 21 March 2021).
- Hurel, S.J.; Thompson, C.J.; Watson, M.J.; Harris, M.M.; Baylis, P.H.; Kendall-Taylor, P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin. Endocrinol. 1996, 44, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Tomlinson, J.W.; Clark, P.M.; Holder, G.; Stewart, P.M. The long-term predictive accuracy of the short Synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J. Clin. Endocrinol. Metab. 2006, 91, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ospina, N.S.; Al Nofal, A.; Bancos, I.; Javed, A.; Benkhadra, K.; Kapoor, E.; Lteif, A.N.; Natt, N.; Murad, M.H. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dorin, R.I.; Qualls, C.R.; Crapo, L.M. Diagnosis of Adrenal Insufficiency. Ann. Intern. Med. 2003, 139, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, M.; Shimshi, M. Diagnosing adrenal insufficiency: Which test is best—The 1-μg or the 250-μg cosyntropin stimulation test? Endocr. Pract. 2008, 14, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.; Meinel, T.; Lahner, H.; Mann, K.; Petersenn, S. Recovery of pituitary function in the late-postoperative phase after pituitary surgery: Results of dynamic testing in patients with pituitary disease by insulin tolerance test 3 and 12 months after surgery. Eur. J. Endocrinol. 2010, 162, 853–859. [Google Scholar] [CrossRef]
- Lopez Schmidt, I.; Lahner, H.; Mann, K.; Petersenn, S. Diagnosis of adrenal insufficiency: Evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J. Clin. Endocrinol. Metab. 2003, 88, 4193–4198. [Google Scholar] [CrossRef]
- Abdu, T.A.M.; Elhadd, T.A.; Neary, R.; Clayton, R.N. Comparison of the low dose short synacthen test (1 μg), the conventional dose short synacthen test (250 μg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J. Clin. Endocrinol. Metab. 1999, 84, 838–843. [Google Scholar] [CrossRef]
- Tordjman, K.; Jaffe, A.; Grazas, N.; Apter, C.; Stern, N. The role of the low dose (1 μg) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J. Clin. Endocrinol. Metab. 1995, 80, 1301–1305. [Google Scholar]
- Kazlauskaite, R.; Evans, A.T.; Villabona, C.V.; Abdu, T.A.M.; Ambrosi, B.; Atkinson, A.B.; Cheung, H.C.; Clayton, R.N.; Courtney, C.H.; Gonc, E.N.; et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J. Clin. Endocrinol. Metab. 2008, 93, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.S.; Helen Kemp, E.; White, A.; Walker, L.; Meredith, S.; Sachdev, P.; Krone, N.P.; Ross, R.J.; Wright, N.P.; Elder, C.J. International survey on high- and low-dose synacthen test and assessment of accuracy in preparing low-dose synacthen. Clin. Endocrinol. 2018, 88, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Baid, S.; Calis, K.; Raff, H.; Sinaii, N.; Nieman, L. Technical details influence the diagnostic accuracy of the 1 μg ACTH stimulation test. Eur. J. Endocrinol. 2010, 162, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Margolin, L.; Cope, D.K.; Bakst-Sisser, R.; Greenspan, J. The Steroid Withdrawal Syndrome: A Review of the Implications, Etiology, and Treatments. J. Pain Symptom Manag. 2007, 33, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Jamilloux, Y.; Liozon, E.; Pugnet, G.; Nadalon, S.; Heang Ly, K.; Dumonteil, S.; Gondran, G.; Fauchais, A.L.; Vidal, E. Recovery of Adrenal Function after Long-Term Glucocorticoid Therapy for Giant Cell Arteritis: A Cohort Study. PLoS ONE 2013, 8, e68713. [Google Scholar] [CrossRef]
- Dinsen, S.; Baslund, B.; Klose, M.; Rasmussen, A.K.; Friis-Hansen, L.; Hilsted, L.; Feldt-Rasmussen, U. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur. J. Intern. Med. 2013, 24, 714–720. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, S.K.; Jung, J.H.; Hahm, J.R.; Jung, J. Recovery of adrenal function in patients with glucocorticoids induced secondary adrenal insufficiency. Endocrinol. Metab. 2016, 31, 153. [Google Scholar] [CrossRef]
- Pofi, R.; Feliciano, C.; Sbardella, E.; Argese, N.; Woods, C.P.; Grossman, A.B.; Jafar-Mohammadi, B.; Gleeson, H.; Lenzi, A.; Isidori, A.M.; et al. The short Synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J. Clin. Endocrinol. Metab. 2018, 103, 3050–3059. [Google Scholar] [CrossRef]
- Esteban, N.V.; Loughlin, T.; Yergey, A.L.; Zawadzki, J.K.; Booth, J.D.; Winterer, J.C.; Loriaux, D.L. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J. Clin. Endocrinol. Metab. 1991, 72, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kraan, G.P.B.; Dullaart, R.P.F.; Pratt, J.J.; Wolthers, B.G.; Drayer, N.M.; De Bruin, R. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J. Clin. Endocrinol. Metab. 1998, 83, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W. The approach to the adult with newly diagnosed adrenal insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 1059–1067. [Google Scholar] [CrossRef]
- Woodcock, T.; Barker, P.; Daniel, S.; Fletcher, S.; Wass, J.A.H.; Tomlinson, J.W.; Misra, U.; Dattani, M.; Arlt, W.; Vercueil, A. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: Guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia 2020, 75, 654–663. [Google Scholar] [CrossRef]
- Prete, A.; Taylor, A.E.; Bancos, I.; Smith, D.J.; Foster, M.A.; Kohler, S.; Fazal-Sanderson, V.; Komninos, J.; O’Neil, D.M.; Vassiliadi, D.A.; et al. Prevention of Adrenal Crisis: Cortisol Responses to Major Stress Compared to Stress Dose Hydrocortisone Delivery. J. Clin. Endocrinol. Metab. 2020, 105, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K. Treatment of Adrenal Insufficiency in Adults. Available online: https://www.uptodate.com/contents/treatment-of-adrenal-insufficiency-in-adults (accessed on 21 March 2021).
| Glucocorticoids | Equivalent Physiological Doses [mg/Day] | Duration of Action [Hours] | Mineralocorticoid Activity Relative to Hydrocortisone |
|---|---|---|---|
| Hydrocortisone | 20 | 8–12 | 1 |
| Prednisone | 5 | 12–36 | 0.8 |
| Methylprednisolone | 4 | 12–36 | 0.5 |
| Triamcinolone | 4 | 12–36 | 0 |
| Dexamethasone | 0.75 | 36–72 | 0 |
| Betamethasone | 0.6 | 36–72 | 0 |
| Probable | Intermediate/Uncertain | Improbable | |
|---|---|---|---|
| Treatment with prednisone (or equivalent) | ≥20 mg/day for >3 weeks or ≥5 mg/day in the evening for more than a few weeks or Cushingoid appearance | 10 to 20 mg/day for >3 weeks or <10 mg/day for more than a few weeks | < 3 weeks or alternate-day approach at a dose <10 mg |
| Suggested approach | |||
| Gradual GC withdrawal | Gradual GC withdrawal | Cessation of GC therapy without previous gradual withdrawal is acceptable | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelewicz, K.; Miśkiewicz, P. Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics 2021, 11, 728. https://doi.org/10.3390/diagnostics11040728
Pelewicz K, Miśkiewicz P. Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics. 2021; 11(4):728. https://doi.org/10.3390/diagnostics11040728
Chicago/Turabian StylePelewicz, Katarzyna, and Piotr Miśkiewicz. 2021. "Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice" Diagnostics 11, no. 4: 728. https://doi.org/10.3390/diagnostics11040728
APA StylePelewicz, K., & Miśkiewicz, P. (2021). Glucocorticoid Withdrawal—An Overview on When and How to Diagnose Adrenal Insufficiency in Clinical Practice. Diagnostics, 11(4), 728. https://doi.org/10.3390/diagnostics11040728






