Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review
Abstract
1. Introduction
2. Cutaneous Endometriosis
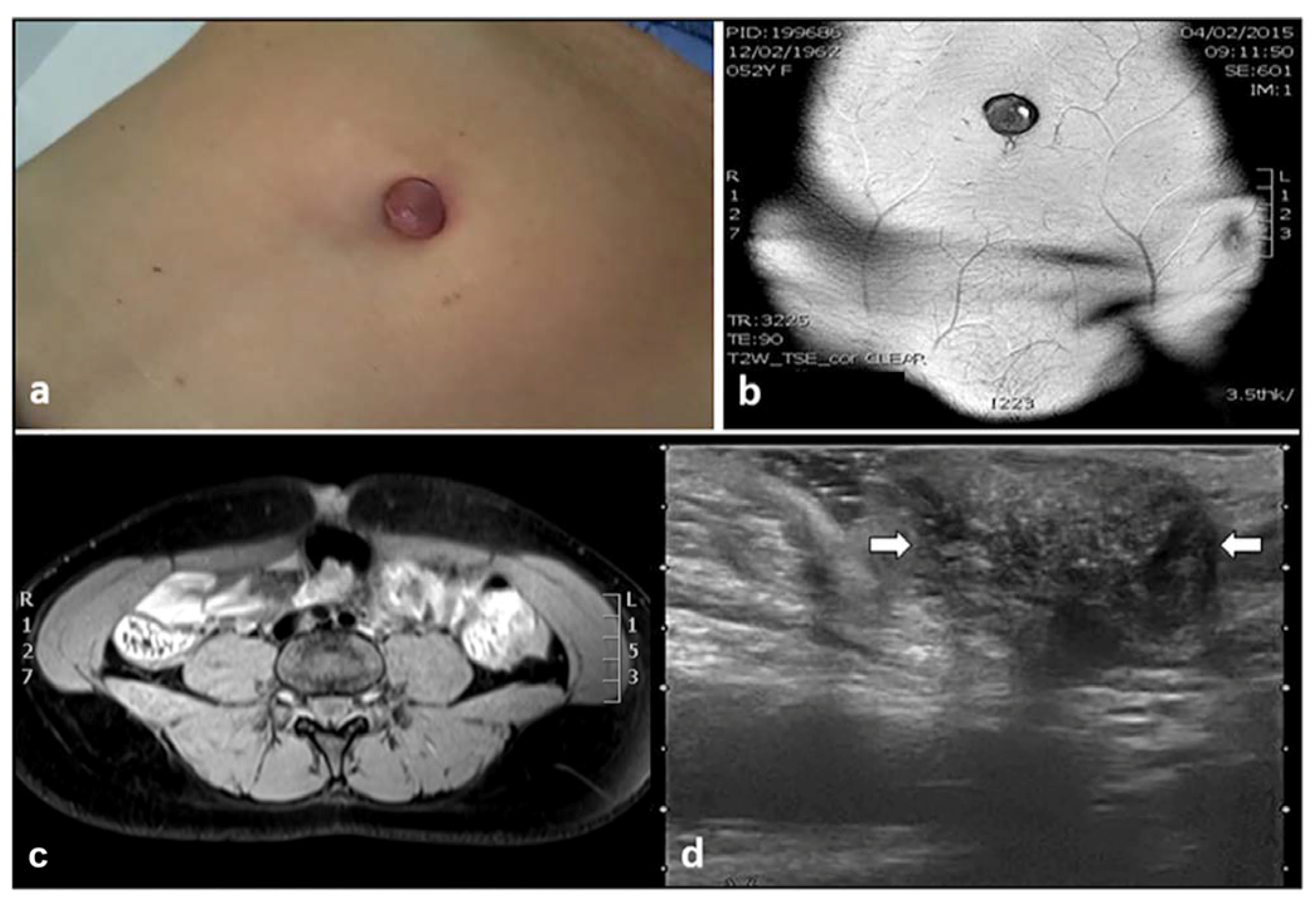
3. Umbilical Endometriosis
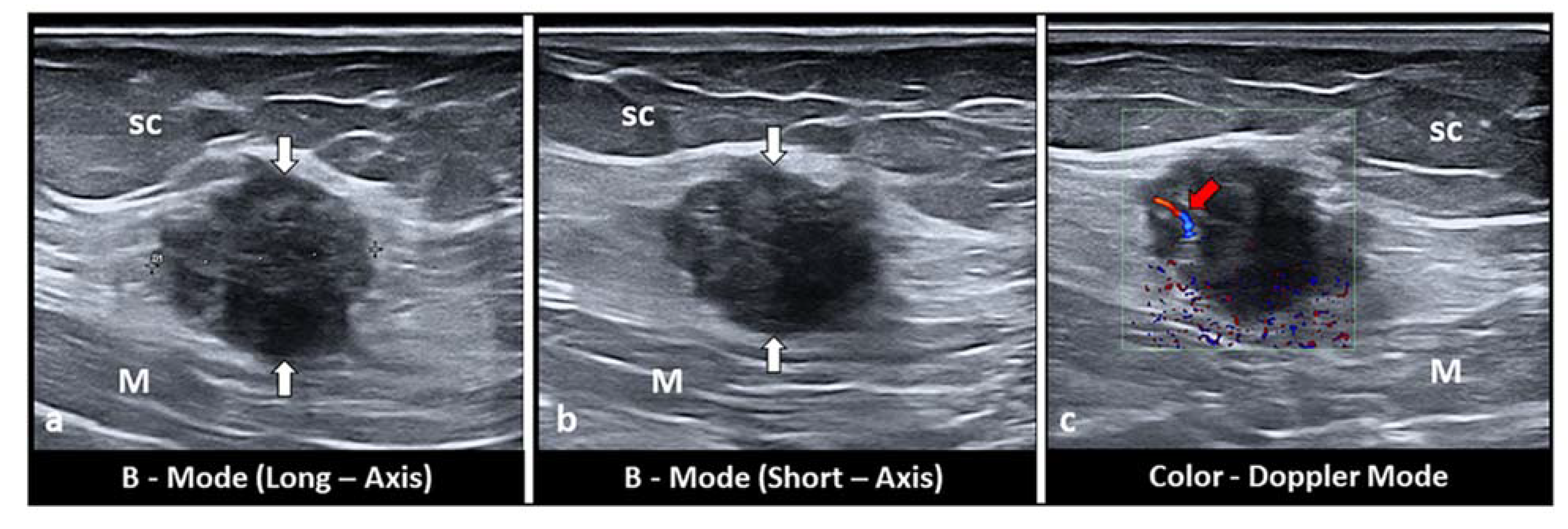
4. Subcutaneous Endometriosis
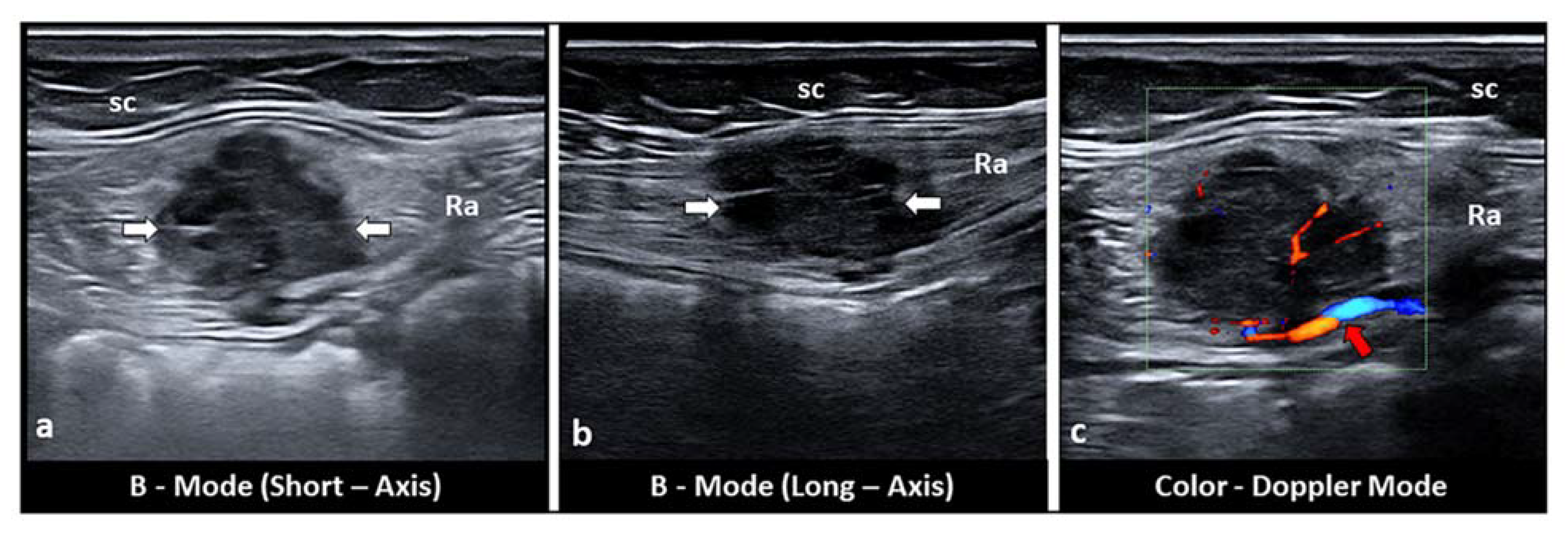
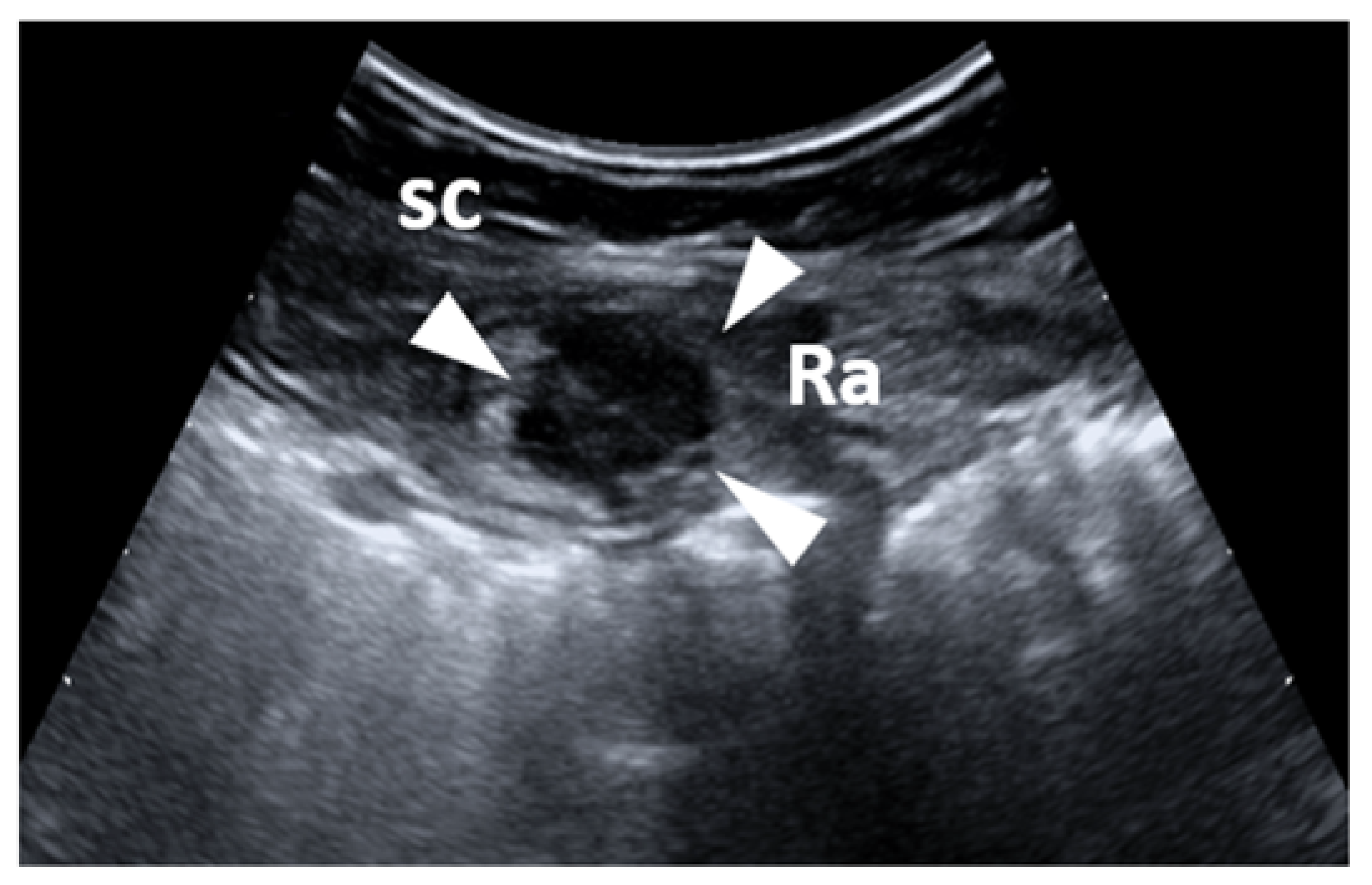
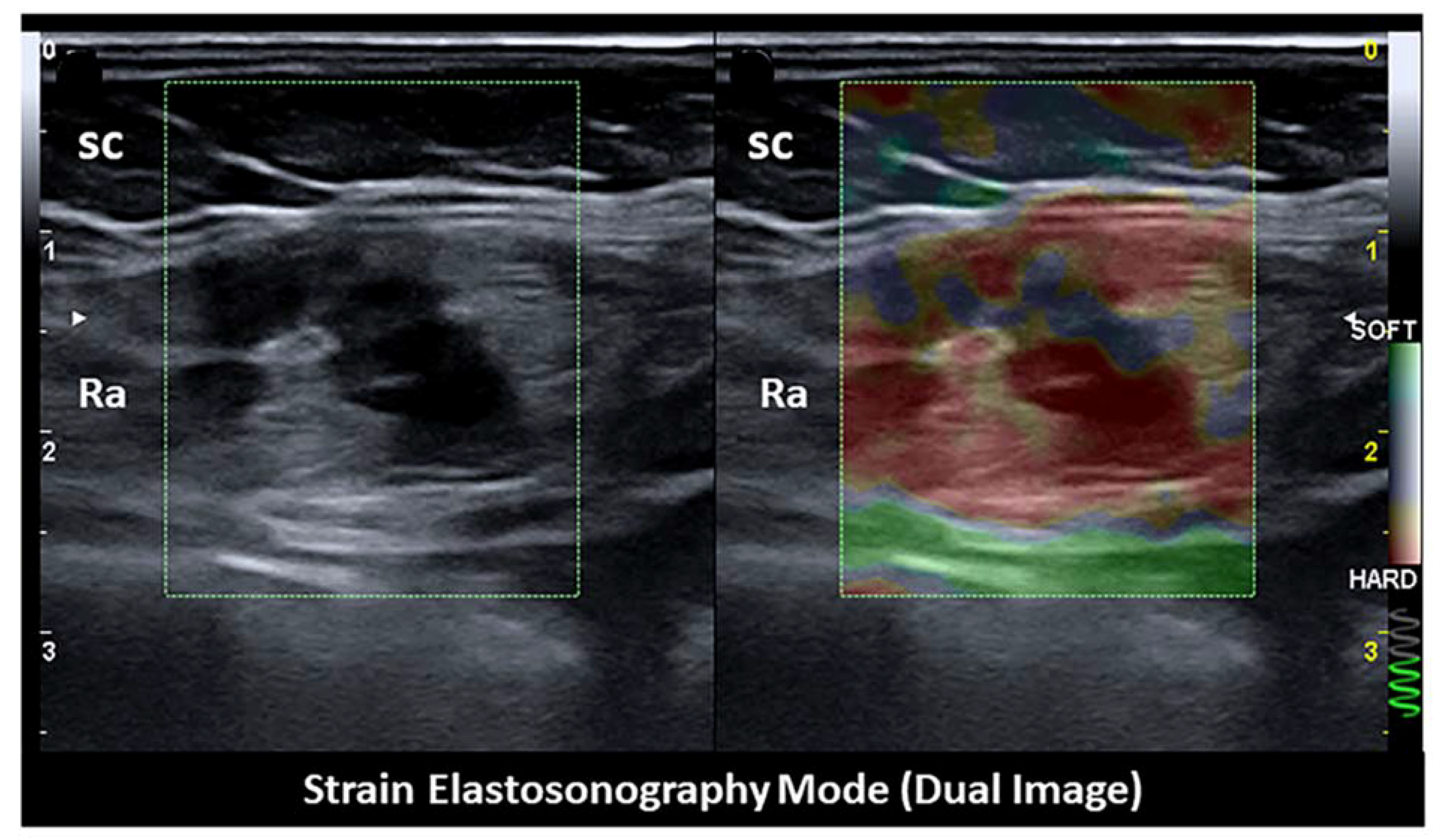
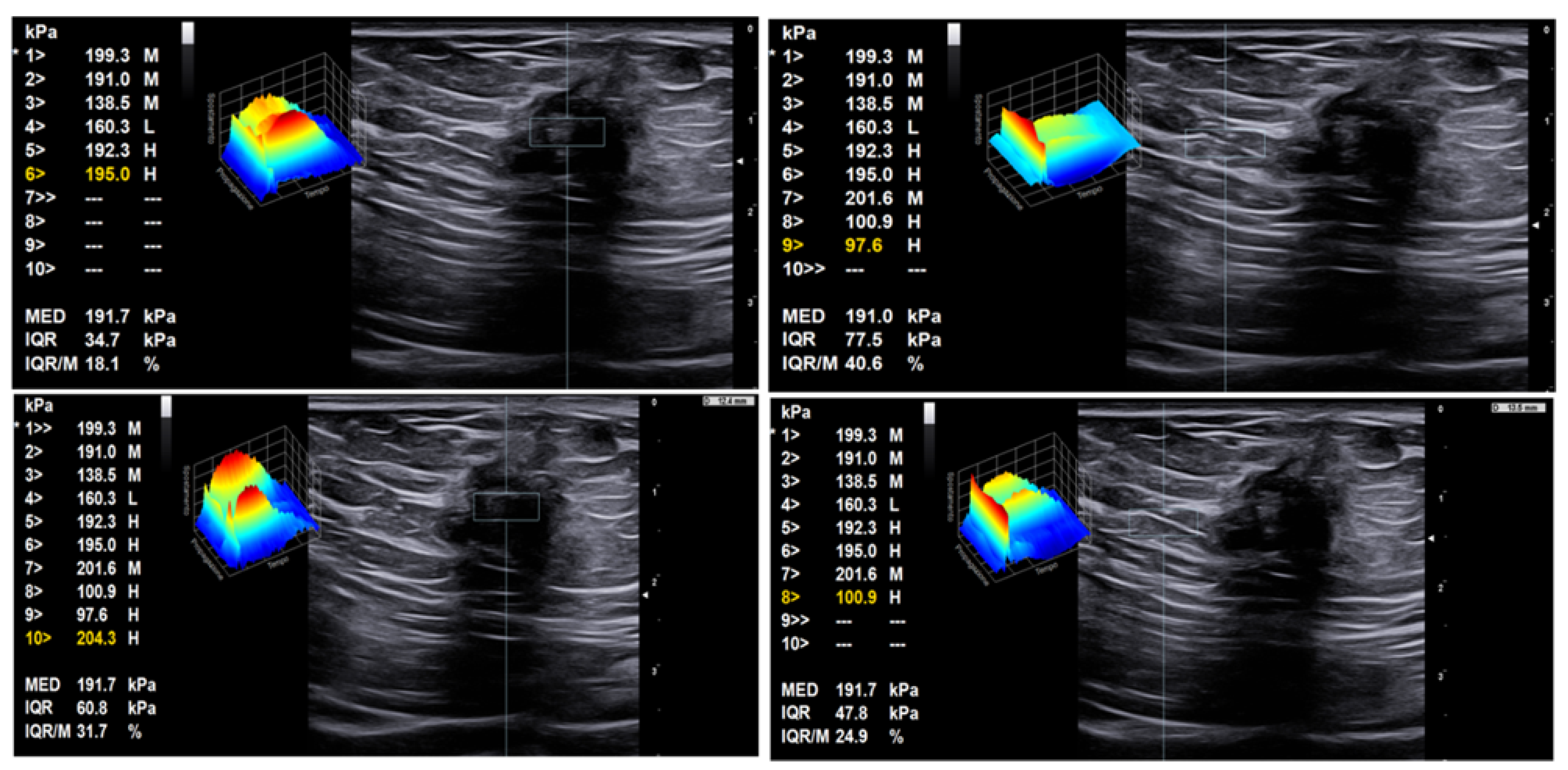
5. Intramuscular Endometriosis
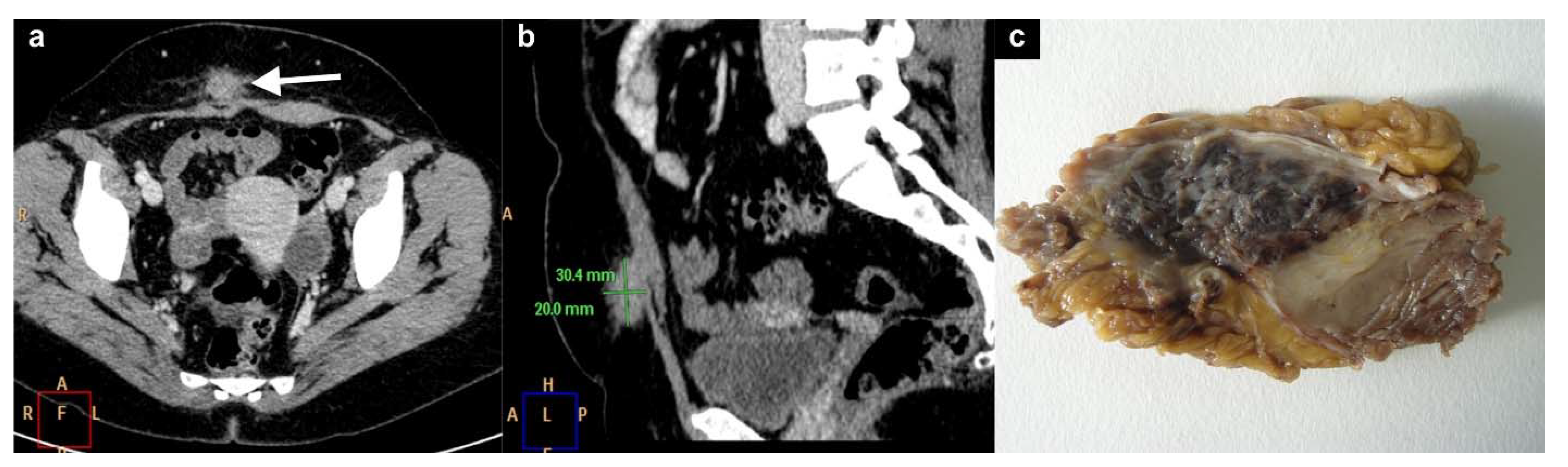
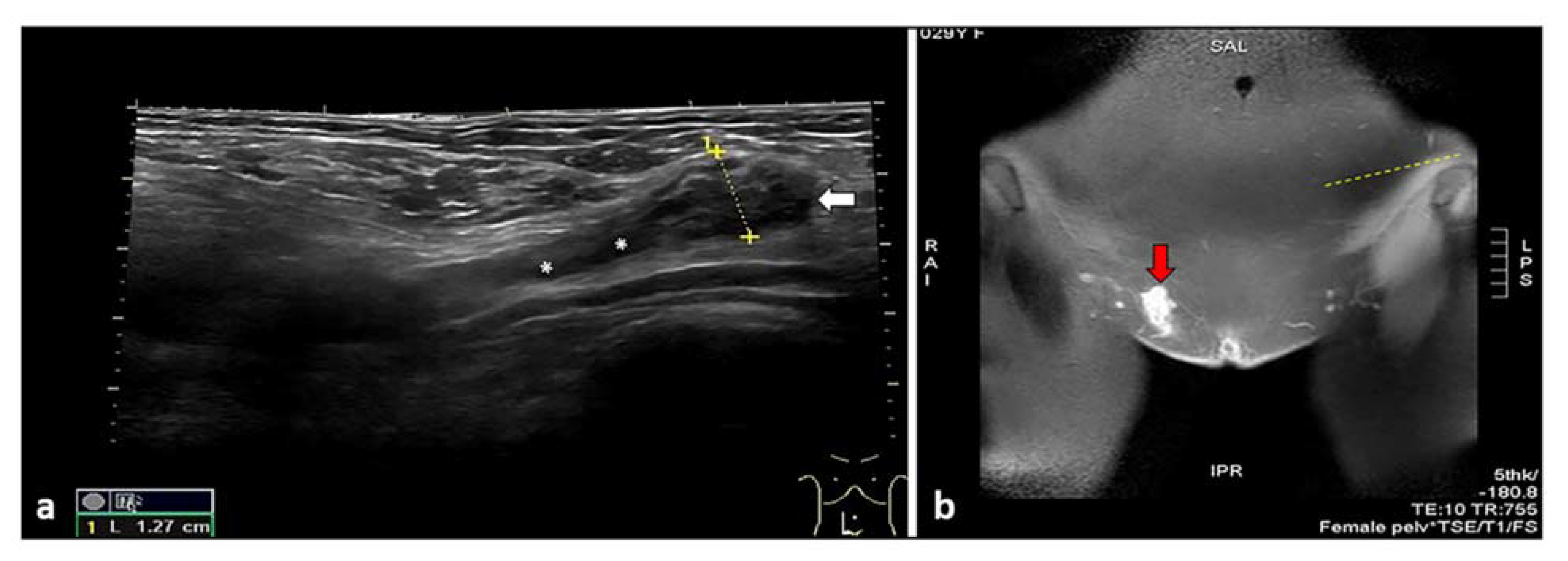
6. Inguinal Canal Endometriosis
7. Discussion
8. Therapeutic Options
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Rokitansky, K. Über Uterusdrüsen-Neubildung. Z. Ges. Aerzte 1860, 16, 577–581. [Google Scholar]
- Missmer, S.A.; Tu, F.F.; Agarwal, S.K.; Chapron, C.; Soliman, A.M.; Chiuve, S.; Eichner, S.; Flores-Caldera, I.; Horne, A.W.; Kimball, A.B.; et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int. J. Gen. Med. 2021, 14, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; De Leire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Scioscia, M.; Bruni, F.; Ceccaroni, M.; Steinkasserer, M.; Stepniewska, A.; Minelli, L. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obs. Gynecol. Scand. 2011, 90, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.C.; Goldberg, J.M. Extrapelvic endometriosis. Semin. Reprod. Med. 2017, 35, 98–101. [Google Scholar]
- Hirsch, M.; Begum, M.R.; Paniz, É.; Barker, C.; Davis, C.J.; Duffy, J.M.N. Diagnosis and management of endometriosis: A systematic review of international and national guidelines. BJOG 2018, 125, 556–564. [Google Scholar] [CrossRef]
- Savelli, L.; Manuzzi, L.; Di Donato, N.; Salfi, N.; Trivella, G.; Ceccaroni, M.; Seracchioli, R. Endometriosis of the abdominal wall: Ultrasonographic and Doppler characteristics. Ultrasound Obstet. Gynecol. 2012, 39, 336–340. [Google Scholar] [CrossRef]
- Betrán, A.P.; Ye, J.; Moller, A.-B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The increasing trend in caesarean section rates: Global, regional and national estimates: 1990–2014. PLoS ONE 2016, 11, e0148343. [Google Scholar]
- Rindos, N.B.; Mansuria, S. Diagnosis and Management of Abdominal Wall Endometriosis: A Systematic Review and Clinical Recommendations. Obstet Gynecol Surv. 2017, 72, 116–122. [Google Scholar] [CrossRef]
- Nominato, N.S.; Prates, L.F.V.S.; Lauar, I.; Morais, J.; Maia, L.; Geber, S. Caesarean section greatly increases risk of scar endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Haak, H.E.; Maas, M.; Lahaye, M.J.; Boellaard, T.N.; Pizzi, A.D.; Mihl, C.; Van Der Zee, D.; Fabris, C.; Van Der Sande, M.E.; Melenhorst, J.; et al. Selection of Patients for Organ Preservation After Chemoradiotherapy: MRI Identifies Poor Responders Who Can Go Straight to Surgery. Ann. Surg. Oncol. 2020, 27, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.D.; Caposiena, D.; Mastrodicasa, D.; Trebeschi, S.; Lambregts, D.; Rosa, C.; Cianci, R.; Seccia, B.; Sessa, B.; Di Flamminio, F.M.; et al. Tumor detectability and conspicuity comparison of standard b1000 and ultrahigh b2000 diffusion-weighted imaging in rectal cancer. Abdom. Radiol. 2019, 44, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.D.; Tavoletta, A.; Narciso, R.; Mastrodicasa, D.; Trebeschi, S.; Celentano, C.; Mastracchio, J.; Cianci, R.; Seccia, B.; Marrone, L.; et al. Prenatal planning of placenta previa: Diagnostic accuracy of a novel MRI-based prediction model for placenta accreta spectrum (PAS) and clinical outcome. Abdom. Radiol. 2019, 44, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Alnafisah, F.; Dawa, S.K.; Alalfy, S. Skin endometriosis at the caesarean section scar: A case report and review of the literature. Cureus 2018, 10, e2063. [Google Scholar] [CrossRef] [PubMed]
- Raffi, L.; Suresh, R.; McCalmont, T.H.; Twigg, A.R. Cutaneous endometriosis. Int. J. Womens Derm. 2019, 5, 384–386. [Google Scholar] [CrossRef]
- Ismael, H.; Ragoza, Y.; Harden, A.; Cox, S. Spontaneous endometriosis associated with an umbilical hernia: A case report and review of the literature. Int. J. Surg. Case Rep. 2017, 30, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Victory, R.; Diamond, M.P.; Johns, D.A. Villar’s nodule: A case report and systematic literature review of endometriosis externa of the umbilicus. J. Minim. Invasive Gynecol. 2007, 14, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mann, L.S.; Clarke, W.R. Endometriosis of the umbilicus. IMJ III Med. J. 1964, 125, 335–336. [Google Scholar]
- Wicherek, L.; Klimek, M.; Skret-Magierlo, J.; Czekierdowski, A.; Banas, T.; Popiela, T.J.; Kraczkowski, J.; Sikora, J.; Oplawski, M.; Nowak, A.; et al. The obstetrical history in patients with Pfannenstiel scar. Gynecol. Obstet. Investig. 2007, 63, 107–113. [Google Scholar] [CrossRef]
- Bagade, P.V.; Guirguis, M.M. Menstruating from the umbilicus as a rare case of primary umbilical endometriosis: A case report. J. Med. Case Rep. 2009, 3, 9326. [Google Scholar] [CrossRef]
- Paramythiotis, D.; Stavrou, G.; Panidis, S.; Panagiotou, D.; Chatzopoulos, K.; Papadopoulos, V.N.; Michalopoulos, A. Concurrent appendiceal and umbilical endometriosis: A case report and review of the literature. J. Med. Case Rep. 2014, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Draghi, F.; Cocco, G.; Richelmi, F.M.; Schiavone, C. Abdominal wall sonography: A pictorial review. J. Ultrasound 2020, 23, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, Y.; Zhang, C.; Yang, Y.; Zhang, L.; Wang, N.; Xu, H. Cesarean scar endometriosis: Presentation of 198 cases and literature review. BMC Womens Health 2019, 19, 14. [Google Scholar] [CrossRef]
- Cocco, G.; Ricci, V.; Boccatonda, A.; Schiavone, C. Focused ultrasound for the diagnosis of non-palpable endometriotic lesions of the abdominal wall: A not-uncommon surgical complication. J. Ultrasound 2020, 23, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Mizumoto, M.; Kinoshita, K. Endometriosis-associated hydrocele of the canal of Nuck with immunohistochemical confirmation: A case report. J. Med. Case Rep. 2017, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Block, R.E. Hydrocele of the canal of Nuck. A report of five cases. Obstet. Gynecol. 1975, 45, 464–466. [Google Scholar]
- Jimenez, M.; Miles, R.M. Inguinal endometriosis. Ann. Surg. 1960, 151, 903–911. [Google Scholar] [CrossRef]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Pandraklakis, A.; Rodolakis, A.; Thomakos, N. Endometriosis of the Canal of Nuck: A Systematic Review of the Literature. Diagnostics 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, M.; Pesci, A.; Scardapane, A.; Noventa, M.; Bonaccorsi, G.; Greco, P.; Zamboni, G. Dye diffusion during laparoscopic tubal patency tests may suggest a lymphatic contribution to dissemination in endometriosis: A prospective, observational study. PLoS ONE 2019, 14, e0226264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Zamora, J.; Vogel, J.P.; Souza, J.P.; Jayaratne, K.; Ganchimeg, T.; Ortiz-Panozo, E.; Hernandez, B.; Oladapo, O.T.; et al. Increases in caesarean delivery rates and change of perinatal outcomes in low- and middle-income countries: A hospital-level analysis of two WHO surveys. Paediatr. Perinat. Epidemiol. 2017, 31, 251–262. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Juang, C.-M.; Chao, H.-T.; Yu, K.-J.; Yuan, C.-C.; Ng, H.-T. Wound endometriosis: Risk factor evaluation and treatment. J. Chin. Med. Assoc. 2003, 66, 113–119. [Google Scholar] [PubMed]
- Oosterlynck, D.J.; Cornillie, F.J.; Waer, M.; Vandeputte, M.; Koninckx, P.R. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril. 1991, 56, 45–51. [Google Scholar] [CrossRef]
- Cocco, G.; Ricci, V.; Boccatonda, A.; Stellin, L.; de Filippis, G.; Soresi, M.; Schiavone, C. Sonographic demonstration of a spontaneous rectus sheath hematoma following a sneeze: A case report and review of the literature. J. Ultrasound 2020. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Ricci, V.; Cocco, N.; Boccatonda, A.; D’Ardes, D.; Basilico, R.; Schiavone, C. Sonography of abdominal wall vascular malformation: A case report and review of the literature. J. Ultrasound 2020, 23, 481–485. [Google Scholar] [CrossRef]
- Cocco, G.; Boccatonda, A.; D’Ardes, D.; Galletti, S.; Schiavone, C. Mantle cell lymphoma: From ultrasound examination to histological diagnosis. J. Ultrasound 2018, 21, 339–342. [Google Scholar] [CrossRef]
- Fàbregas, F.F.; Guimferrer, M.C.; Casas, F.T.; Caballero, S.B.; Xauradó, R.F. Malignant transformation of abdominal wall endometriosis with lymph node metastasis: Case report and review of literature. Gynecol. Oncol. Case Rep. 2014, 8, 10–13. [Google Scholar] [CrossRef][Green Version]
- Graur, F.; Mois, E.; Elisei, R.; Furcea, L.; Dragota, M.; Zaharie, T.; Al Hajjar, N. Malignant endometriosis of the abdominal wall. Ann. Ital. Chir. 2017, 6, S2239253X17026895. [Google Scholar]
- Ferrandina, G.; Palluzzi, E.; Fanfani, F.; Gentileschi, S.; Valentini, A.L.; Mattoli, M.V.; Pennacchia, I.; Scambia, G.; Zannoni, G. Endometriosis-associated clear cell carcinoma arising in caesarean section scar: A case report and review of the literature. World J. Surg. Oncol. 2016, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, X.; Zhan, J.; Ren, Y.; Wang, W. Potential role of strain elastography for detection of the extent of large-scar endometriosis. J. Ultrasound Med. 2013, 32, 1635–1642. [Google Scholar] [CrossRef]
- Viganò, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, M.; Orlandi, S.; Trivella, G.; Portuese, A.; Bettocchi, S.; Pontrelli, G.; Bocus, P.; Anna Virgilio, B. Sonographic Differential Diagnosis in Deep Infiltrating Endometriosis: The Bowel. BioMed Res. Int. 2019, 2019, 5958402. [Google Scholar] [CrossRef]
- Carsote, M.; Terzea, D.C.; Valea, A.; Gheorghisan-Galateanu, A.A. Abdominal wall endometriosis (a narrative review). Int. J. Med. Sci. 2020, 17, 536–542. [Google Scholar] [CrossRef]
- Lopez-Soto, A.; Sanchez-Zapata, M.I.; Martinez-Cendan, J.P.; Ortiz Reina, S.; Bernal Mañas, C.M.; Remezal Solano, M. Cutaneous endometriosis: Presentation of 33 cases and literature review. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 221, 58–63. [Google Scholar] [CrossRef]
- Bozkurt, M.; Çil, A.S.; Bozkurt, D.K. Intramuscular abdominal wall endometriosis treated by ultrasound-guided ethanol injection. Clin. Med. Res. 2014, 12, 160–165. [Google Scholar] [CrossRef]
- Xiao-Ying, Z.; Hua, D.; Jin-Juan, W.; Ying-Shu, G.; Jiu-Mei, C.; Hong, Y.; Chun-Yi, Z. Clinical analysis of high-intensity focussed ultrasound ablation for abdominal wall endometriosis: A 4-year experience at a specialty gynecological institution. Int. J. Hyperth. 2019, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Deng, Y.; Wei, Q.; Chen, J.; Zhao, C. Comparison of ultrasound-guided high-intensity focused ultrasound ablation and surgery for abdominal wall endometriosis. Int. J. Hyperth. 2018, 35, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, Y.-J.; Hong, G.-Y.; Nam, S.-K.; Kim, T.-E. Abdominal wall endometriosis treatment by ultrasound-guided high-intensity focused ultrasound ablation: A case report. Gynecol. Endocrinol. 2019, 35, 109–111. [Google Scholar] [CrossRef]
- Welch, B.T.; Ehman, E.C.; Van Buren, W.M.; Cope, A.G.; Welch, T.L.; A Woodrum, D.; Kurup, A.N.; Burnett, T.L. Percutaneous cryoablation of abdominal wall endometriosis: The Mayo Clinic approach. Abdom. Radiol. 2020, 45, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- Dibble, E.H.; D’Amico, K.C.; Bandera, C.A.; Littrup, P.J. Cryoablation of Abdominal Wall Endometriosis: A Minimally Invasive Treatment. AJR Am. J. Roentgenol. 2017, 209, 690–696. [Google Scholar] [CrossRef]
- Maillot, J.; Brun, J.L.; Dubuisson, V.; Bazot, M.; Grenier, N.; Cornelis, F.H. Mid-term outcomes after percutaneous cryoablation of symptomatic abdominal wall endometriosis: Comparison with surgery alone in a single institution. Eur. Radiol. 2017, 27, 4298–4306. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, G.; Delli Pizzi, A.; Scioscia, M.; Ricci, V.; Boccatonda, A.; Candeloro, M.; Tana, M.; Balconi, G.; Romano, M.; Schiavone, C. Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review. Diagnostics 2021, 11, 609. https://doi.org/10.3390/diagnostics11040609
Cocco G, Delli Pizzi A, Scioscia M, Ricci V, Boccatonda A, Candeloro M, Tana M, Balconi G, Romano M, Schiavone C. Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review. Diagnostics. 2021; 11(4):609. https://doi.org/10.3390/diagnostics11040609
Chicago/Turabian StyleCocco, Giulio, Andrea Delli Pizzi, Marco Scioscia, Vincenzo Ricci, Andrea Boccatonda, Matteo Candeloro, Marco Tana, Giuseppe Balconi, Marcello Romano, and Cosima Schiavone. 2021. "Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review" Diagnostics 11, no. 4: 609. https://doi.org/10.3390/diagnostics11040609
APA StyleCocco, G., Delli Pizzi, A., Scioscia, M., Ricci, V., Boccatonda, A., Candeloro, M., Tana, M., Balconi, G., Romano, M., & Schiavone, C. (2021). Ultrasound Imaging of Abdominal Wall Endometriosis: A Pictorial Review. Diagnostics, 11(4), 609. https://doi.org/10.3390/diagnostics11040609








