Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Sample Acquisition
2.3. Proteomic Analysis
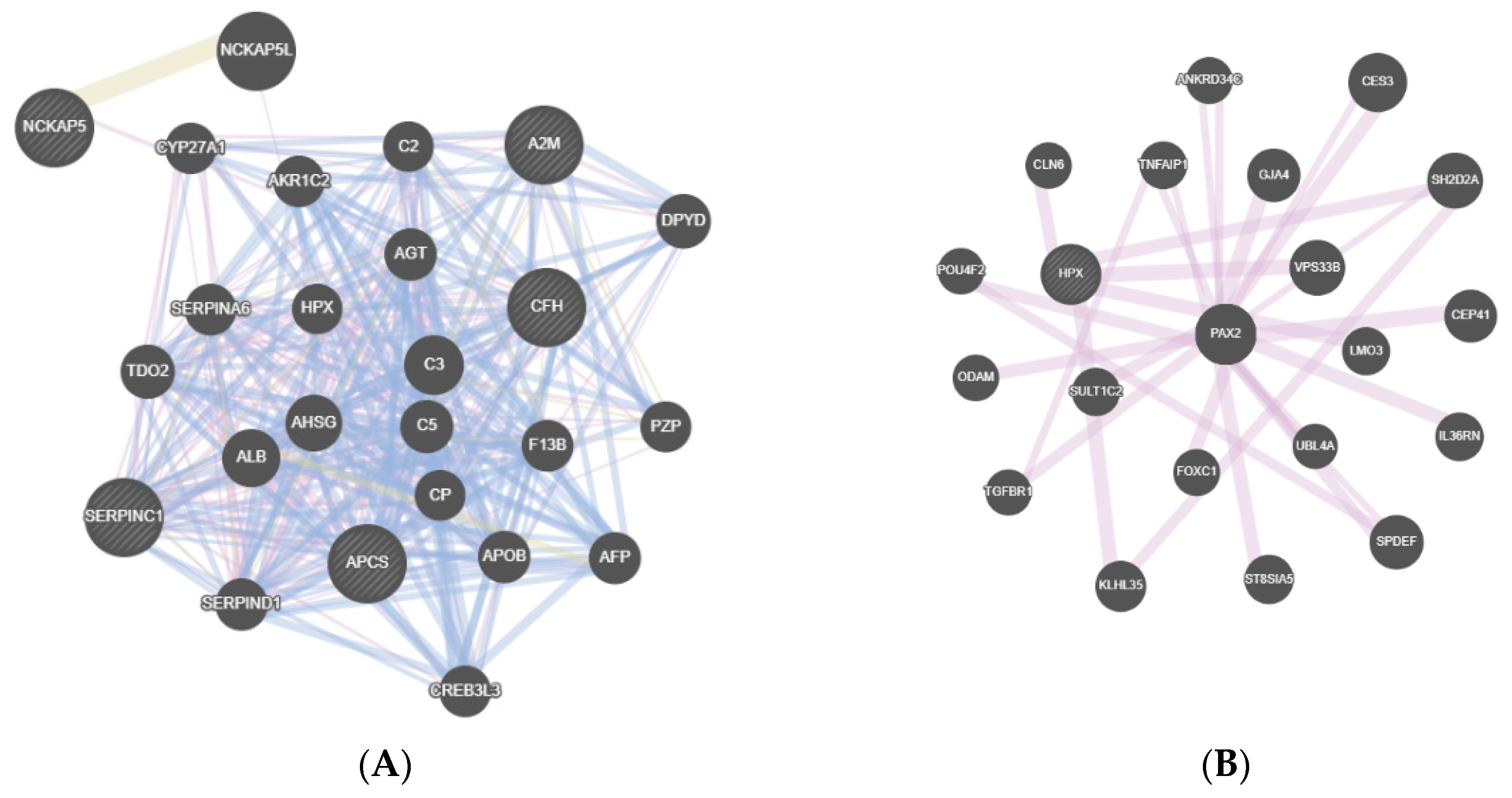
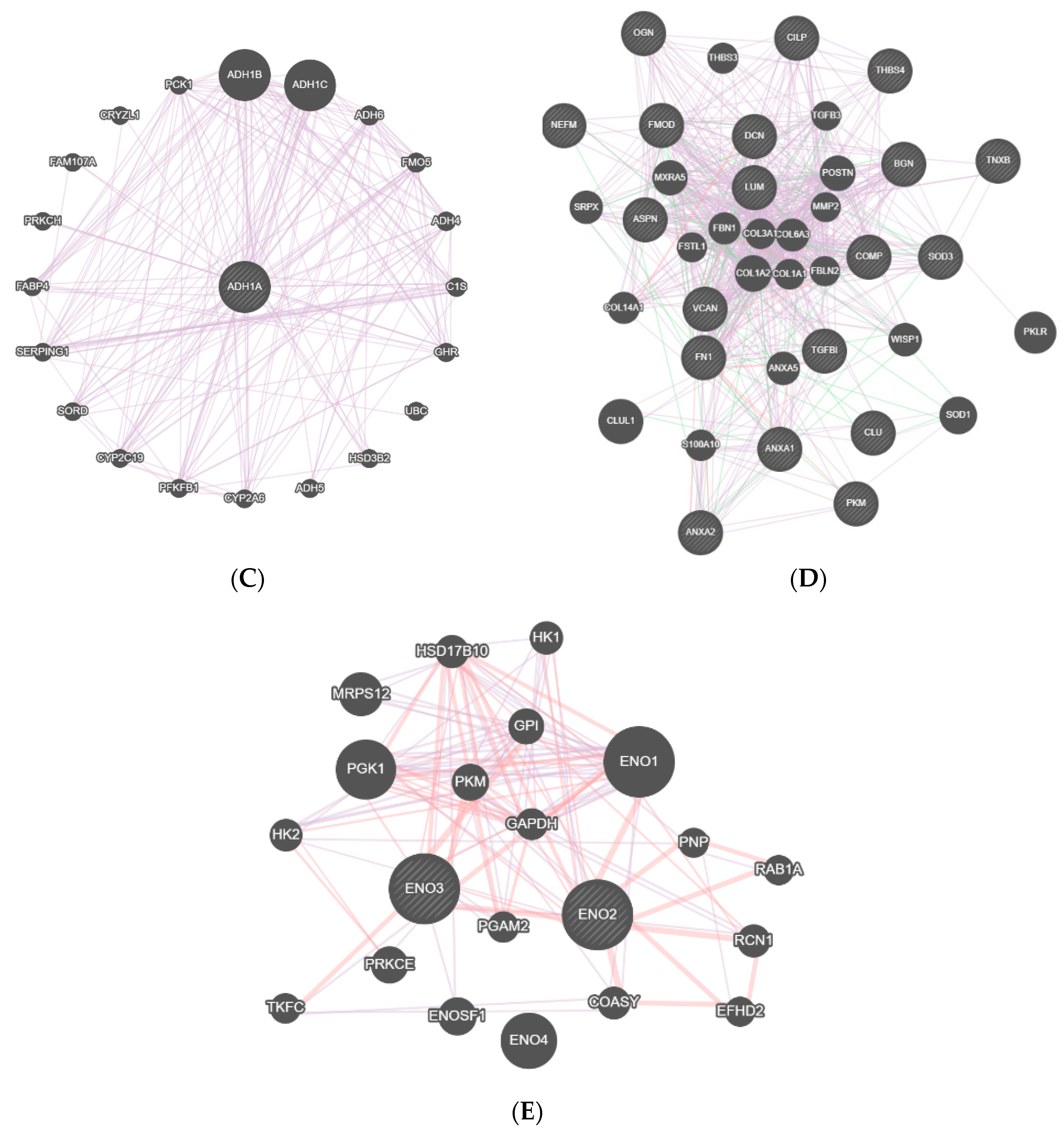
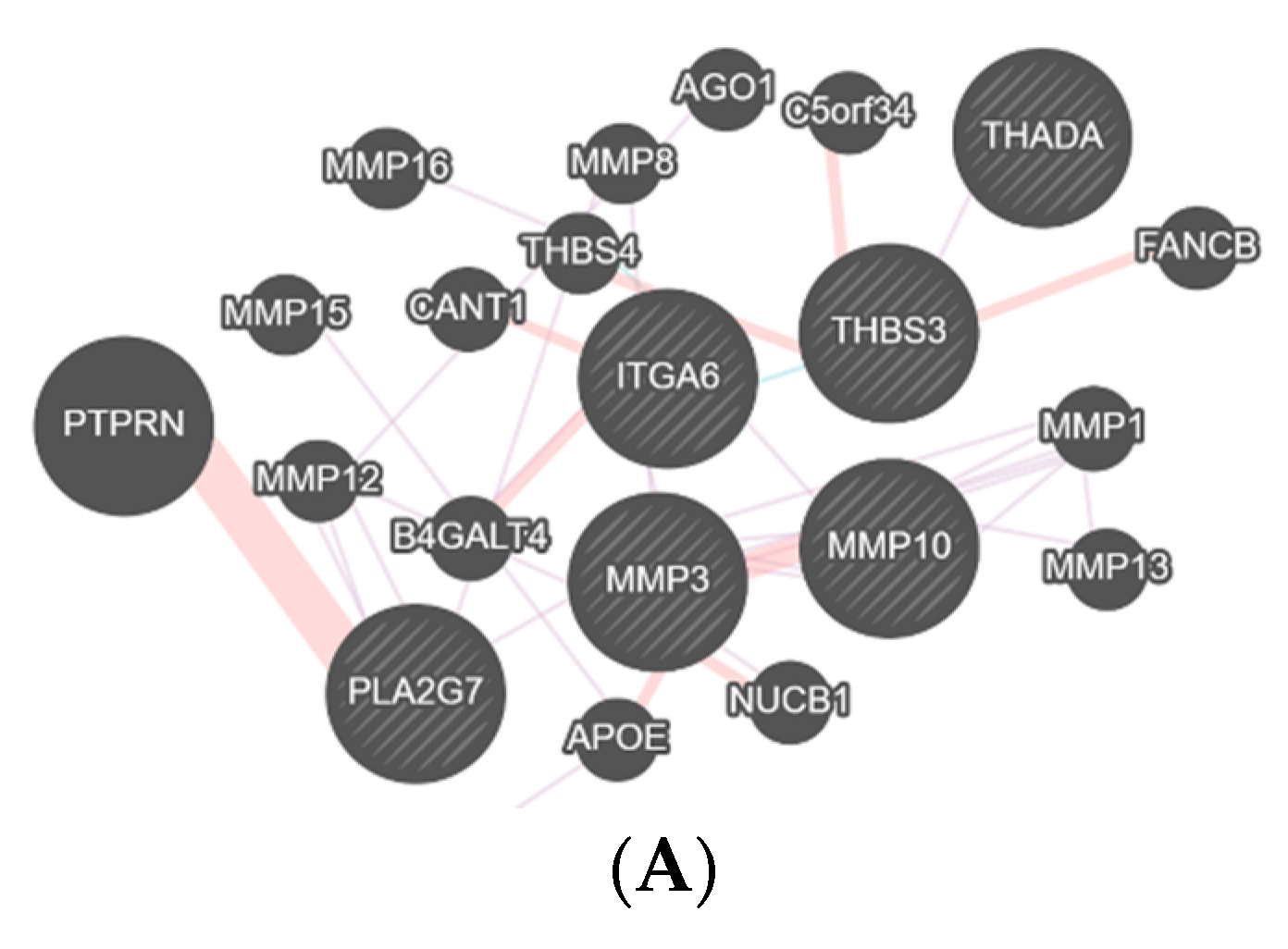
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slade, G.D.; Ohrbach, R.; Greenspan, J.D.; Fillingim, R.B.; Bair, E.; Sanders, A.E.; Dubner, R.; Diatchenko, L.; Meloto, C.B.; Smith, S.; et al. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res. 2016, 95, 1084–1092. [Google Scholar] [CrossRef]
- National Institute of Health. Available online: https://www.nidcr.nih.gov/health-info/tmj (accessed on 3 October 2019).
- Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef]
- Minervini, G.; Lucchese, A.; Perillo, L.; Serpico, R.; Minervini, G. Unilateral superior condylar neck fracture with disloca-tion in a child treated with an acrylic splint in the upper arch for functional repositioning of the mandible. Case Rep. Cranio 2017, 35, 337–341. [Google Scholar]
- Eberhard, D.; Bantleon, H.; Steger, W. The efficacy of anterior repositioning splint therapy studied by magnetic resonance imaging. Eur. J. Orthod. 2002, 24, 343–352. [Google Scholar] [CrossRef]
- Supplement, D.; Minervini, G.; Nucci, L.; Lanza, A.; Femiano, F.; Contaldo, M.; Grassia, V. Temporomandibular disc displacement with reduction treated with anterior repositioning splint: A 2-year clinical and magnetic resonance imaging (MRI) follow-up. Case Rep. J. Biol. Regul. Homeost. Agents 2020, 34, 151–160. [Google Scholar]
- Talaat, W.M.; Adel, O.I.; Al Bayatti, S. Prevalence of temporomandibular disorders discovered incidentally during routine dental examination using the Research Diagnostic Criteria for Temporomandibular Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Cakarer, S.; Isler, S.; Yalcin, B.; Şitilci, T. Management of the bilateral chronic temporomandibular joint dislocation. Ann. Maxillofac. Surg. 2018, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Poluha, R.L.; Canales, G.D.L.T.; Costa, Y.M.; Grossmann, E.; Bonjardim, L.R.; Conti, P.C.R. Temporomandibular joint disc displacement with reduction: A review of mechanisms and clinical presentation. J. Appl. Oral Sci. 2019, 27, e20180433. [Google Scholar] [CrossRef]
- Fayed, M.M.S.; El-Mangoury, N.H.; El-Bokle, D.N.; Belal, I.A. Occlusal splint therapy and magnetic resonance imaging. World J. Orthod. 2004, 5, 133–140. [Google Scholar]
- Prechel, U.; Ottl, P.; Ahlers, O.M.; Neff, A. The Treatment of Temporomandibular Joint Dislocation: A Systematic Review. Dtsch. Arztebl. Int. 2018, 115, 59–64. [Google Scholar]
- Nitzan, D.W.; Katsnelson, A.; Bermanis, I.; Brin, I.; Casap, N. The clinical characteristics of condylar hyperplasia: Expe-rience with 61 patients. J. Oral Maxillofac. Surg. 2008, 66, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, P.G.; Karssemakers, L.H.; Tuinzing, D.B. Female Predominance and Effect of Gender on Unilateral Condylar Hyperplasia: A Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2012, 70, 72–76. [Google Scholar] [CrossRef]
- Mahajan, M. Unilateral condylar hyperplasia—A genetic link? Case reports. Nat. J. Maxillofacial Surg. 2017, 8, 58–63. [Google Scholar] [CrossRef]
- Herr, M.M.; Fries, K.M.; Upton, L.G.; Edsberg, L.E. Potential Biomarkers of Temporomandibular Joint Disorders. J. Oral Maxillofac. Surg. 2011, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Demerjian, G.G.; Sims, A.B.; Stack, B.C. Proteomic signature of Temporomandibular Joint Disorders (TMD): Toward di-agnostically predictive biomarkers. Bioinformation 2011, 6, 282–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DuPree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implant. 2013, 28, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Olate, S.; Netto, H.D.; Rodriguez-Chessa, J.; Alister, J.P.; Albergaria-Barbosa, J.P.; de Moraes, J. Mandible condylar hy-perplasia: A review of diagnosis and treatment protocol. Int J. Clin. Exp. Med. 2013, 6, 727–737. [Google Scholar] [PubMed]
- Mehra, P.; Wolford, L.M. Serum nutrient deficiencies in the patient with complex temporomandibular joint problems. Bayl. Univ. Med. Cent. Proc. 2008, 21, 243–247. [Google Scholar] [CrossRef]
- Alstergren, P.; Benavente, C.; Kopp, S. Interleukin-1beta, interleukin-1 receptor antagonist, and interleukin-1 soluble receptor II in temporomandibular joint synovial fluid from patients with chronic polyarthritides. J. Oral Maxillofac. Surg. 2003, 61, 1171–1178. [Google Scholar] [CrossRef]
- Cassiano, L.P.; Ventura, T.M.; Silva, C.M.; Leite, A.L.; Magalhães, A.C.; Pessan, J.P.; Buzalaf, M.A.R. Protein Profile of the Acquired Enamel Pellicle after Rinsing with Whole Milk, Fat-Free Milk, and Water: An in vivo Study. Caries Res. 2018, 52, 288–296. [Google Scholar] [CrossRef]
- Universal Protein Resource. Available online: http://www.uniprot.org (accessed on 5 September 2020).
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, 214–220. [Google Scholar] [CrossRef]
- Fredriksson, L.; Alstergren, P.; Kopp, S. Tumor Necrosis Factor-α in Temporomandibular Joint Synovial Fluid Predicts Treatment Effects on Pain by Intra-Articular Glucocorticoid Treatment. Mediat. Inflamm. 2006, 2006, 59425. [Google Scholar] [CrossRef]
- Fujita, H.; Morisugi, T.; Tanaka, Y.; Kawakami, T.; Kirita, T.; Yoshimura, Y. MMP-3 activation is a hallmark indicating an early change in TMJ disorders, and is related to nitration. Int. J. Oral Maxillofac. Surg. 2009, 38, 70–78. [Google Scholar] [CrossRef]
- Tiilikainen, P.; Pirttiniemi, P.; Kainulainen, T.; Pernu, H.; Raustia, A. MMP-3 and -8 expression is found in the condylar surface of temporomandibular joints with internal derangement. J. Oral Pathol. Med. 2005, 34, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Filetti, V.; Almeida, L.E.; Rosa, G.R.; Leonardi, R.; Grippaudo, C.; Giudice, A. MMP-7 and MMP-9 are over-expressed in the synovial tissue from severe temporomandibular joint dysfunction. Eur. J. Histochem. 2020, 64, 3113. [Google Scholar] [CrossRef]
- Da Costa, G.F.A.; Souza, R.D.C.; de Araújo, G.M.; Gurgel, B.C.V.; Barbosa, G.A.S.; Calderon, P.D.S. Does TGF-beta play a role in degenerative temporomandibular joint diseases? A systematic review. Cranio 2017, 35, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Tudzarovski, N.; Kruger-Weiner, C.; Lund, B. Synovial Tissue Proteins and Patient-Specific Variables as Predictive Factors for Temporomandibular Joint Surgery. Diagnostics 2020, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Feizbakhsh, M.; Razavi, M.; Minaian, M.; Teimoori, F.; Dadgar, S.; Maghsoodi, S. The effect of local injection of the human growth hormone on the mandibular condyle growth in rabbit. Dent. Res. J. 2014, 11, 436–441. [Google Scholar]
- Koyama, E.; Saunders, C.; Salhab, I.; Decker, R.; Chen, I.; Um, H.; Pacifici, M.; Nah, H. Lubricin is Required for the Structural Integrity and Post-natal Maintenance of TMJ. J. Dent. Res. 2014, 93, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Stocum, D.L.; Roberts, W.E. Part I: Development and Physiology of the Temporomandibular Joint. Curr. Osteoporos. Rep. 2018, 16, 360–368. [Google Scholar] [CrossRef]
- Berkovitz, B.K.B.; Holland, G.R.; Moxham, B.J. Oral Anatomy, Histology and Embryology, 4th ed.; Mosby: St. Louis, MD, USA, 2009. [Google Scholar]
- Gage, J.; Virdi, A.; Triffitt, J.; Howlett, C.; Francis, M. Presence of type III collagen in disc attachments of human temporomandibular joints. Arch. Oral Biol. 1990, 35, 283–288. [Google Scholar] [CrossRef]
- De Moraes, L.O.; Lodi, F.R.; Gomes, T.S.; Marques, S.R.; Oshima, C.T.; Lancellotti, C.L.; Rodríguez-Vázquez, J.F.; Mé-rida-Velasco, J.R.; Alonso, L.G. Immunohistochemical expression of types I and III collagen antibodies in the tem-poromandibular joint disc of human foetuses. Eur. J. Histochem. 2011, 55, 24. [Google Scholar] [CrossRef]
- Kondoh, T.; Hamada, Y.; Iino, M.; Takahashi, T.; Kikuchi, T.; Fujikawa, K.; Seto, K. Regional differences of type II collagen synthesis in the human temporomandibular joint disc: Immunolocalization study of carboxy-terminal type II procollagen peptide (chondrocalcin). Arch. Oral Biol. 2003, 48, 621–625. [Google Scholar] [CrossRef]
- De Moraes, L.O.; Lodi, F.R.; Gomes, T.S.; Marques, S.R.; Fernandes Junior, J.A.; Oshima, C.T.; Alonso, L.G. Immuno-histochemical expression of collagen type IV antibody in the articular disc of the temporomandibular joint of human fe-tuses. Ital. J. Anat. Embryol. 2008, 113, 91–95. [Google Scholar] [PubMed]
- Chu, W.C.; Zhang, S.; Sng, T.J.; Ong, Y.J.; Tan, W.-L.; Ang, V.Y.; Foldager, C.B.; Toh, W.S. Distribution of pericellular matrix molecules in the temporomandibular joint and their chondroprotective effects against inflammation. Int. J. Oral Sci. 2017, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.H.; Xu, J.; Cai, H.X.; Fang, W.; Long, X. Effect of temporomandibular joint disc perforation on expression of type ? collagen in temporomandibular joint disc cells. Chin. J. Stomatol. 2017, 52, 274–277. [Google Scholar]
- Ciavarella, D.; Mastrovincenzo, M.; Sabatucci, A.; Campisi, G.; Di Cosola, M.; Suriano, M.; Muzio, L.L. Primary and secondary prevention procedures of temporo-mandibular joint disease in the evolutive age. Minerva Pediatr. 2009, 61, 93–97. [Google Scholar]

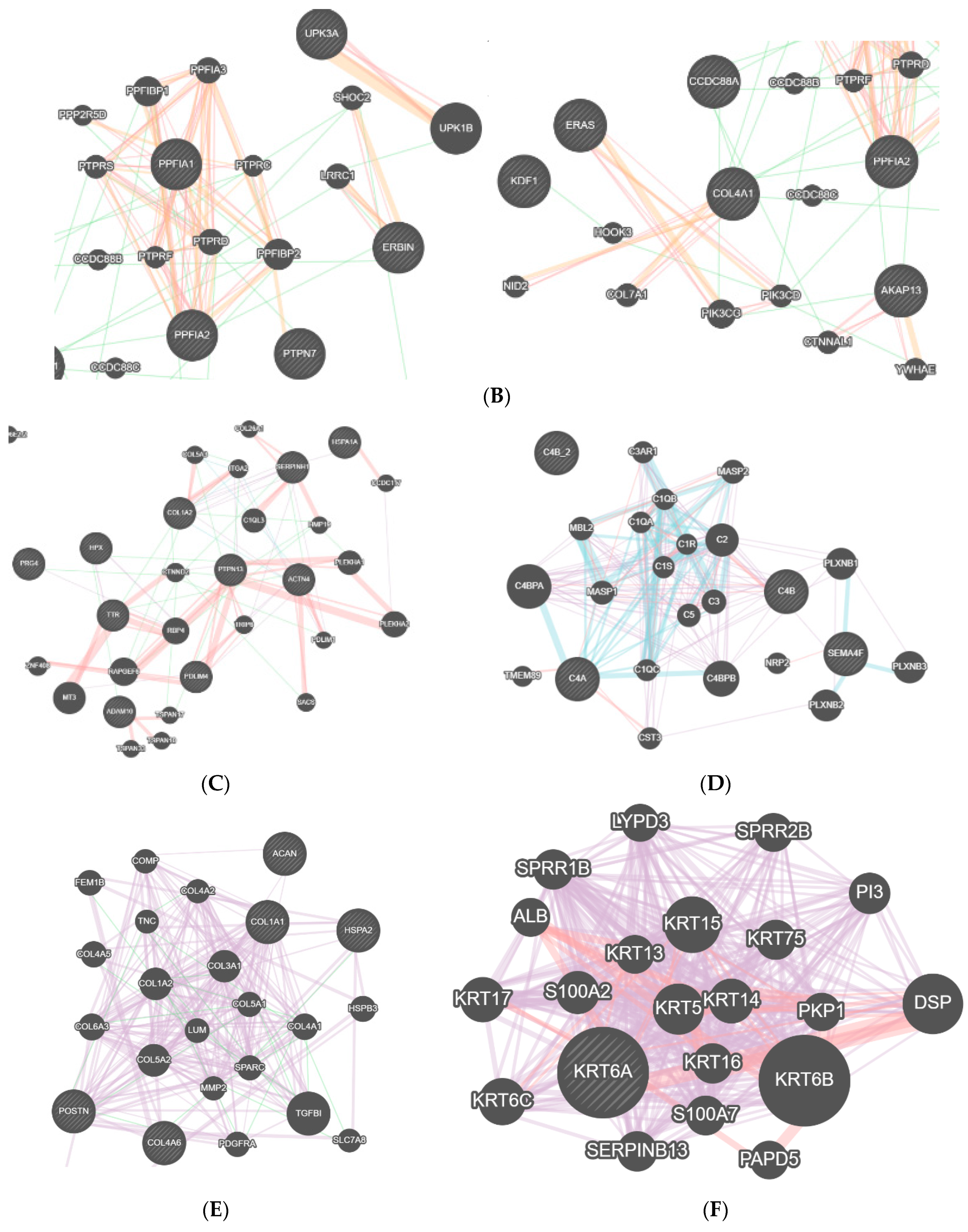
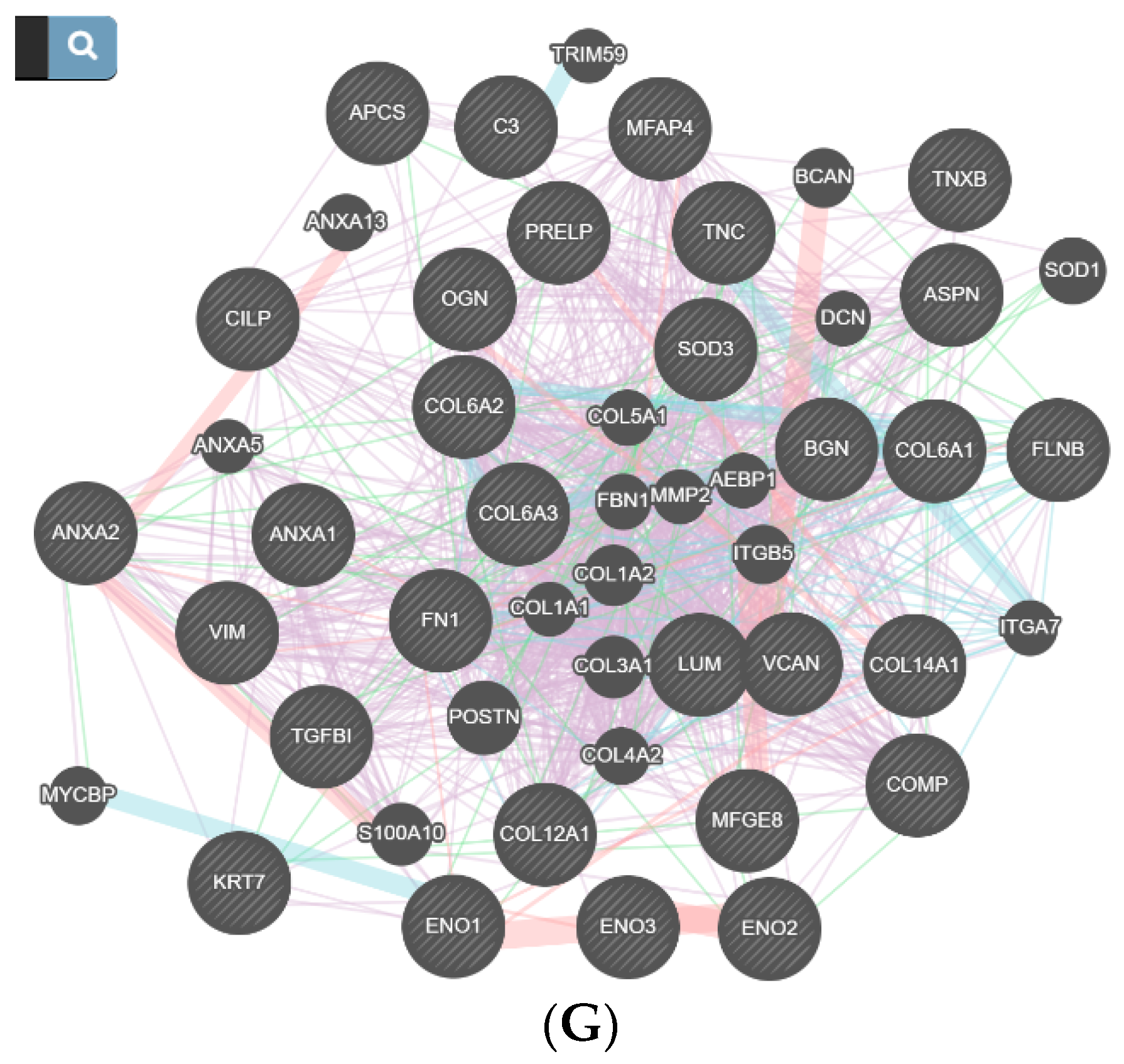
| Number | Age | Diagnostic |
|---|---|---|
| 1 | 18 | Condylar Hyperplasia |
| 2 | 20 | Condylar Hyperplasia |
| 3 | 38 | Mandibular Dislocation |
| 4 | 38 | Mandibular Dislocation |
| 5 | 36 | Condylar Hyperplasia |
| 6 | 29 | Condylar Hyperplasia |
| 7 | 25 | Disc Displacement Without Reduction |
| 8 | 25 | Disc Displacement Without Reduction |
| 9 | 52 | Disc Displacement Without Reduction |
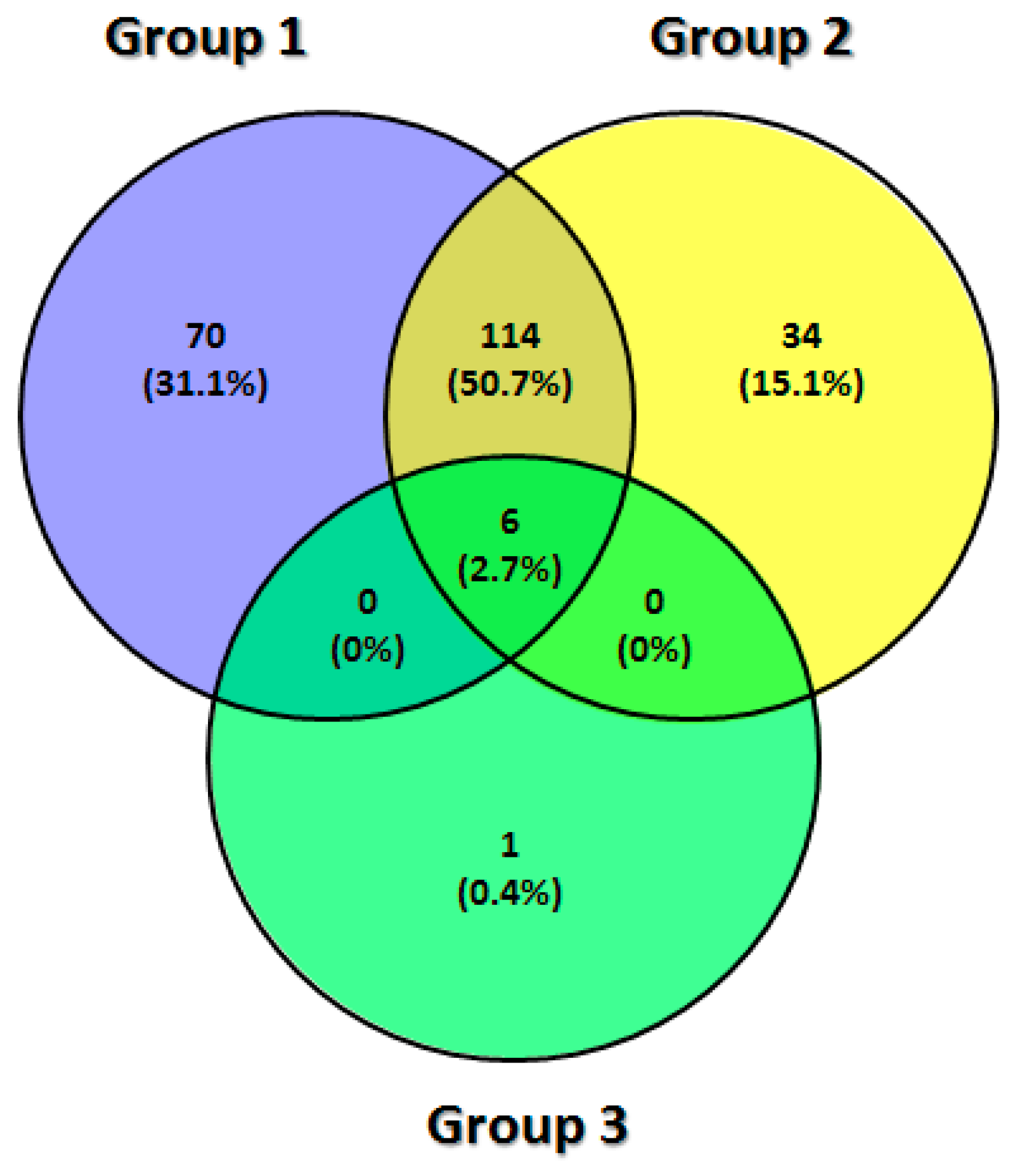
| Protein Expressed in Each Group of TMJ Synovial Fluid Sample (n = 225) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DDWoR (n = 70) | MD (n = 34) | CH (n = 1) | DDWoR and MD (n = 114) | DDWoR and CH (n = 0) | MD and CH (n = 0) | DDWoR, MD and CH (n = 6) | |||||
| Code | Name | Code | Name | Code | Name | Code | Name | X | X | Code | Name |
| A2M | Alpha-2-Macroglobulin | ACTR3B | Actin Related Protein 3B | ADH1 | Alcohol Dehydrogenase Subunit Alpha | ABI3BP | ABI Family Member 3 Binding Protein | ENO1 | Enolase 1 | ||
| ANXA5 | Annexin A5 | ACTR3C | Actin Related Protein 3C | ACTA1 | Actin Alpha 1, Skeletal Muscle | ENO2 | Enolase 2 | ||||
| APCS | Amyloid P Component | AKNA | AT-Hook Transcription Factor | ACTA2 | Actin Alpha 2, Smooth Muscle | ENO3 | Enolase 3 | ||||
| APOH | Apolipoprotein H | ALDH1L1 | Aldehyde Dehydrogenase 1 Family Member L1 | ACTB | Actin Beta | MYH16 | Myosin Heavy Chain 16 Pseudogene | ||||
| ARHGAP21 | Rho GTPase Activating Protein 21 | C4A | Complement C4A (Rodgers Blood Group) | ACTBL2 | Actin Beta Like 2 | RPL7L1 | Ribosomal Protein L7 Like 1 | ||||
| CFH | Complement Factor H | C4B_2 | Complement Component 4B | ACTC1 | Actin Alpha Cardiac Muscle 1 | SHLD3 | Shieldin Complex Subunit 3 | ||||
| CHD8 | Chromodomain Helicase DNA Binding Protein 8 | C7orf57 | Complement C7 | ACTG1 | Actin Gamma 1 | ||||||
| CILP2 | Cartilage Intermediate Layer Protein | CAGE1 | Cancer Antigen 1 | ACTG2 | Actin Gamma 2, Smooth Muscle | ||||||
| CNOT6L | CCR4-NOT Transcription Complex Subunit 6 Like | CPSF2 | Cleavage And Polyadenylation Specific Factor 2 | ALB | Albumin | ||||||
| DAGLA | Diacylglycerol Lipase Alpha | DCAF4L2 | DDB1 And CUL4 Associated Factor 4 Like 2 | ANXA1 | Annexin A1 | ||||||
| DPYSL2 | Dihydropyrimidinase Like 2 | DHRS11 | Dehydrogenase/Reductase 11 | ANXA2 | Annexin A2 | ||||||
| DPYSL3 | Dihydropyrimidinase Like 3 | DMD | Dystrophin | ANXA2P2 | Annexin A2 Pseudogene 2 | ||||||
| DYM | Dymeclin | FLNA | Filamin A | APOA1 | Apolipoprotein A1 | ||||||
| DYNC1H1 | Dynein Cytoplasmic 1 Heavy Chain | HPR | Haptoglobin-Related Protein | ASPN | Asporin | ||||||
| ENPP3 | Ectonucleotide Pyrophosphatase/Phosphodiesterase 3 | HPX | Hemopexin | ATP5F1B | ATP Synthase F1 Subunit Beta | ||||||
| FGFR2 | Fibroblast Growth Factor Receptor 2 | IFT122 | Intraflagellar Transport 122 | BGN | Biglycan | ||||||
| GPSM2 | G Protein Signaling Modulator 2 | LMO7 | LIM Domain 7 | C3 | Complement C3 | ||||||
| GPX3 | Glutathione Peroxidase 3 | MYO6 | Myosin VI | CILP | Cartilage Intermediate Layer Protein | ||||||
| GSTP1 | Glutathione S-Transferase Pi 1 | PDIA3 | Protein Disulfide Isomerase Family A Member 3 | CLU | Clusterin | ||||||
| H2BC1 | H2B Clustered Histone 1 | PPFIA1 | PTPRF Interacting Protein Alpha 1 | COL12A1 | Collagen Type XII Alpha 1 Chain | ||||||
| H2BE1 | H2B.E Variant Histone 1 | PPFIA2 | PTPRF Interacting Protein Alpha 2 | COL14A1 | Collagen Type XIV Alpha 1 Chain | ||||||
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | PRDX1 | Peroxiredoxin 1 | COL1A1 | Collagen Type I Alpha 1 Chain | ||||||
| HSPA1B | Heat Shock Protein Family A (Hsp70) Member 1B | PRDX2 | Peroxiredoxin 2 | COL6A1 | Collagen Type VI Alpha 1 Chain | ||||||
| HSPA1L | Heat Shock Protein Family A (Hsp70) Member 1 Like | RGMB | Repulsive Guidance Molecule BMP Co-Receptor B | COL6A2 | Collagen Type VI Alpha 2 Chain | ||||||
| HSPA2 | Heat Shock Protein Family A (Hsp70) Member 2 | SACM1L | SAC1 Like Phosphatidylinositide Phosphatase | COL6A3 | Collagen Type VI Alpha 3 Chain | ||||||
| HSPA8 | Heat Shock Protein Family A (Hsp70) Member 8 | SERPINA9 | Serpin Family A Member 9 | COMP | Thrombospondin-5 | ||||||
| IGLC1 | Immunoglobulin Lambda Constant 1 | SERPINH1 | Serpin Family H Member 1 | DCN | Decorin | ||||||
| IGLC2 | Immunoglobulin Lambda Constant | SLC4A1 | Solute Carrier Family 4 Member 1 | DES | Desmin | ||||||
| IGLC3 | Immunoglobulin Lambda Constant 3 | SMPD3 | Sphingomyelin Phosphodiesterase 3 | DPT | Dermatopontin | ||||||
| Immunoglobulin Lambda Constant 6 | Teneurin Transmembrane Protein 4 | Fibrillin 1 | |||||||||
| IGLC6 | Immunoglobulin Lambda Constant 7 | TENM4 | Transmembrane O-Mannosyltransferase Targeting Cadherins 3 | FBN1 | Fibrinogen Alpha Chain | ||||||
| IGLC7 | Immunoglobulin Lambda Like Polypeptide 1 | TMTC3 | Testis Specific 10 | FGA | Fibrinogen Beta Chain | ||||||
| IGLL1 | Immunoglobulin Lambda Like Polypeptide 5 | TSGA10 | Transthyretin | FGB | Fibrinogen Gamma Chain | ||||||
| IGLL5 | Interferon Regulatory Factor 7 | TTR | Ubiquitin Specific Peptidase 10 | FGG | Fibromodulin | ||||||
| IRF7 | Kalirin RhoGEF Kinase | USP10 | Actin Related Protein 3B | FMOD | Fibronectin 1 | ||||||
| KALRN | Kelch Repeat And BTB Domain Containing 11 | FN1 | Glyceraldehyde-3-Phosphate Dehydrogenase | ||||||||
| KBTBD11 | Keratocan | GAPDH | Gelsolin | ||||||||
| KERA | Keratin 18 | GSN | H2B Clustered Histone 11 | ||||||||
| KRT18 | Keratin 7 | H2BC11 | H2B Clustered Histone 12 | ||||||||
| KRT7 | Keratin 8 | H2BC12 | H2B Clustered Histone 13 | ||||||||
| KRT8 | Keratin 84 | H2BC13 | H2B Clustered Histone 14 | ||||||||
| KRT84 | Putative Uncharacterized Protein | H2BC14 | H2B Clustered Histone 15 | ||||||||
| LOC400499 | Leucine Rich Repeat Containing 9 | H2BC15 | H2B Clustered Histone 17 | ||||||||
| LRRC9 | Mitogen-Activated Protein Kinase Kinase Kinase 7 | H2BC17 | H2B Clustered Histone 18 | ||||||||
| MAP3K7 | Microfibril Associated Protein 5 | H2BC18 | H2B Clustered Histone 21 | ||||||||
| MFAP5 | Myosin Light Chain 6B | H2BC21 | H2B Clustered Histone 3 | ||||||||
| MYL6B | NCK Associated Protein 5 | H2BC3 | H2B Clustered Histone 5 | ||||||||
| NCKAP5 | Nik Related Kinase | H2BC5 | H2B Clustered Histone 9 | ||||||||
| NRK | Pericentriolar Material 1 | H2BC9 | H2B.S Histone 1 | ||||||||
| PCM1 | Procollagen C-Endopeptidase Enhancer | H2BS1 | H2B.U Histone 1 | ||||||||
| PCOLCE | RAD54 Like | H2BU1 | Hemoglobin Subunit Alpha 1 | ||||||||
| RAD54L | Retinol Dehydrogenase 5 | HBA1 | Hemoglobin Subunit Alpha 2 | ||||||||
| RDH5 | Ret Proto-Oncogene | HBA2 | Hemoglobin Subunit Beta | ||||||||
| RET | Regulatory Factor X1 | HBB | Hemoglobin Subunit Delta | ||||||||
| RFX1 | RPTOR Independent Companion Of MTOR Complex 2 | HBD | Hemoglobin Subunit Epsilon 1 | ||||||||
| RICTOR | RIMS Binding Protein 3 | HBE1 | Hemoglobin Subunit Gamma 1 | ||||||||
| RIMBP3 | RUN And FYVE Domain Containing 2 | HBG1 | Hemoglobin Subunit Gamma 2 | ||||||||
| RUFY2 | Serpin Family C Member 1 | HBG2 | Haptoglobin | ||||||||
| SERPINC1 | Serpin Family F Member 1 | HP | Heat Shock Protein Family B (Small) Member 1 | ||||||||
| SERPINF1 | SEC14 And Spectrin Domain Containing 1 | HSPB1 | Immunoglobulin Heavy Constant Alpha 1 | ||||||||
| SESTD1 | Small Nuclear Ribonucleoprotein U5 Subunit 200 | IGHA1 | Immunoglobulin Heavy Constant Alpha 2 (A2m Marker) | ||||||||
| SNRNP200 | SVOP Like | IGHA2 | Immunoglobulin Heavy Constant Gamma 1 (G1m Marker | ||||||||
| SVOPL | Transcription Elongation Factor, Mitochondrial | IGHG1 | Immunoglobulin Heavy Constant Gamma 2 | ||||||||
| TEFM | Thrombospondin 3 | IGHG2 | Immunoglobulin Heavy Constant Gamma 3 | ||||||||
| THBS3 | Tenascin C | IGHG3 | Immunoglobulin Heavy Constant Gamma 4 | ||||||||
| TNC | Trio Rho Guanine Nucleotide Exchange Factor | IGHG4 | Immunoglobulin Kappa Constant | ||||||||
| TRIO | Tubulin Beta 1 Class VI | IGKC | Internexin Neuronal Intermediate Filament Protein Alpha | ||||||||
| TUBB1 | Ubiquitin Specific Peptidase 42 | INA | Galectin 1 | ||||||||
| USP42 | WW Domain Binding Protein 1 Like | LGALS1 | Lamin | ||||||||
| WBP1L | Zinc Finger ZZ-Type And EF-Hand Domain Containing 1 | LMNA | Lumican | ||||||||
| ZZEF1 | H2B Clustered Histone 1 | LUM | Microfibril Associated Protein 4 | ||||||||
| MFAP4 | Myosin Light Chain 6 | ||||||||||
| MYL6 | Myocilin | ||||||||||
| MYOC | Neurofilament Heavy | ||||||||||
| NEFH | Neurofilament Light | ||||||||||
| NEFL | Neurofilament Medium | ||||||||||
| NEFM | Osteoglycin | ||||||||||
| OGN | Pellino E3 Ubiquitin Protein Ligase Family Member 3 | ||||||||||
| PELI3 | Pyruvate Kinase M1/2 | ||||||||||
| PKM | POTE Ankyrin Domain Family Member E | ||||||||||
| POTEE | POTE Ankyrin Domain Family Member F | ||||||||||
| POTEF | POTE Ankyrin Domain Family Member I | ||||||||||
| POTEI | POTE Ankyrin Domain Family Member J | ||||||||||
| POTEJ | POTE Ankyrin Domain Family Member K, Pseudogene | ||||||||||
| POTEKP | Peptidylprolyl Isomerase A | ||||||||||
| PPIA | Proline And Arginine Rich End Leucine Rich Repeat Protein | ||||||||||
| PRELP | Peripherin | ||||||||||
| PRPH | S100 Calcium Binding Protein A10 | ||||||||||
| S100A10 | Serpin Family A Member 1 | ||||||||||
| SERPINA1 | Superoxide Dismutase 3 | ||||||||||
| SOD3 | Transferrin | ||||||||||
| TF | Transforming Growth Factor Beta Induced | ||||||||||
| TGFBI | Thrombospondin 4 | ||||||||||
| THBS4 | Tenascin XA | ||||||||||
| TNXA | Tenascin XB | ||||||||||
| TNXB | Tubulin Alpha 1a | ||||||||||
| TUBA1A | Tubulin Alpha 1b | ||||||||||
| TUBA1B | Tubulin Alpha 1c | ||||||||||
| TUBA1C | Tubulin Alpha 3c | ||||||||||
| TUBA3C | Tubulin Alpha 3d | ||||||||||
| TUBA3D | Tubulin Alpha 3e | ||||||||||
| TUBA3E | Tubulin Alpha 4a | ||||||||||
| TUBA4A | Tubulin Alpha 8 | ||||||||||
| TUBA8 | Tubulin Beta Class I | ||||||||||
| TUBB | Tubulin Beta 2A Class IIa | ||||||||||
| TUBB2A | Tubulin Beta 2B Class IIb | ||||||||||
| TUBB2B | Tubulin Beta 3 Class III | ||||||||||
| TUBB3 | Tubulin Beta 4A Class IVa | ||||||||||
| TUBB4A | Tubulin Beta 4B Class IVb | ||||||||||
| TUBB4B | Tubulin Beta 6 Class V | ||||||||||
| TUBB6 | Tubulin Beta 8 Class VIII | ||||||||||
| TUBB8 | Tubulin Beta 8B | ||||||||||
| TUBB8B | Versican | ||||||||||
| VCAN | VIM | ||||||||||
| VIM | ABI Family Member 3 Binding Protein | ||||||||||
| Protein Expressed in Each Group of TMJ Disc Sample (n = 379) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDWoR (n= 66) | MD (n = 38) | CH (n = 89) | DDWoR and MD (n = 9) | DDWoR and CH (n = 28) | MD and CH (n = 17) | DDWoR, MD and CH (n = 132) | |||||||
| Code | Name | Code | Name | Code | Name | Code | Name | Code | Name | Code | Name | Code | Name |
| ABCC9 | ATP Binding Cassette Subfamily C Member 9 | AFTPH | Aftiphilin | ACTN1 | Actinin Alpha 1 | ATP7B | ATPase Copper Transporting Beta | ACAN | Aggrecan | ATP5F1B | ATP Synthase F1 Subunit Beta | A2M | Alpha-2-Macroglobulin |
| ACSS3 | Acyl-CoA Synthetase Short Chain Family Member 3 | AKAP13 | A-kinase anchor protein 13 | ACTN4 | Actinin Alpha 4 | AXIN2 | Axin 2 | APOH | Apolipoprotein H | GFAP | Glial Fibrillary Acidic Protein | ABI3BP | ABI Family Member 3 Binding Protein |
| AGO4 | Argonaute RISC Component 4 | ALDH3A2 | Aldehyde dehydrogenase family 3 member A2 | ACTR3 | Actin Related Protein 3 | C4A | Complement C4A | BRD3 | Bromodomain Containing 3 | KRT3 | Keratin 3 | ACTA1 | Actin Alpha 1, Skeletal Muscle |
| AMBP | Alpha-1-Microglobulin/Bikunin Precursor | ANKRD44 | Serine/threonine-protein phosphatase 6 regulatory ankyrin repeat subunit B | ADAM10 | ADAM Metallopeptidase Domain 10 | C4B | Complement C4B | CLTC | Clathrin Heavy Chain | KRT5 | Keratin 5 | ACTA2 | Actin Alpha 2, Smooth Muscle |
| ANKRD17 | Ankyrin Repeat Domain 17 | ANKRD52 | Serine/threonine-protein phosphatase 6 regulatory ankyrin repeat subunit C | ADSL | Adenylosuccinate Lyase | C4B_2 | Complement Component 4B | COL1A1 | Collagen Type I Alpha 1 Chain | KRT6A | Keratin 6A | ACTB | Actin Beta |
| ARHGAP35 | Rho GTPase Activating Protein 35 | ARMH3 | Armadillo-like helical domain-containing protein 3 | ALDOA | Aldolase, Fructose-Bisphosphate A | KERA | Keratocan | COL4A6 | Collagen Type IV Alpha 6 Chain | KRT6B | Keratin 6B | ACTBL2 | Actin Beta Like 2 |
| ARHGEF10 | Rho Guanine Nucleotide Exchange Factor 10 | CCDC88A | Girdin | ALDOC | Aldolase, Fructose-Bisphosphate C | KIAA0556 | Katanin Interacting Protein | DNAH8 | Defensin Alpha 1 | KRT6C | Keratin 6C | ACTC1 | Actin Alpha Cardiac Muscle 1 |
| ATAD2B | ATPase Family AAA Domain Containing 2B | CLUH | Clustered mitochondria protein homolog | ANKMY1 | Ankyrin Repeat And MYND Domain Containing 1 | MAP4 | Microtubule Associated Protein 4 | EEF1A1 | Dynein Axonemal Heavy Chain 8 | KRT75 | Keratin 75 | ACTG1 | Actin Gamma 1 |
| BCAS2 | BCAS2 Pre-MRNA Processing Factor | COL4A1 | Collagen alpha-1(IV) chain | ANXA5 | Annexin A5 | SEMA4F | Semaphorin 4F | EEF1A1P5 | Eukaryotic Translation Elongation Factor 1 Alpha 1 | KRT76 | Keratin 76 | ACTG2 | Actin Gamma 2 |
| CARNS1 | Carnosine Synthase 1 | DOCK10 | Dedicator of cytokinesis protein 10 | ANXA6 | Annexin A6 | EEF1A2 | Eukaryotic Translation Elongation Factor 1 Alpha 1 Pseudogene 5 | KRT78 | Keratin 78 | ALB | Albumin | ||
| CCDC187 | Coiled-Coil Domain Containing 187 | DTHD1 | Death domain-containing protein 1 | ASXL1 | ASXL Transcriptional Regulator 1 | HMCN2 | Eukaryotic Translation Elongation Factor 1 Alpha 2 | KRT79 | Keratin 79 | ANXA1 | Annexin A1 | ||
| CDCP1 | CUB Domain Containing Protein 1 | ERAS | GTPase ERas | ATP2C1 | ATPase Secretory Pathway Ca2+ Transporting 1 | HSPA2 | Hemicentin 2 | KRT81 | Keratin 81 | ANXA2 | Annexin A2 | ||
| CDH3 | Cadherin 3 | ERBIN | Erbin | BLOC1S1 | Biogenesis Of Lysosomal Organelles Complex 1 Subunit 1 | HSPA8 | Heat Shock Protein Family A (Hsp70) Member 2 | KRT83 | Keratin 83 | ANXA2P2 | Annexin A2 Pseudogene 2 | ||
| CHD7 | Chromodomain Helicase DNA Binding Protein 7 | FLNA | Filamin-A | BRCA2 | BRCA2 DNA Repair Associated | HYDIN | Heat Shock Protein Family A (Hsp70) Member 8 | KRT85 | Keratin 85 | APCS | Amyloid P Component | ||
| CHD8 | Chromodomain Helicase DNA Binding Protein 8 | GOT1L1 | Putative aspartate aminotransferase, cytoplasmic 2 | CABP5 | Calcium Binding Protein 5 | IGLC1 | HYDIN Axonemal Central Pair Apparatus Protein | KRT86 | Keratin 86 | APOA1 | Apolipoprotein A1 | ||
| CHD9 | Chromodomain Helicase DNA Binding Protein 9 | HHLA1 | HERV-H LTR-associating protein 1 | CACNA2D3 | Calcium Voltage-Gated Channel Auxiliary Subunit Alpha2delta 3 | IGLC2 | Immunoglobulin Lambda Constant 1 | PKM | Pyruvate Kinase M1/2 | ASPN | Asporin | ||
| CSTF2T | Cleavage Stimulation Factor Subunit 2 Tau Variant | IGHV3OR16–9 | Immunoglobulin heavy variable 3/OR16–9 (non-functional) | CCDC18 | Coiled-Coil Domain Containing 18 | IGLC3 | Immunoglobulin Lambda Constant 2 | TTBK2 | Tau Tubulin Kinase 2 | BGN | Biglycan | ||
| ECH1 | Enoyl-CoA Hydratase 1 | KDF1 | Keratinocyte differentiation factor 1 | CDC20 | Cell Division Cycle 20 | IGLC6 | Immunoglobulin Lambda Constant 3 | C3 | Complement C3 | ||||
| ELAVL3 | ELAV Like RNA Binding Protein 3 | L1CAM | Neural cell adhesion molecule L1 | CENPF | Centromere Protein F | IGLC7 | Immunoglobulin Lambda Constant 6 | CILP | Cartilage Intermediate Layer Protein | ||||
| EML4 | EMAP Like 4 | MARK1 | Serine/threonine-protein kinase MARK1 | CFAP20DC | CFAP20 Domain Containing | IGLL1 | Immunoglobulin Lambda Constant 7 | CILP2 | Cartilage Intermediate Layer Protein 2 | ||||
| FARP2 | FERM, ARH/RhoGEF And Pleckstrin Domain Protein 2 | NEIL3 | Endonuclease 8-like 3 | CNTN1 | Contactin 1 | IGLL5 | Immunoglobulin Lambda Like Polypeptide 1 | CLU | Clusterin | ||||
| FBN1 | Fibrillin 1 | NOL8 | Nucleolar protein 8 | COQ8B | Coenzyme Q8B | LOC441081 | Immunoglobulin Lambda Like Polypeptide 5 | COL12A1 | Collagen Type XII Alpha 1 Chain | ||||
| GALK2 | Galactokinase 2 | NUFIP1 | Nuclear fragile X mental retardation-interacting protein 1 | CTNNA3 | Catenin Alpha 3 | MIS18BP1 | POM121 Membrane Glycoprotein (Rat) Pseudogene | COL14A1 | Collagen Type XIV Alpha 1 Chain | ||||
| GPR162 | G Protein-Coupled Receptor 162 | NUMA1 | Nuclear mitotic apparatus protein 1 | DPYSL2 | Dihydropyrimidinase Like 2 | MYO15B | MIS18 Binding Protein 1 | COL6A1 | Collagen Type VI Alpha 1 Chain | ||||
| GPRASP1 | G Protein-Coupled Receptor Associated Sorting Protein 1 | PARP10 | Protein mono-ADP-ribosyltransferase PARP10 | EHD2 | EH Domain Containing 2 | POSTN | Myosin XVB | COL6A2 | Collagen Type VI Alpha 2 Chain | ||||
| IKBKE | Inhibitor Of Nuclear Factor Kappa B Kinase Subunit Epsilon | PCDHA4 | Protocadherin alpha-4 | EYS | Eyes Shut Homolog | SERPINA9 | Periostin | COL6A3 | Collagen Type VI Alpha 3 Chain | ||||
| INS | Insulin | POLD1 | DNA polymerase delta catalytic subunit | F13A1 | Coagulation Factor XIII A Chain | VTN | Serpin Family A Member 9 | COMP | Cartilage Oligomeric Matrix Protein | ||||
| IRF2BPL | Interferon Regulatory Factor 2 Binding Protein Like | POM121L2 | POM121-like protein 2 | GOLGA4 | Golgin A4 | DCN | Decorin | ||||||
| ITGA6 | Integrin Subunit Alpha 6 | PPFIA1 | Liprin-alpha-1 | GSTP1 | Glutathione S-Transferase Pi 1 | DES | Desmin | ||||||
| KRT26 | Keratin 26 | PPFIA2 | Liprin-alpha-2 | GVINP1 | GTPase, Very Large Interferon Inducible Pseudogene 1 | DMD | Dystrophin | ||||||
| LEMD2 | LEM Domain Nuclear Envelope Protein 2 | PRR14L | Protein PRR14L | H3-2 | H3.2 Histone (Putative) | DPT | Dermatopontin | ||||||
| MAP3K21 | Mitogen-Activated Protein Kinase Kinase Kinase 21 | PTPN7 | Tyrosine-protein phosphatase non-receptor type 7 | H3-3A | H3.3 Histone A | ENO1 | Enolase 1 | ||||||
| MDGA1 | MAM Domain Containing Glycosylphosphatidylinositol Anchor 1 | RASSF10 | Ras association domain-containing protein 10 | H3-3B | H3.3 Histone B | ENO2 | Enolase 2 | ||||||
| MMP10 | Matrix Metallopeptidase 10 | RPS6KA6 | Ribosomal protein S6 kinase alpha-6 | H3-4 | H3.4 Histone | ENO3 | Enolase 3 | ||||||
| MMP27 | Matrix Metallopeptidase 27 | TRIO | TRIO and F-actin-binding protein | H3-5 | H3.5 Histone | FBLN1 | Fibulin 1 | ||||||
| MMP3 | Matrix Metallopeptidase 3 | TSC1 | Hamartin | HEATR6 | HEAT Repeat Containing 6 | FGA | Fibrinogen Alpha Chain | ||||||
| MOS | MOS Proto-Oncogene, Serine/Threonine Kinas | UPK3A | Uroplakin-3a | HPX | Hemopexin | FGB | Fibrinogen Beta Chain | ||||||
| MYL6 | Myosin Light Chain 6 | UROD | Uroporphyrinogen decarboxylase | HSP90B1 | Heat Shock Protein 90 Beta Family Member 1 | FGG | Fibrinogen Gamma Chain | ||||||
| MYO7B | Myosin VIIB | HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | FLNB | Filamin B | ||||||||
| NT5E | 5’-Nucleotidase Ecto | HSPA1B | Heat Shock Protein Family A (Hsp70) Member 1B | FMOD | Fibromodulin | ||||||||
| OLFML1 | Olfactomedin Like 1 | HSPA1L | Heat Shock Protein Family A (Hsp70) Member 1 Like | FN1 | Fibronectin 1 | ||||||||
| PGM5 | Phosphoglucomutase 5 | HSPA5 | Heat Shock Protein Family A (Hsp70) Member 5 | GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | ||||||||
| PHKA2 | Phosphorylase Kinase Regulatory Subunit Alpha 2 | IGFN1 | Immunoglobulin Like And Fibronectin Type III Domain Containing 1 | GPX3 | Glutathione Peroxidase 3 | ||||||||
| PLA2G7 | Phospholipase A2 Group VII | INF2 | Inverted Formin 2 | GSN | Angiotensin I Converting Enzyme 2 | ||||||||
| POR | Cytochrome P450 Oxidoreductase | L3MBTL4 | L3MBTL Histone Methyl-Lysine Binding Protein 4 | H2BC1 | H2B Clustered Histone 1 | ||||||||
| RANBP17 | RAN Binding Protein 17 | LMNB1 | Lamin B1 | H2BC11 | H2B Clustered Histone 11 | ||||||||
| RGS22 | Regulator Of G Protein Signaling 22 | LMNB2 | Lamin B2 | H2BC12 | H2B Clustered Histone 12 | ||||||||
| RIF1 | Replication Timing Regulatory Factor 1 | MFAP5 | Microfibril Associated Protein 5 | H2BC13 | H2B Clustered Histone 13 | ||||||||
| RTN4 | Reticulon 4 | MRPL50 | Mitochondrial Ribosomal Protein L50 | H2BC14 | H2B Clustered Histone 14 | ||||||||
| SARS2 | Seryl-TRNA Synthetase 2, Mitochondrial | MS4A6A | Membrane Spanning 4-Domains A6A | H2BC15 | H2B Clustered Histone 15 | ||||||||
| SEPHS2 | Selenophosphate Synthetase 2 | MUC4 | Mucin 4, Cell Surface Associated | H2BC17 | H2B Clustered Histone 17 | ||||||||
| SLFN13 | Schlafen Family Member 13 | MYH14 | Myosin Heavy Chain 14 | H2BC18 | H2B Clustered Histone 18 | ||||||||
| SLK | STE20 Like Kinase | MYL6B | Myosin Light Chain 6B | H2BC21 | H2B Clustered Histone 21 | ||||||||
| SPATA20 | Spermatogenesis Associated 20 | NEK10 | NIMA Related Kinase 10 | H2BC3 | H2B Clustered Histone 3 | ||||||||
| SPATA5 | Spermatogenesis Associated 5 | PAK3 | P21 (RAC1) Activated Kinase 3 | H2BC5 | H2B Clustered Histone 5 | ||||||||
| SPTA1 | Spectrin Alpha, Erythrocytic 1 | PAPOLA | Poly(A) Polymerase Alpha | H2BC9 | H2B Clustered Histone 9 | ||||||||
| SQLE | Squalene Epoxidase | PAPOLG | Poly(A) Polymerase Gamma | H2BS1 | H2B.S Histone 1 | ||||||||
| ST20-AS1 | ST20 Antisense RNA 1 | PDIA3 | Protein Disulfide Isomerase Family A Member 3 | H2BU1 | H2B.U Histone 1 | ||||||||
| STIL | STIL Centriolar Assembly Protein | PDLIM4 | PDZ And LIM Domain 4 | HBA1 | Hemoglobin Subunit Alpha 1 | ||||||||
| TACC2 | Transforming Acidic Coiled-Coil Containing Protein 2 | RALBP1 | RalA Binding Protein 1 | HBA2 | Hemoglobin Subunit Alpha 2 | ||||||||
| TAP1 | Transporter 1, ATP Binding Cassette Subfamily B Member | RNF213 | Ring Finger Protein 213 | HBB | Hemoglobin Subunit Beta | ||||||||
| THADA | THADA Armadillo Repeat Containing | SBF2 | SET Binding Factor 2 | HBD | Hemoglobin Subunit Delta | ||||||||
| THBS3 | Thrombospondin 3 | SERPINF1 | Serpin Family F Member 1 | HBE1 | Hemoglobin Subunit Epsilon 1 | ||||||||
| UQCRC1 | Ubiquinol-Cytochrome C Reductase Core Protein 1 | SERPINH1 | Serpin Family H Member 1 | HBG1 | Hemoglobin Subunit Gamma 1 | ||||||||
| VWA3A | Von Willebrand Factor A Domain Containing 3A | SLC4A5 | Solute Carrier Family 4 Member 5 | HBG2 | Hemoglobin Subunit Gamma 2 | ||||||||
| ZNF333 | Zinc Finger Protein 333 | SLIT2 | Slit Guidance Ligand 2 | HBZ | Hemoglobin Subunit Zeta | ||||||||
| SMPD3 | Sphingomyelin Phosphodiesterase 3 | HP | Haptoglobin | ||||||||||
| TAPT1 | Transmembrane Anterior Posterior Transformation 1 | HPR | Haptoglobin-Related Protein | ||||||||||
| TBX22 | T-Box Transcription Factor 22 | HSPB1 | Heat Shock Protein Family B (Small) Member 1 | ||||||||||
| TDRD1 | Tudor Domain Containing 1 | IGHA1 | Immunoglobulin Heavy Constant Alpha 1 | ||||||||||
| TENM4 | Teneurin Transmembrane Protein 4 | IGHA2 | Immunoglobulin Heavy Constant Alpha 2 (A2m Marker) | ||||||||||
| THBS1 | Thrombospondin 1 | IGHG1 | Immunoglobulin Heavy Constant Gamma 1 (G1m Marker) | ||||||||||
| TJP2 | Tight Junction Protein 2 | IGHG2 | Immunoglobulin Heavy Constant Gamma 2 (G2m Marker) | ||||||||||
| TTR | Transthyretin | IGHG3 | Immunoglobulin Heavy Constant Gamma 3 (G3m Marker) | ||||||||||
| UBP1 | Upstream Binding Protein 1 | IGHG4 | Immunoglobulin Heavy Constant Gamma 4 (G4m Marker) | ||||||||||
| WHRN | Whirlin | IGKC | Immunoglobulin Kappa Constant | ||||||||||
| ZNF155 | Zinc Finger Protein 155 | INA | Internexin Neuronal Intermediate Filament Protein Alpha | ||||||||||
| ZNF221 | Zinc Finger Protein 221 | KRT7 | Keratin 7 | ||||||||||
| KRT8 | Keratin 8 | ||||||||||||
| KRT84 | Keratin 84 | ||||||||||||
| LGALS1 | Galectin 1 | ||||||||||||
| LMNA | Lamin A/C | ||||||||||||
| LUM | Lumican | ||||||||||||
| MFAP4 | Microfibril Associated Protein 4 | ||||||||||||
| MFGE8 | Milk Fat Globule EGF And Factor V/VIII Domain Containing | ||||||||||||
| MYH16 | Myosin Heavy Chain 16 Pseudogene | ||||||||||||
| MYOC | Myocilin | ||||||||||||
| NEFH | Neurofilament Heavy | ||||||||||||
| NEFL | Neurofilament Light | ||||||||||||
| NEFM | Neurofilament Medium | ||||||||||||
| OGN | Osteoglycin | ||||||||||||
| POTEE | POTE Ankyrin Domain Family Member E | ||||||||||||
| POTEF | POTE Ankyrin Domain Family Member F | ||||||||||||
| POTEI | POTE Ankyrin Domain Family Member I | ||||||||||||
| POTEJ | POTE Ankyrin Domain Family Member J | ||||||||||||
| POTEKP | POTE Ankyrin Domain Family Member K, Pseudogene | ||||||||||||
| PPIA | Peptidylprolyl Isomerase A | ||||||||||||
| PRDX1 | Peroxiredoxin 1 | ||||||||||||
| PRDX2 | Peroxiredoxin 2 | ||||||||||||
| PRELP | Proline And Arginine Rich End Leucine Rich Repeat Protein | ||||||||||||
| PRPH | Peripherin | ||||||||||||
| RPL7L1 | Ribosomal Protein L7 Like 1 | ||||||||||||
| S100A10 | S100 Calcium Binding Protein A10 | ||||||||||||
| SALL3 | Spalt Like Transcription Factor 3 | ||||||||||||
| SERPINA1 | Serpin Family A Member | ||||||||||||
| SHLD3 | Shieldin Complex Subunit 3 | ||||||||||||
| SLC4A1 | Solute Carrier Family 4 Member 1 | ||||||||||||
| SOD3 | Superoxide Dismutase 3 | ||||||||||||
| TF | Transferrin | ||||||||||||
| TGFBI | Transforming Growth Factor Beta Induced | ||||||||||||
| THBS4 | Thrombospondin 4 | ||||||||||||
| TNC | Tenascin C | ||||||||||||
| TNXA | Tenascin XA (Pseudogene) | ||||||||||||
| TNXB | Tenascin XB | ||||||||||||
| TUBA1A | Tubulin Alpha 1a | ||||||||||||
| TUBA1B | Tubulin Alpha 1b | ||||||||||||
| TUBA1C | Tubulin Alpha 1c | ||||||||||||
| TUBA3E | Tubulin Alpha 3e | ||||||||||||
| TUBA4A | Tubulin Alpha 4a | ||||||||||||
| TUBA8 | Tubulin Alpha 8 | ||||||||||||
| TUBB | Tubulin Beta Class I | ||||||||||||
| TUBB1 | Tubulin Beta 1 Class VI | ||||||||||||
| TUBB2A | Tubulin Beta 2A Class IIa | ||||||||||||
| TUBB2B | Tubulin Beta 2B Class IIb | ||||||||||||
| TUBB3 | Tubulin Beta 3 Class III | ||||||||||||
| TUBB4A | Tubulin Beta 4A Class IVa | ||||||||||||
| TUBB4B | Tubulin Beta 4B Class IVb | ||||||||||||
| TUBB6 | Tubulin Beta 6 Class V | ||||||||||||
| TUBB8 | Tubulin Beta 8 Class VIII | ||||||||||||
| TUBB8B | Tubulin Beta 8B | ||||||||||||
| Protein Expressed in Each Group of TMJ Synovial Fluid and Disc Samples (n = 11) | ||||||
|---|---|---|---|---|---|---|
| DDWoR (n= 2) | MD (n = 3) | CH (n = 0) | DDWoR and MD (n = 0) | DDWoR and CH (n = 0) | MD and CH (n = 0) | DDWoR, MD and CH (n = 6) |
| CHD8 | FLNA | ENO1 | ||||
| MYL6B | PPFIA1 | ENO2 | ||||
| PPFIA2 | ENO3 | |||||
| MYH16 | ||||||
| RPL7L1 | ||||||
| SHLD3 | ||||||
| Synovial Fluid Sample | ||
|---|---|---|
| Code | Name | Function |
| DDWoR | ||
| A2M | Alpha-2-Macroglobulin | Inhibits inflammatory cytokines. |
| APCS | Amyloid P Component, Serum | Binds to apoptotic cells at an early stage. |
| GPSM2 | G Protein Signaling Modulator 2 | Involved in the development of normal hearing. |
| KRT18 | Keratin 18 | Is involved in interleukin-6-mediated barrier protection. |
| MAP3K7 | Mitogen-Activated Protein Kinase Kinase Kinase 7 | Mediates signal transduction various cytokines including interleukin-1, transforming growth factor-beta, bone morphogenetic protein 2 and 4, Toll-like receptors, tumor necrosis factor receptor CD40 and B-cell receptor. |
| SERPINC1 | Serpin Family C Member 1 | This protein inhibits thrombin and it regulates the blood coagulation cascade. |
| MD | ||
| ALDH1L1 | Aldehyde Dehydrogenase 1 Family Member L1 | Associated with decreased apoptosis, increased cell motility, and cancer progression. |
| C4A | Complement C4A (Rodgers Blood Group) | An antimicrobial peptide and a mediator of local inflammation. |
| HPX | Hemopexin | Acute phase protein that transports heme from the plasma to the liver and may be involved in protecting cells from oxidative stress. |
| IFT122 | Intraflagellar Transport 122 | Involved in cell cycle progression, signal transduction, apoptosis, and gene regulation. |
| MYO6 | Myosin VI | This protein maintains the structural integrity of inner ear hair cells and mutations in this gene cause hearing loss. |
| PRDX1 | Peroxiredoxin 1 | Has an antioxidant protective role in cells and may contribute to the antiviral activity of CD8(+) T-cells. |
| SERPINH1 | Serpin Family H Member 1 | Plays a role in collagen biosynthesis as a collagen-specific molecular chaperone. |
| SMPD3 | Sphingomyelin Phosphodiesterase 3 | Mediates cellular functions, such as apoptosis and growth arrest. |
| CH | ||
| ADH1 | Alcohol Dehydrogenase Subunit Alpha | Catalyzes the oxidation of alcohols to aldehydes. |
| DDWoR and MD | ||
| ANXA1 | Annexin A1 | Inhibits phospholipase A2 and has anti-inflammatory activity. |
| ANXA2 | Annexin A2 | Functions as an autocrine factor which heightens osteoclast formation and bone resorption. |
| ASPN | Asporin | Regulate chondrogenesis by inhibiting transforming growth factor-beta 1-induced gene expression in cartilage. May induce collagen mineralization. |
| BGN | Biglycan | Plays a role in bone growth, muscle development and regeneration, and collagen fibril assembly in multiple tissues. This protein may also regulate inflammation and innate immunity. |
| CILP | Cartilage Intermediate Layer Protein | This protein is present in the cartilage intermediate layer protein (CILP), which increases in early osteoarthrosis cartilage. |
| CLU | Clusterin | Under stress conditions can be found in the cell cytosol. May be involved in cell death, tumor progression, and neurodegenerative disorders |
| COMP | Thrombospondin-5 | Present in rheumatoid arthritis, is a noncollagenous extracellular matrix protein. |
| DCN | Decorin | Has a stimulatory effect on autophagy and inflammation and an inhibitory effect on angiogenesis and tumorigenesis. |
| FMOD | Fibromodulin | May also regulate TGF-beta activities by sequestering TGF-beta into the extracellular matrix. |
| FN1 | Fibronectin 1 | Fibronectin is involved in cell adhesion and migration processes including embryogenesis, wound healing, blood coagulation, host defense. |
| IGHG1 | Immunoglobulin Heavy Constant Gamma 1 (G1m Marker) | Involved in pathways of Interleukin-4 and 13 signaling and IL4-mediated signaling events. |
| DDWoR and CH | ||
| x | x | x |
| MD and CH | ||
| x | x | x |
| DDWoR, MD and CH | ||
| ENO2 | Enolase 2 | Found in mature neurons and cells of neuronal origin. |
| ENO3 | Enolase 3 | May play a role in muscle development and regeneration. |
| Disc Sample | ||
|---|---|---|
| Code | Name | Function |
| DDWoR | ||
| AMBP | Alpha-1-Microglobulin/Bikunin Precursor | Regulation of the inflammatory process. |
| MMP10 | Matrix Metallopeptidase 10 | Breakdown of extracellular matrix. |
| MMP27 | Matrix Metallopeptidase 27 | Breakdown of extracellular matrix. |
| MMP3 | Matrix Metallopeptidase 3 | Breakdown of extracellular matrix. |
| PLA2G7 | Phospholipase A2 Group VII | Inflammatory and oxidative stress response. |
| THADA | THADA Armadillo Repeat Containing | Apoptosis pathway. |
| THBS3 | Thrombospondin 3 | Matrix interactions. |
| MD | ||
| AKAP13 | A-kinase anchor protein 13 | Regulation of apoptotic process. |
| CCDC88A | Girdin | Vascular endothelial growth factor receptor 2 binding. |
| COL4A1 | Collagen alpha-1(IV) chain | Extracellular matrix structural constituent. |
| ERAS | GTPase ERas | Tumor-like growth properties of embryonic stem cells. |
| ERBIN | Erbin | Inhibits NOD2-dependent NF-kappa-B signaling and proinflammatory cytokine secretion. |
| PARP10 | Protein mono-ADP-ribosyltransferase PARP10 | Negative regulation of fibroblast proliferation. |
| PPFIA1 | Liprin-alpha-1 | Cell–matrix adhesion. |
| PPFIA2 | Liprin-alpha-2 | Cell–matrix adhesion. |
| PTPN7 | Tyrosine-protein phosphatase non-receptor type 7 | Regulation of T and B-lymphocyte development and signal transduction. |
| UPK3A | Uroplakin-3a | Epithelial cell differentiation. |
| CH | ||
| ACTN4 | Actinin Alpha 4 | Transcriptional coactivator. |
| ADAM10 | ADAM Metallopeptidase Domain 10 | Responsible for the FasL ectodomain shedding. |
| COQ8B | Coenzyme Q8B | Biosynthesis of coenzyme Q. |
| HPX | Hemopexin | Protect cells from oxidative stress. |
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | Protection of the proteome from stress. |
| NEK10 | NIMA Related Kinase 10 | Cellular response to UV irradiation. |
| PDLIM4 | PDZ And LIM Domain 4 | Involved in bone development. |
| SERPINH1 | Serpin Family H Member 1 | Chaperone in the biosynthetic pathway of collagen. |
| TTR | Transthyretin | Thyroid hormone-binding protein. |
| COL1A2 | Collagen Type I Alpha 2 Chain | Fibril-forming collagen abundant in bone. |
| PRG4 | Proteoglycan 4 | This protein contains both chondroitin sulfate and keratan sulfate glycosaminoglycans. |
| PTPN13 | Protein Tyrosine Phosphatase Non-Receptor Type 13 | Regulates negatively FasL induced apoptosis. |
| DDWoR and MD | ||
| C4A | Complement C4A | Antimicrobial peptide and a mediator of local inflammation. |
| C4B | Complement C4B | Mediator of local inflammation. |
| C4B_2 | Complement Component 4B | Mediator of local inflammatory process. |
| SEMA4F | Semaphorin 4F | Plays a role in neural development. |
| Code | Name | Function |
| DDWoR and CH | ||
| ACAN | Aggrecan | Part of the extracellular matrix that withstands compression in cartilage. |
| COL1A1 | Collagen Type I Alpha 1 Chain | Collagen component. |
| COL4A6 | Collagen Type IV Alpha 6 Chain | Major structural component of basement membranes. |
| HSPA2 | Heat Shock Protein Family A (Hsp70) Member 2 | Protection of the proteome from stress. |
| POSTN | Periostin | Extracellular matrix protein that functions in tissue development and regeneration, including wound healing. |
| MD and CH | ||
| KRT6A | Keratin 6A | Epidermis-specific type I keratin involved in wound healing. |
| DDWoR, MD and CH | ||
| ANXA1 | Annexin A1 | Anti-inflammatory activity. |
| ANXA2 | Annexin A2 | Heightens osteoclast formation and bone resorption. |
| ANXA2P2 | Annexin A2 Pseudogene 2 | May be involved in heat-stress response. |
| APCS | Amyloid P Component | Is involved in dealing with apoptotic cells in vivo. |
| ASPN | Asporin | Regulates chondrogenesis by inhibiting transforming growth factor-beta 1-induced gene expression in cartilage |
| BGN | Biglycan | Plays a role in bone growth, and collagen fibril assembly in multiple tissues. This protein may also regulate inflammation and innate immunity. |
| C3 | Complement C3 | Modulates inflammation and possesses antimicrobial activity. |
| CILP | Cartilage Intermediate Layer Protein | Increases in early osteoarthrosis cartilage. |
| COL12A1 | Collagen Type XII Alpha 1 Chain | Type XII collagen. |
| COL14A1 | Collagen Type XIV Alpha 1 Chain | Type XIV collagen. |
| COL6A1 | Collagen Type VI Alpha 1 Chain | Collagen VI. |
| COL6A2 | Collagen Type VI Alpha 2 Chain | Type VI collagen. |
| COL6A3 | Collagen Type VI Alpha 3 Chain | Ttype VI collagen. |
| COMP | Cartilage Oligomeric Matrix Protein | Degradation of the extracellular matrix. |
| ENO1 | Enolase 1 | Tumor suppressor. |
| ENO2 | Enolase 2 | Found in mature neurons and cells of neuronal origin. |
| ENO3 | Enolase 3 | Plays a role in muscle development and regeneration. |
| FN1 | Fibronectin 1 | Involved in wound healing, blood coagulation, host defense. |
| KRT7 | Keratin 7 | Co-expressed during differentiation of simple and stratified epithelial tissues. |
| LUM | Lumican | May regulate collagen fibril organization, epithelial cell migration and tissue repair. |
| MFAP4 | Microfibril Associated Protein 4 | Extracellular matrix protein which is involved in cell adhesion or intercellular interactions. |
| MFGE8 | Milk Fat Globule EGF And Factor V/VIII Domain Containing | Promotes phagocytosis of apoptotic cells. This protein has also been implicated in wound healing, autoimmune disease, and cancer. |
| OGN | Osteoglycin | Induces ectopic bone formation in conjunction with transforming growth factor beta and may regulate osteoblast differentiation. |
| SOD3 | Superoxide Dismutase 3 | Antioxidant enzymes that protect tissues from oxidative stress. |
| TGFBI | Transforming Growth Factor Beta Induced | May be involved in endochondrial bone formation in cartilage. |
| TNC | Tenascin C | Modulation of inflammatory cytokine. |
| TNXB | Tenascin XB | Accelerates collagen fibril formation. |
| VCAN | Versican | A large chondroitin sulfate proteoglycan and is a major component of the extracellular matrix. |
| VIM | Vimentin | Involved in the stabilization of type I collagen mRNAs for CO1A1 and CO1A2. |
| Name | Function | Disc | Synovial Fluid |
|---|---|---|---|
| Amyloid P Component, Serum | Is involved in dealing with apoptotic cells in vivo. | DDWoR, MD and CH | DDWoR |
| Annexin A1 | Anti-inflammatory activity. | DDWoR, MD and CH | DDWoR and MD |
| Annexin A2 | Heightens osteoclast formation and bone resorption. | DDWoR, MD and CH | DDWoR and MD |
| Asporin | Regulates chondrogenesis. | DDWoR, MD and CH | DDWoR and MD |
| Biglycan | Plays a role in bone growth, and collagen fibril assembly in multiple tissues. | DDWoR, MD and CH | DDWoR and MD |
| Cartilage Intermediate Layer Protein | Increases in early osteoarthrosis cartilage. | DDWoR, MD and CH | DDWoR and MD |
| Complement C4A | Antimicrobial peptide and a mediator of local inflammation. | DDWoR and MD | MD |
| Enolase 2 | Found in mature neurons and cells of neuronal origin. | DDWoR, MD and CH | DDWoR, MD and CH |
| Enolase 3 | Play a role in muscle development and regeneration. | DDWoR, MD and CH | DDWoR, MD and CH |
| Fibronectin 1 | Involved in wound healing, blood coagulation, host defense. | DDWoR, MD and CH | DDWoR and MD |
| Hemopexin | Protect cells from oxidative stress. | CH | MD |
| Lumican | May regulate collagen fibril organization, epithelial cell migration and tissue repair. | DDWoR, MD and CH | DDWoR and MD |
| Osteoglycin | Regulate osteoblast differentiation. | DDWoR, MD and CH | DDWoR and MD |
| Serpin Family H Member 1 | Chaperones in the biosynthetic pathway of collagen. | CH | MD |
| Superoxide Dismutase 3 | Antioxidant enzymes that protect tissues from oxidative stress. | DDWoR, MD and CH | DDWoR and MD |
| Tenascin XB | Modulation of inflammatory cytokine. | DDWoR, MD and CH | DDWoR and MD |
| Transforming Growth Factor Beta Induced | May be involved in endochondral bone formation in cartilage. | DDWoR, MD and CH | DDWoR and MD |
| Versican | A large chondroitin sulfate proteoglycan and is a major component of the extracellular matrix. | DDWoR, MD and CH | DDWoR and MD |
| Type of Collagen Identified in Each Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DDWoR | MD | CH | DDWoR and MD | DDWoR and CH | MD and CH | DDWoR, MD and CH | ||||
| x | Code | Name | Code | Name | x | Code | Name | x | Code | Name |
| COL4A1 | Collagen Type IV Alpha 1 Chain | COL1A2 | Collagen Type I Alpha 2 Chain | COL1A1 | Collagen Type I Alpha 1 Chain | COL12A1 | Collagen Type XII Alpha 1 Chain | |||
| COL4A6 | Collagen Type IV Alpha 6 Chain | COL14A1 | Collagen Type XIV Alpha 1 Chain | |||||||
| COL6A1 | Collagen Type VI Alpha 1 Chain | |||||||||
| COL6A2 | Collagen Type VI Alpha 2 Chain | |||||||||
| COL6A3 | Collagen Type VI Alpha 3 Chain | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doetzer, A.D.; Herai, R.H.; Buzalaf, M.A.R.; Trevilatto, P.C. Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction. Diagnostics 2021, 11, 601. https://doi.org/10.3390/diagnostics11040601
Doetzer AD, Herai RH, Buzalaf MAR, Trevilatto PC. Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction. Diagnostics. 2021; 11(4):601. https://doi.org/10.3390/diagnostics11040601
Chicago/Turabian StyleDoetzer, Andrea Duarte, Roberto Hirochi Herai, Marília Afonso Rabelo Buzalaf, and Paula Cristina Trevilatto. 2021. "Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction" Diagnostics 11, no. 4: 601. https://doi.org/10.3390/diagnostics11040601
APA StyleDoetzer, A. D., Herai, R. H., Buzalaf, M. A. R., & Trevilatto, P. C. (2021). Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction. Diagnostics, 11(4), 601. https://doi.org/10.3390/diagnostics11040601







