Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
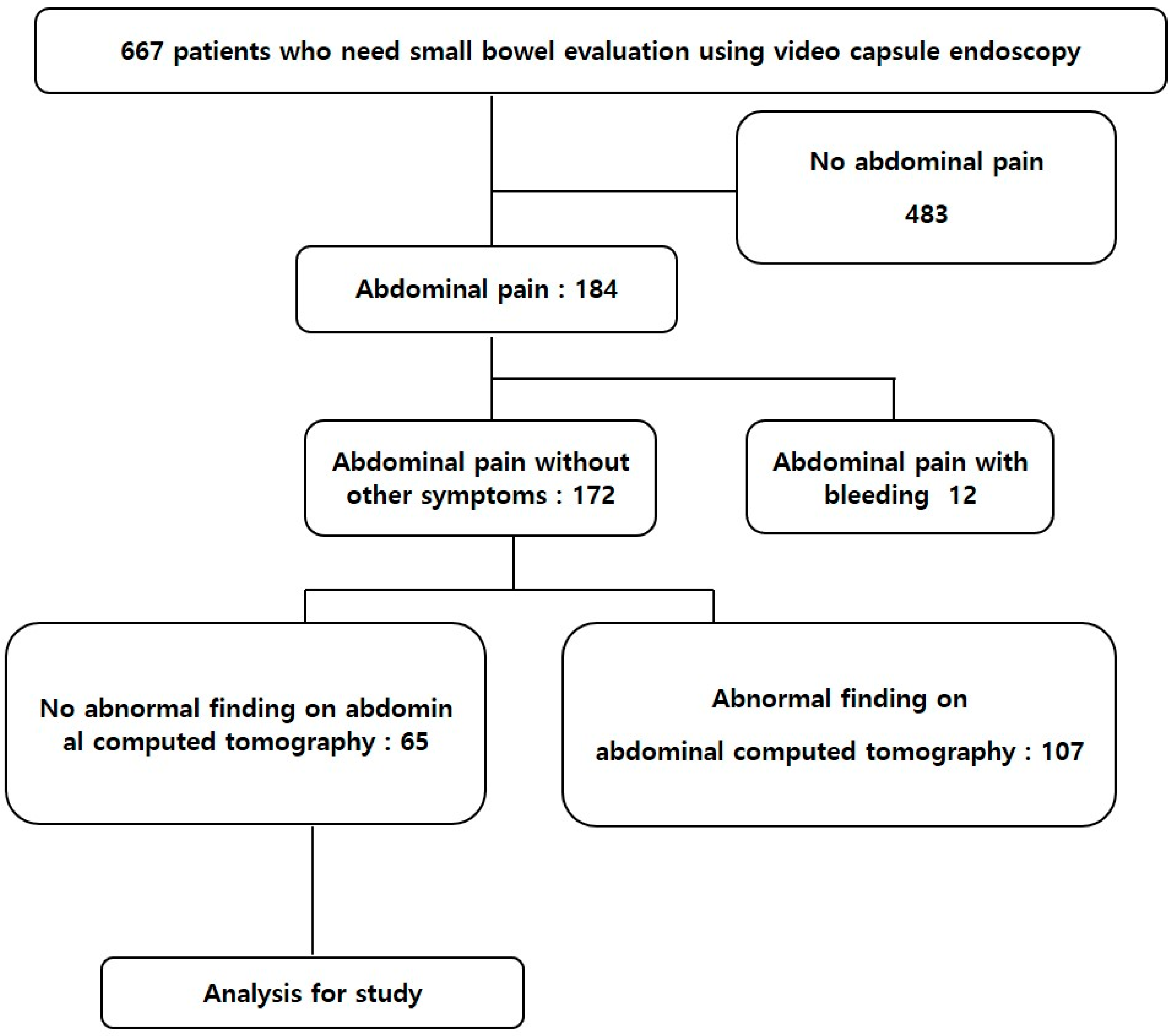
2.1. Patients
2.2. Video Capsule Endoscopy
2.3. Study Search Strategy
2.4. Eligible Criteria and Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
3.2. Findings of Capsule Endoscopy
3.3. Comparison of Clinical and Laboratory Findings between the Capsule Positive and Capsule Negative Groups
3.4. Elevated ESR Was a Significant Predictor of Positive VCE
3.5. Result of Meta-Analysis for Relevant Factors of VCE Positive Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.Y.; Moon, C.M.; Shim, K.-N.; Cheung, D.Y.; Lee, H.S.; Lim, Y.J.; Jeon, S.R.; Park, S.J.; Kim, K.O.; Song, H.J. Positive Fecal Occult Blood Test is a Predictive Factor for Gastrointestinal Bleeding after Capsule Endoscopy in Patients with Unexplained Iron Deficiency Anemia: A Korean Multicenter CAPENTRY Study. Clin. Endosc. 2020, 53, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Hosoe, N.; Takabayashi, K.; Ogata, H.; Kanai, T. Capsule endoscopy for small-intestinal disorders: Current status. Dig. Endosc. 2019, 31, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.Y.; Lee, B.J.; Kim, W.S.; Kim, S.M.; Kim, S.H.; Joo, M.K.; Kim, H.J.; Park, J.-J. Clinicopathological features of small bowel tumors diagnosed by video capsule endoscopy and balloon-assisted enteroscopy: A single center experience. Clin. Endosc. 2021, 54, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Bjarnason, I. Drug-induced small bowel injury: A challenging and often forgotten clinical condition. Curr. Gastroenterol. Rep. 2019, 21, 55. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Nabi, Z.; Shava, U.; Ganesh, G.; Reddy, D.N. Very early onset inflammatory bowel disease in a South Asian country where inflammatory bowel disease is emerging: A distinct clinical phenotype from later onset disease. Intest. Res. 2021, 19, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, I.; Kobayashi, T. Capsule endoscopy in inflammatory bowel disease: When and how. Intest. Res. 2020, 18, 265–274. [Google Scholar] [CrossRef]
- Bang, S.; Park, J.Y.; Jeong, S.; Kim, Y.H.; Shim, H.B.; Kim, T.S.; Lee, D.H.; Song, S.Y. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: Electric-field propagation. Gastrointest. Endosc. 2009, 69, 253–259. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Z.; Tai, Y.; Wang, P.; Hu, B.; Tang, C. The small bowel diseases detected by capsule endoscopy in patients with chronic abdominal pain: A retrospective study. Medicine 2018, 97, e0025. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.-N.; Kim, Y.-S.; Kim, K.-J.; Kim, Y.-H.; Kim, T.-I.; Do, J.-H.; Ryu, J.-K.; Moon, J.-S.; Park, S.-H.; Hee Park, C. Abdominal pain accompanied by weight loss may increase the diagnostic yield of capsule endoscopy: A Korean multicenter study. Scand. J. Gastroenterol. 2006, 41, 983–988. [Google Scholar] [CrossRef]
- Katsinelos, P.; Fasoulas, K.; Beltsis, A.; Chatzimavroudis, G.; Paroutoglou, G.; Maris, T.; Mimidis, K.; Koufokotsios, A.; Terzoudis, S.; Atmatzidis, S. Diagnostic yield and clinical impact of wireless capsule endoscopy in patients with chronic abdominal pain with or without diarrhea: A Greek multicenter study. Eur. J. Intern. Med. 2011, 22, e63–e66. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Milluzzo, S.M.; Cesaro, P.; Grazioli, L.M.; Olivari, N.; Spada, C. Artificial intelligence in lower gastrointestinal endoscopy: The current status and future perspective. Clin. Endosc. 2021, 54, 329–339. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Meron, G.D. The development of the swallowable video capsule (M2A). Gastrointest. Endosc. 2000, 52, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.S.; Swain, P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest. Endosc. 2002, 56, 349–353. [Google Scholar] [CrossRef]
- Pennazio, M.; Santucci, R.; Rondonotti, E.; Abbiati, C.; Beccari, G.; Rossini, F.P.; De Franchis, R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: Report of 100 consecutive cases. Gastroenterology 2004, 126, 643–653. [Google Scholar] [CrossRef]
- Apostolopoulos, P.; Liatsos, C.; Gralnek, I.; Giannakoulopoulou, E.; Alexandrakis, G.; Kalantzis, C.; Gabriel, P.; Kalantzis, N. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy 2006, 38, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, P.; Liatsos, C.; Gralnek, I.M.; Kalantzis, C.; Giannakoulopoulou, E.; Alexandrakis, G.; Tsibouris, P.; Kalafatis, E.; Kalantzis, N. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest. Endosc. 2007, 66, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Urbain, D.; De Looze, D.; Demedts, I.; Louis, E.; Dewit, O.; Macken, E.; Van Gossum, A. Video capsule endoscopy in small-bowel malignancy: A multicenter Belgian study. Endoscopy 2006, 38, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.A.; Debinski, H.S.; Appleyard, M.N.; Remedios, M.L.; Hooper, J.E.; Walsh, A.J.; Selby, W.S. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: A three-center Australian experience. Off. J. Am. Coll. Gastroenterol. ACG 2006, 101, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Schmidt, H.; Bolz, G.; Schilling, D.; Kinzel, F.; Eickhoff, A.; Huschner, W.; Möller, K.; Jakobs, R.; Reitzig, P. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest. Endosc. 2005, 61, 826–832. [Google Scholar] [CrossRef]
- Imagawa, H.; Oka, S.; Tanaka, S.; Noda, I.; Higashiyama, M.; Sanomura, Y.; Shishido, T.; Yoshida, S.; Chayama, K. Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: A pilot study. Scand. J. Gastroenterol. 2011, 46, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Tanaka, S.; Shishido, T.; Takemura, Y.; Oka, S.; Chayama, K. Comparison of detectability of small-bowel lesions between capsule endoscopy and double-balloon endoscopy for patients with suspected small-bowel disease. Gastrointest. Endosc. 2009, 69, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Spada, C.; Adler, S.; May, A.; Despott, E.J.; Koulaouzidis, A.; Panter, S.; Domagk, D.; Fernandez-Urien, I.; Rahmi, G. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2018, 50, 423–446. [Google Scholar] [CrossRef] [Green Version]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.-F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J. Crohn’s Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Soncini, M.; Girelli, C.M.; de Franchis, R.; Rondonotti, E. Small-bowel capsule endoscopy in clinical practice: Has anything changed over 13 years? Dig. Dis. Sci. 2018, 63, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, V.P.; Nos, P.; Bastida, G.; Beltrán, B.; Argüello, L.; Aguas, M.; Rubín, A.; Pertejo, V.; Sala, T. Evaluation of postsurgical recurrence in Crohn’s disease: A new indication for capsule endoscopy? Gastrointest. Endosc. 2007, 66, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Dong, Z.; Dai, C. The diagnostic and predictive value of fecal calprotectin and capsule endoscopy for small-bowel Crohn’s disease: A systematic review and meta-analysis. Rev. Esp. Enferm. Dig. 2021, 113, 193–201. [Google Scholar] [PubMed]
- Enns, R.A.; Hookey, L.; Armstrong, D.; Bernstein, C.N.; Heitman, S.J.; Teshima, C.; Leontiadis, G.I.; Tse, F.; Sadowski, D. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 2017, 152, 497–514. [Google Scholar] [CrossRef] [Green Version]
- Flemming, J.; Cameron, S. Small bowel capsule endoscopy: Indications, results, and clinical benefit in a University environment. Medicine 2018, 97, e0148. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Noh, C.-K.; Lim, S.G.; Lee, K.M.; Lee, K.J. Non-steroidal anti-inflammatory drug-induced enteropathy. Intest. Res. 2017, 15, 446–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiden, L.; Thjodleifsson, B.; Seigal, A.; Bjarnason, I.I.; Scott, D.; Birgisson, S.; Bjarnason, I. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 2007, 5, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Maiden, L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J. Gastroenterol. 2009, 44, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Gudis, K.; Takahashi, Y.; Seo, T.; Yamada, Y.; Ehara, A.; Kobayashi, T.; Mitsui, K.; Yonezawa, M.; Tanaka, S. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur. J. Clin. Investig. 2010, 40, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Tziomalos, K.; Fasoulas, K.; Paroutoglou, G.; Koufokotsios, A.; Mimidis, K.; Terzoudis, S.; Maris, T.; Beltsis, A.; Geros, C. Can capsule endoscopy be used as a diagnostic tool in the evaluation of nonbleeding indications in daily clinical practice? A prospective study. Med. Princ. Pract. 2011, 20, 362–367. [Google Scholar] [CrossRef] [PubMed]



| Variable | Capsule Negative (n = 38) | Capsule Positive (n = 27) | p |
|---|---|---|---|
| Age (year) | 49.42 ± 16.57 | 54.11 ± 18.15 | 0.28 |
| Gender (M/F) | 18/20 | 11/16 | 0.06 |
| BMI (kg/m2) | 22.80 ± 3.75 | 23.40 ± 3.16 | 0.54 |
| Smoking | 29 | 23 | 0.09 |
| Alcohol | 26 | 18 | 0.81 |
| Medication | 0.62 | ||
| Anti-PLT agent | 5 | 2 | |
| NSAIDs | 2 | 4 | |
| Comorbidities | 0.64 | ||
| Cardiovascular comorbidity | 10 | 7 | |
| DM | 3 | ||
| No history | 9 | 13 | |
| Etc * | 8 | 17 | |
| Hb (g/dL) | 13.47 ± 1.44 | 12.74 ± 1.87 | 0.08 |
| WBC (103/uL) | 6.13 ± 2.60 | 6.46 ± 2.63 | 0.61 |
| PLT (103/uL) | 197.66 ± 46.68 | 242.0 ± 93.11 | 0.03 |
| Protein (g/dL) | 7.02 ± 0.54 | 7.04 ± 0.77 | 0.94 |
| Albumin (g/dL) | 4.21 ± 0.29 | 4.08 ± 0.37 | 0.14 |
| CRP (mg/L) | 1.84 ± 4.33 | 19.10 ± 29.94 | 0.01 |
| ESR (mm/hr) | 13.54 ± 12.35 | 31.25 ± 28.37 | 0.01 |
| Finding of Capsule Endoscopy | n = 65 |
|---|---|
| Negative | 38 (58.6%) |
| Mucosal inflammatory lesions | |
| Ulcer | 10 (15.4%) |
| Erosions | 8 (12.3%) |
| Ulcer with stricture | 4 (6.2%) |
| Erythema with edema | 1 (1.5%) |
| Lymphagiectasia | 1 (1.5%) |
| Tumorous lesions | |
| SET * | 2 (3.0%) |
| Multiple polyps | 1 (1.5%) |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.017 | 0.987–1.047 | 0.28 |
| Gender | 0.764 | 0.282–2.071 | 0.597 |
| BMI | 1.051 | 0.898–1.230 | 0.537 |
| Hb | 0.752 | 0.541–1.047 | 0.091 |
| PLT | 1.01 | 1.001–1.018 | 0.023 |
| BUN | 1.041 | 0.922–1.175 | 0.515 |
| Cr | 0.154 | 0.006–3.919 | 0.257 |
| Protein | 1.033 | 0.468–2.279 | 0.936 |
| Albumin | 0.28 | 0.054–1.469 | 0.133 |
| CRP | 1.117 | 1.004–1.243 | 0.042 |
| ESR | 1.045 | 1.013–1.079 | 0.006 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.99 | 0.95–1.05 | 0.86 |
| Gender | 2.62 | 0.21–33.27 | 0.46 |
| BMI | 1.02 | 0.81–1.28 | 0.89 |
| CRP | 1.1 | 0.95–1.25 | 0.213 |
| ESR | 1.057 | 1.02–1.1 | 0.004 |
| PLT | 0.99 | 0.98–1.01 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Lee, B.; Yoo, A.; Kim, S.; Joo, M.; Park, J.-J. Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis. Diagnostics 2021, 11, 2123. https://doi.org/10.3390/diagnostics11112123
Kim W, Lee B, Yoo A, Kim S, Joo M, Park J-J. Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis. Diagnostics. 2021; 11(11):2123. https://doi.org/10.3390/diagnostics11112123
Chicago/Turabian StyleKim, Wonshik, Beomjae Lee, Ahyoung Yoo, Seunghan Kim, Moonkyung Joo, and Jong-Jae Park. 2021. "Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis" Diagnostics 11, no. 11: 2123. https://doi.org/10.3390/diagnostics11112123
APA StyleKim, W., Lee, B., Yoo, A., Kim, S., Joo, M., & Park, J.-J. (2021). Predictors of Positive Video Capsule Endoscopy Findings for Chronic Unexplained Abdominal Pain: Single-Center Retrospective Study and Meta-Analysis. Diagnostics, 11(11), 2123. https://doi.org/10.3390/diagnostics11112123






