Electrocution Stigmas in Organ Damage: The Pathological Marks
Abstract
1. Introduction
Pathophysiology of Electrocution Damage
2. Materials and Methods
3. Results and Discussion (Main Histological Findings and Differential Diagnosis)
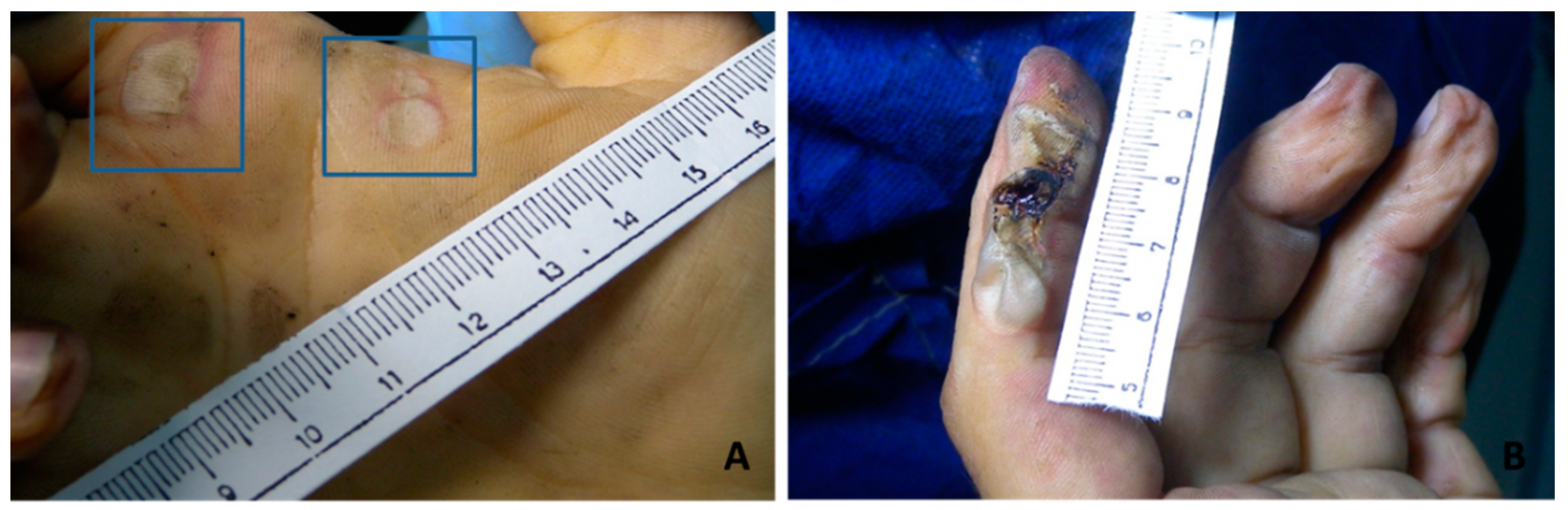
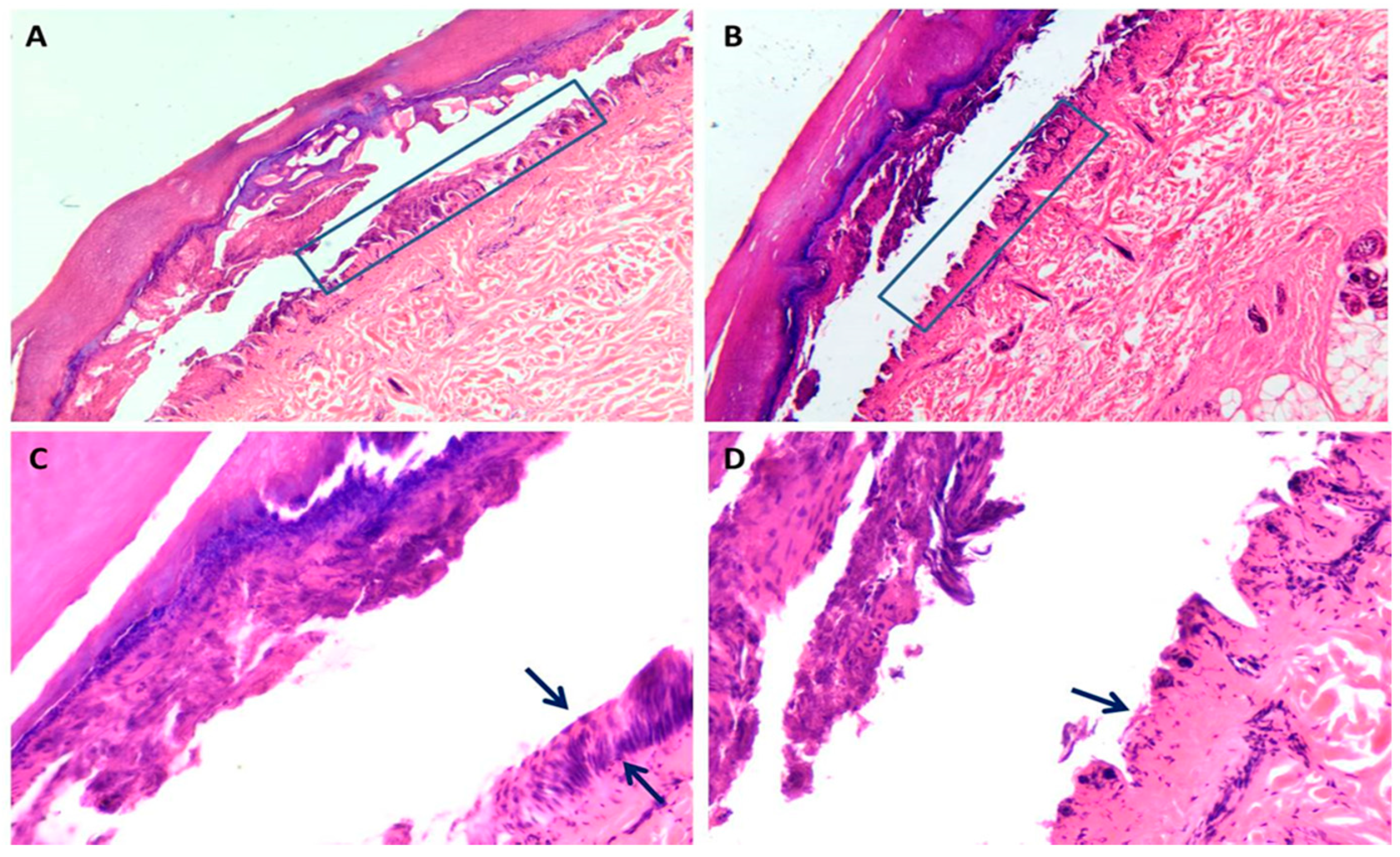
3.1. Skin Damage
3.2. Central Nervous System Damage
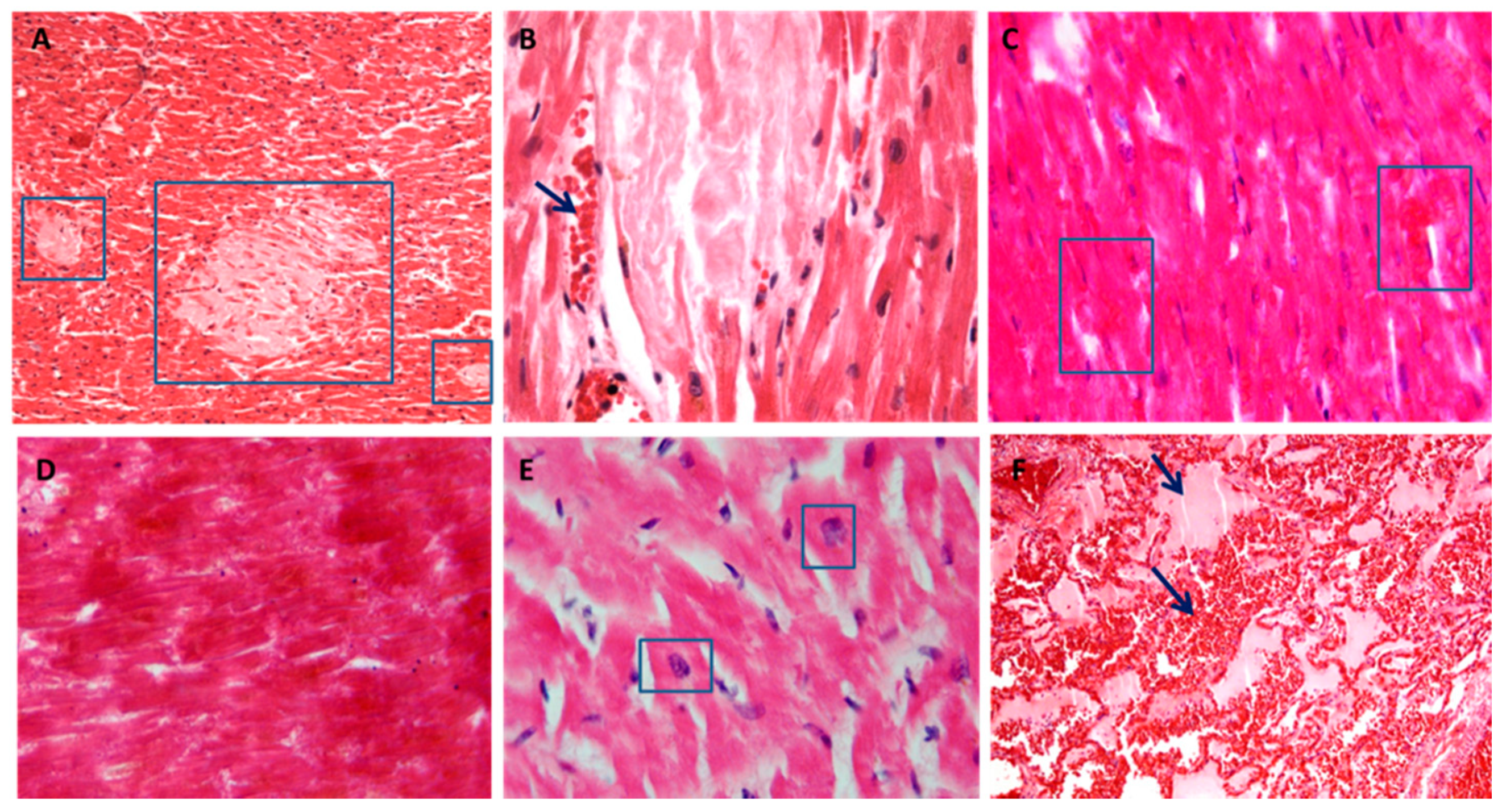
3.3. Cardiac Damage
3.4. Pulmonary Damage
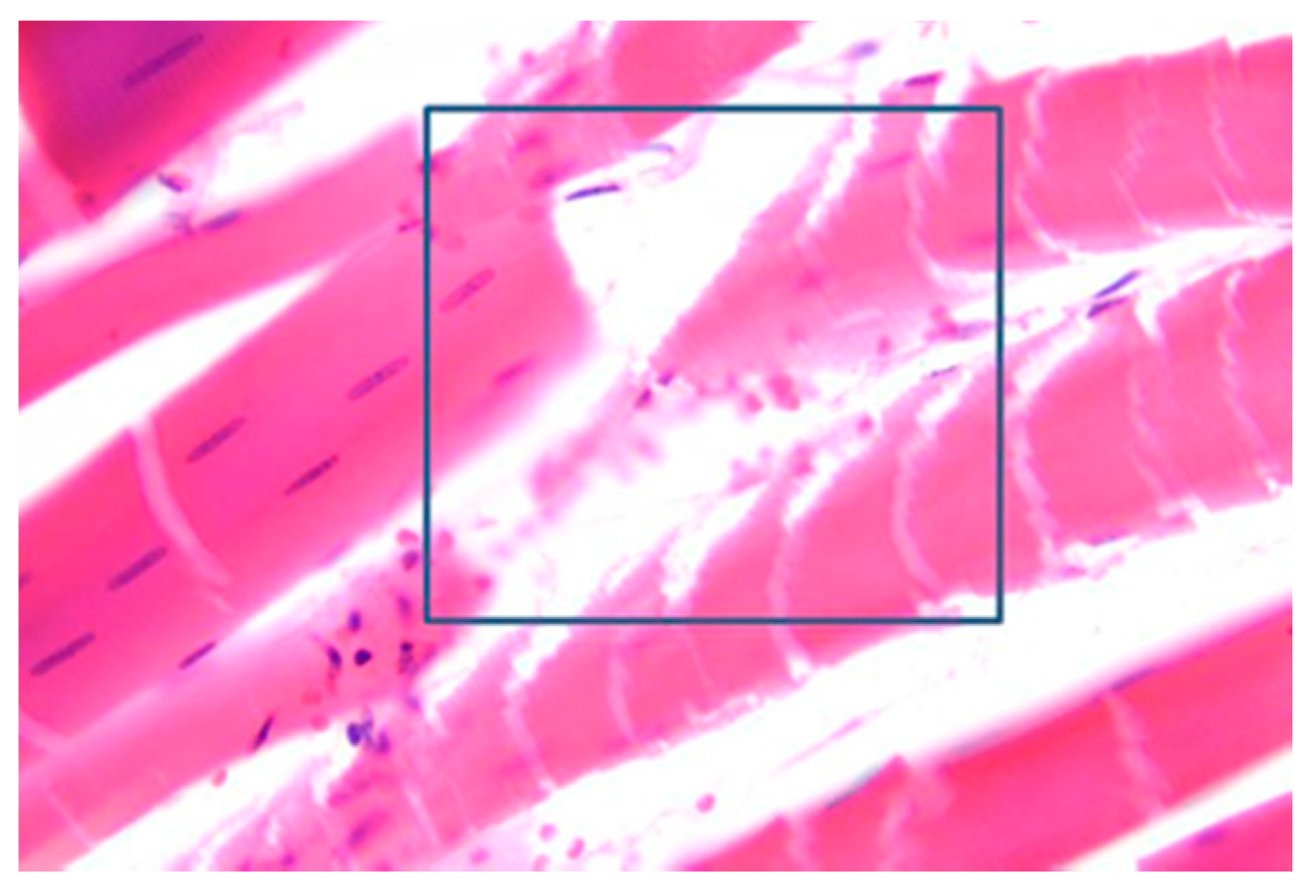
3.5. Muscles Damage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trivedi, T.K.; Liu, C.; Antonio, A.L.M.; Wheaton, N.; Kreger, V.; Yap, A.; Schriger, D.; Elmore, J.G. Injuries Associated With Standing Electric Scooter Use. JAMA Netw. Open 2019, 2, e187381. [Google Scholar] [CrossRef] [PubMed]
- Zemaitis, M.R.; Foris, L.A.; Lopez, R.A.; Huecker, M.R. Electrical Injuries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448087/ (accessed on 10 January 2021).
- Waldmann, V.; Narayanan, K.; Combes, N.; Jost, D.; Jouven, X.; Marijon, E. Electrical cardiac injuries: Current concepts and management. Eur. Heart J. 2018, 39, 1459–1465. [Google Scholar] [CrossRef]
- Parakkattil, J.; Kandasamy, S.; Das, S.; Devnath, G.P.; Chaudhari, V.A.; Shaha, K.K. Atypical Exit Wound in High-Voltage Electrocution. Am. J. Forensic Med. Pathol. 2017, 38, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shikui, C. Study on electrocution death by low-voltage. Forensic Sci. Int. 1995, 76, 115–119. [Google Scholar] [CrossRef]
- Wright, R.K. Death or Injury Caused by Electrocution. Clin. Lab. Med. 1983, 3, 343–353. [Google Scholar] [CrossRef]
- Aquila, I.; Gratteri, S.; Amirante, C.; Fineschi, V.; Frati, P.; Ricci, P. Electric or traumatic injury? The role of histopathological investigations. Med. Leg. J. 2018, 86, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Mondello, C.; Micali, A.; Cardia, L.; Argo, A.; Zerbo, S.; Spagnolo, E.V. Forensic tools for the diagnosis of electrocution death: Case study and literature review. Med. Leg. J. 2018, 86, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Nizhu, L.N.; Hasan, M.J.; Rabbani, R. High-voltage electrocution-induced pulmonary injury and cerebellar hemorrhage with fractures in atlas. Trauma Case Rep. 2020, 25, 100267. [Google Scholar] [CrossRef] [PubMed]
- Dechent, D.; Emonds, T.; Stunder, D.; Schmiedchen, K.; Kraus, T.; Driessen, S. Direct current electrical injuries: A systematic review of case reports and case series. Burns 2020, 46, 267–278. [Google Scholar] [CrossRef]
- Paternoster, M.; Capasso, E.; Di Lorenzo, P.; Mansueto, G. Fatal exertional rhabdomyolysis. Literature review and our experience in forensic thanatology. Leg. Med. (Tokyo Jpn.) 2018, 35, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Benincasa, G.; Capasso, E.; Graziano, V.; Russo, M.; Niola, M.; Napoli, C.; Buccelli, C. Autoptic findings of sudden cardiac death (SCD) in patients with arrhythmogenic ventricular cardiomiopathy (AVC) from left ventricle and biventricular involvement. Pathol. Res. Pract. 2020, 216, 153269. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Niola, M.; Napoli, C. Can COVID 2019 induce a specific cardiovascular damage or it exacerbates pre-existing cardiovascular diseases? Pathol. Res. Pract. 2020, 216, 153086. [Google Scholar] [CrossRef]
- Bellini, E.; Gambassi, G.; Nucci, G.; Benvenuti, M.; Landi, G.; Gabbrielli, M.; Vanezis, P. Death by electrocution: Histological technique for copper detection on the electric mark. Forensic Sci. Int. 2016, 264, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.V.; Sacca, F.; Paternoster, M.; Buonomo, A.R.; Gentile, I.; Scotto, R.; Morra, V.B.; Mansueto, G. Post-mortem diagnosis of invasive pulmonary aspergillosis after alemtuzumab treatment for multiple sclerosis. Mult. Scler. J. 2020, 26, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Zhang, D.; Hannig, J. Biophysical Injury Mechanisms in Electrical Shock Trauma. Annu. Rev. Biomed. Eng. 2000, 2, 477–509. [Google Scholar] [CrossRef]
- Jain, S.; Bandi, V. ELECTRICAL AND LIGHTNING INJURIES. Crit. Care Clin. 1999, 15, 319–331. [Google Scholar] [CrossRef]
- Koumbourlis, A.C. Electrical injuries. Crit. Care Med. 2002, 30, S424–S430. [Google Scholar] [CrossRef]
- Wright, R.K.; Davis, J.H. The investigation of electrical deaths: A report of 220 fatalities. J. Forensic Sci. 1980, 25, 514–521. [Google Scholar] [CrossRef]
- Ramati, A.; Pliskin, N.H.; Keedy, S.; Erwin, R.J.; Fink, J.W.; Bodnar, E.N.; Lee, R.C.; Cooper, M.A.; Kelley, K.; Sweeney, J.A. Alteration in Functional Brain Systems After Electrical Injury. J. Neurotrauma 2009, 26, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Cherington, M. Spectrum of neurologic complications of lightning injuries. Neurorehabilition 2005, 20, 3–8. [Google Scholar] [CrossRef]
- Davis, C.; Engeln, A.; Johnson, E.L.; McIntosh, S.E.; Zafren, K.; Islas, A.A.; McStay, C.; Smith, W.R.; Cushing, T. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Lightning Injuries: 2014 Update. Wilderness Environ. Med. 2014, 25, S86–S95. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Engeln, A.; Johnson, E.; McIntosh, S.E.; Zafren, K.; Islas, A.A.; McStay, C.; Smith, W.; Cushing, T. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Lightning Injuries. Wilderness Environ. Med. 2012, 23, 260–269. [Google Scholar] [CrossRef]
- Wang, T.; Zou, D.; Zhang, J.; Chen, Y. Application of Microbeam X-Ray Fluorescence Spectrometry in the Diagnosis of Suspected Electrocution by High-Voltage Direct Current: A Case Report. Am. J. Forensic Med. Pathol. 2016, 37, 190–193. [Google Scholar] [CrossRef]
- Akyildiz, E.; Uzun, I.; Inanici, M.A.; Baloglu, H. Computerized Image Analysis in Differentiation of Skin Lesions Caused by Electrocution, Flame Burns, and Abrasion. J. Forensic Sci. 2009, 54, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, W.; Lin, H.; Wang, Z.; Dong, H. Identification of Skin Electrical Injury Using Infrared Imaging: A Possible Complementary Tool for Histological Examination. PLoS ONE 2017, 12, e0170844. [Google Scholar] [CrossRef]
- Uzun, I.; Akyildiz, E.; Inanici, M.A. Histopathological differentiation of skin lesions caused by electrocution, flame burns and abrasion. Forensic Sci. Int. 2008, 178, 157–161. [Google Scholar] [CrossRef]
- Sangita, C.; Garima, G.; Jayanthi, Y.; Arneet, A.; Neelkamal, K. Histological indicators of cutaneous lesions caused by electrocution, flame burn and impact abrasion. Med. Sci. Law 2018, 58, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Visona, S.D.; Chen, Y.; Bernardi, P.; Andrello, L.; Osculati, A. Diagnosis of electrocution: The application of scanning electron microscope and energy-dispersive X-ray spectroscopy in five cases. Forensic Sci. Int. 2018, 284, 107–116. [Google Scholar] [CrossRef]
- Pfeiffer, H.; Du Chesne, A.; Brinkmann, B. An unusual case of homicidal near drowning followed by electrocution. Int. J. Leg. Med. 2006, 120, 36–41. [Google Scholar] [CrossRef]
- Shaha, K.K.; Joe, A.E. Electrocution-related mortality: A retrospective review of 118 deaths in Coimbatore, India, between January 2002 and December 2006. Med. Sci. Law 2010, 50, 72–74. [Google Scholar] [CrossRef]
- Shetty, B.S.K.; Kanchan, T.; Acharya, J.; Naik, R. Cardiac pathology in fatal electrocution. Burns 2014, 40, e45–e46. [Google Scholar] [CrossRef]
- Gentile, G.; Andreola, S.; Bailo, P.; Boracchi, M.; Fociani, P.; Piccinini, A.; Zoja, R. A Pilot Study on the Diagnosis of Fatal Electrocution by the Detection of Myocardial Microhemorrhages. J. Forensic Sci. 2020, 65, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Karch, S.B.; D’Errico, S.; Pomara, C.; Riezzo, I.; Turillazzi, E. Cardiac pathology in death from electrocution. Int. J. Leg. Med. 2006, 120, 79–82. [Google Scholar] [CrossRef]
- Michiue, T.; Ishikawa, T.; Zhao, D.; Kamikodai, Y.; Zhu, B.-L.; Maeda, H. Pathological and biochemical analysis of the pathophysiology of fatal electrocution in five autopsy cases. Leg. Med. 2009, 11 (Suppl. 1), S549–S552. [Google Scholar] [CrossRef]
- Debono, R. A histological analysis of a high voltage electric current injury to an upper limb. Burns 1999, 25, 541–547. [Google Scholar] [CrossRef]
- Blumenthal, R. A retrospective descriptive study of electrocution deaths in Gauteng, South Africa: 2001–2004. Burns 2009, 35, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Peters, M.; Baumgartner, W.; Wohlsein, P. Electrical Injuries in Animals: Causes, Pathogenesis, and Morphological Findings. Vet. Pathol. 2016, 53, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, A.; Acar, K.; Adiguzel, E.; Boz, B. Hippocampal neuron loss due to electric injury in rats: A stereological study. Leg. Med. 2009, 11, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kokatnur, L.; Rudrappa, M. Acute Stroke due to Electrocution: Uncommon or Unrecognized? Case Rep. Neurol. Med. 2016, 2016, 9510863. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lo, R. Electric injury with cerebral venous thrombosis. Case report and review of the literature. Stroke 1993, 24, 903–905. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Dell’Erba, A.S.; Addante, A.; Zotti, F.; Marzullo, A.; Colonna, M.F. Sudden Cardiac Death and Myocardial Ischemia Indicators: A comparative study of four immunohistochemical markers. Am. J. Forensic Med. Pathol. 2008, 29, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Baroldi, G.; Silver, M.D.; Parolini, M.; Pomara, C.; Turillazzi, E.; Fineschi, V. Myofiberbreak-up: A marker of ventricular fibrillation in sudden cardiac death. Int. J. Cardiol. 2005, 100, 435–441. [Google Scholar] [CrossRef]
- Aimo, A.; Di Paolo, M.; Castiglione, V.; Modena, M.; Barison, A.; Benvenuti, M.; Bugelli, V.; Campobasso, C.P.; Guidi, B.; Camici, P.G.; et al. Scared to Death: Emotional Stress Causing Fatal Myocardial Infarction With Nonobstructed Coronary Arteries in Women. JACC Case Rep. 2020, 2, 2400–2403. [Google Scholar] [CrossRef]
- Singh, S.; Sankar, J.; Dubey, N. Non-cardiogenic pulmonary oedema following accidental electrocution in a toddler. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Michiue, T.; Ishikawa, T.; Zhu, B.-L.; Maeda, H. Combined analyses of creatine kinase MB, cardiac troponin I and myoglobin in pericardial and cerebrospinal fluids to investigate myocardial and skeletal muscle injury in medicolegal autopsy cases. Leg. Med. 2011, 13, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, V.J.; Di Maio, D. Forensic Pathology, 2rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Saukko, P.; Knight, B. Knight’s Forensic Pathology; CRC Press: London, UK, 2015. [Google Scholar]

| Current Intensity | Effect |
|---|---|
| 1 mA | Tingling sensation; almost not perceptible |
| 3–5 mA | “Let-go” current for an average child |
| 6–9 mA | “Let-go” current for an average adult |
| 16 mA | Maximum current a person can grasp and “let go” |
| 16–20 mA | Tetany of skeletal muscles |
| 20–50 mA | Paralysis of respiratory muscles; respiratory arrest |
| 50–100 mA | Threshold for ventricular fibrillation |
| >2 A | Asystole |
| 15–30 A | Common household circuit breakers |
| 240 A | Maximal intensity of household current (U.S.) |
| Study | Current Intensity | Skin | CNS | Heart | Lung | Other |
|---|---|---|---|---|---|---|
| Wang, et al. [24] | LV | Slight elongation of the epidermal cell nuclei. Detachment in corneous layer | ||||
| Akyildiz et al. [25] | N | Greater nuclear elongation in electrocution than flame burns, and abrasion | ||||
| Zhang J, et al. [26] | HV | Dermal-epidermal detachment; elongated and polarized epidermal cells with darkly nuclei | ||||
| Mondello, et al. [8] | HV | Elongated and polarized epidermal cells. Dermis coagulative necrosis | ||||
| Bellini, et al. [14] | HV/LV | Palisade nuclei in the spinous layer and lysis of the granular layer; incomplete dermo-epidermal detachment; iron and copper deposit | ||||
| Uzun, et al. [27] | HV | Coagulative necrosis of the epidermis; intra-epidermal and sub-epidermal detachment; nuclear elongation with darkly nuclei Homogenized collagen like dermis | ||||
| Sangita, et al. [28] | HV | Intra-epidermal and subepidermal detachment; epidermis coagulative necrosis; elongated and polarized epidermal cells with dark nuclei Dermal homogenization collagen like with vascular dilatation, congestion, hemorrage and thrombosis | ||||
| Visonà, et al. [29] | HV | Horny layer vacuolization, epidemic cells elongation Fragmentation and necrosis of the elastic dermal fibers | Subarachnoid hemorrhage from trauma | Hemopericardium | Hemorrhage from contusion | Visceral edema and congestion |
| Pfeiffer, et al. [30] | HV | Dermopidermis detachment with basal cells elongation | Fresh myocardial fiber necrosis | Hemorrhages | Kidney hemorrhage; hemoglobin detection | |
| Wang, et al. [24] | HV | Aorta and pulmonary artery perforation (Electronic microscopy) | ||||
| Shaha, et al. [31] | N | Edema | Edema | |||
| Shetty, et al. [32] | N | Myocardium necrosis without inflammatory reaction; myocardial fragmentation and contraction bands; pericardial surface hemorrhage | ||||
| Gentile, et al. [33] | HV | Myocardial hemorrhages | ||||
| Fineschi, et al. [34]. | N | Myofiber break-up; square nucleus; bundles of hyper-contracted myocites (electrocution compared with cocaine and other trauma) | ||||
| Michiue, et al. [35] | HV | Myocardial fibers fragmentation; cardiomyolysis | Hemorrhage; edema | |||
| DeBono, et al. [36] | HV | Muscle fiber fragmentation; red blood cell extravasation; neural damage; |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansueto, G.; Di Napoli, M.; Mascolo, P.; Carfora, A.; Zangani, P.; Pietra, B.D.; Campobasso, C.P. Electrocution Stigmas in Organ Damage: The Pathological Marks. Diagnostics 2021, 11, 682. https://doi.org/10.3390/diagnostics11040682
Mansueto G, Di Napoli M, Mascolo P, Carfora A, Zangani P, Pietra BD, Campobasso CP. Electrocution Stigmas in Organ Damage: The Pathological Marks. Diagnostics. 2021; 11(4):682. https://doi.org/10.3390/diagnostics11040682
Chicago/Turabian StyleMansueto, Gelsomina, Mario Di Napoli, Pasquale Mascolo, Anna Carfora, Pierluca Zangani, Bruno Della Pietra, and Carlo Pietro Campobasso. 2021. "Electrocution Stigmas in Organ Damage: The Pathological Marks" Diagnostics 11, no. 4: 682. https://doi.org/10.3390/diagnostics11040682
APA StyleMansueto, G., Di Napoli, M., Mascolo, P., Carfora, A., Zangani, P., Pietra, B. D., & Campobasso, C. P. (2021). Electrocution Stigmas in Organ Damage: The Pathological Marks. Diagnostics, 11(4), 682. https://doi.org/10.3390/diagnostics11040682








