Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy
Abstract
:1. Introduction
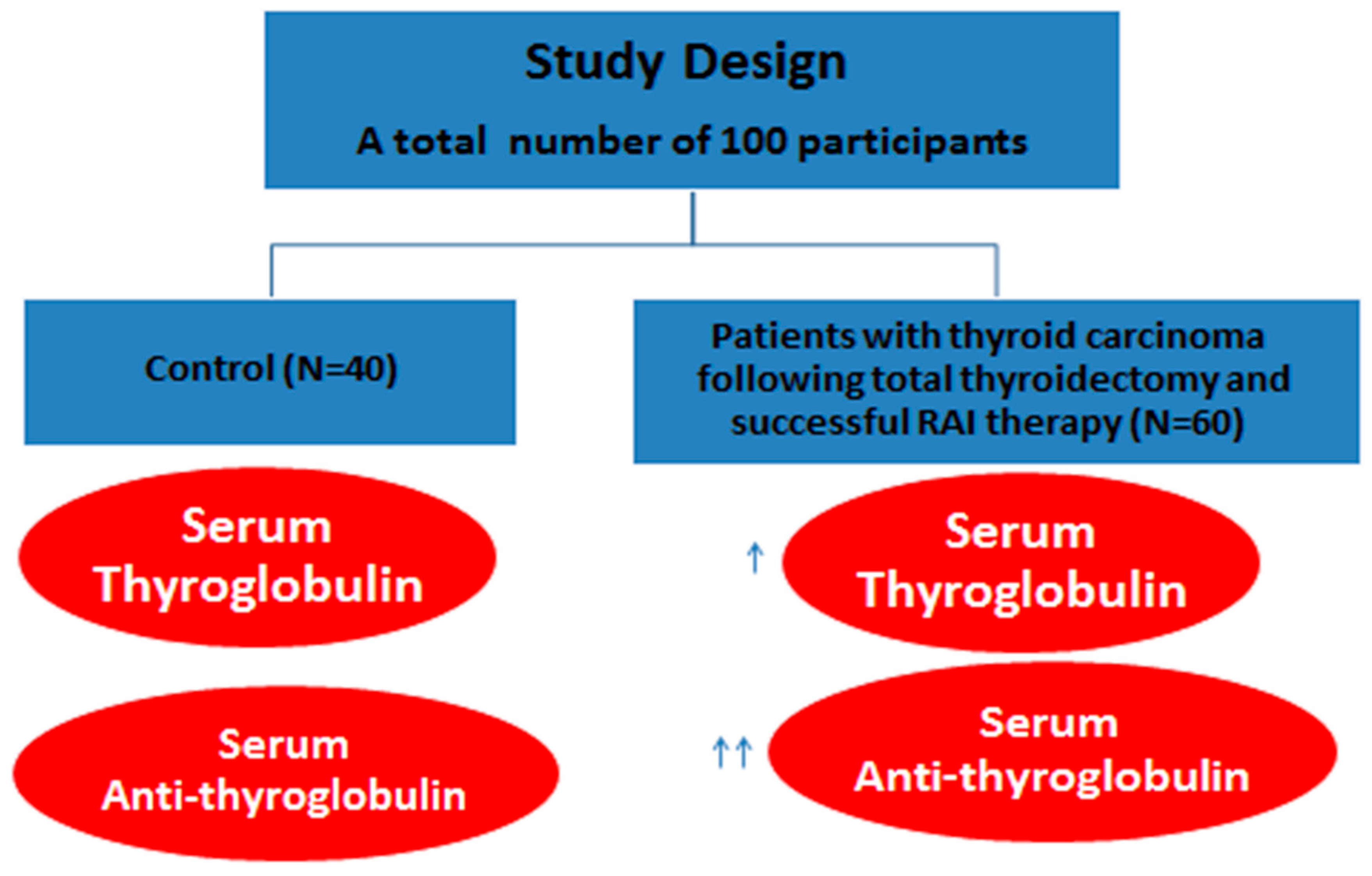
2. Subjects and Methods
2.1. Ethical Approval and Informed Consent Statement
2.2. Sampling
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Determination of Serum TG Levels Using the ELISA Technique
2.6. Determination of Serum TGAb Levels Using the ELISA Technique
2.7. Immunohistochemistry
2.7.1. Immunohistochemical Detection of TTFI
2.7.2. Detection of TG Immunohistochemically in the Tissue after Total Thyroidectomy
2.8. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawin, M.; Kurczyk, A.; Stobiecka, E.; Frątczak, K.; Polańska, J.; Pietrowska, M.; Widłak, P. Molecular heterogeneity of papillary thyroid cancer: Comparison of primary tumors and synchronous metastases in regional lymph nodes by mass spectrometry imaging. Endocr. Pathol. 2019, 30, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Siraj, A.K.; Masoodi, T.; Bu, R.; Beg, S.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Dawish, M.; Alkuraya, F.S.; Al-Kuraya, K.S. Genomic profiling of thyroid cancer reveals a role for thyroglobulin in metastasis. Am. J. Hum. Genet. 2016, 98, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Sabry, S.; El Hakim Ramadan, A.; Abd Elghany, M.; Okda, T.; Hasan, A. Formulation, characterization, and evaluation of the anti-tumor activity of nanosized galangin loaded niosomes on chemically induced hepatocellular carcinoma in rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102163. [Google Scholar] [CrossRef]
- Okda, T.M.; Abd-Εlghaffar, S.K.; Katary, M.A.; Abd-Alhaseeb, M.M. Chemopreventive and anticancer activities of indomethacin and vitamin D combination on colorectal cancer induced by 1, 2-dimethylhydrazine in rats. Biomed. Rep. 2021, 14, 1. [Google Scholar] [CrossRef]
- Tsushima, Y.; Miyauchi, A.; Ito, Y.; Kudo, T.; Masuoka, H.; Yabuta, T.; Fukushima, M.; Kihara, M.; Higashiyama, T.; Takamura, Y. Prognostic significance of changes in serum thyroglobulin antibody levels of pre-and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr. J. 2013, 60, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Turanli, S.; Mersin, H.H. Serum antithyroglobulin antibody levels are not a good predictive factor on detection of disease activity in patients with papillary thyroid carcinoma. J. Cancer Res. Ther. 2020, 16, 624. [Google Scholar] [CrossRef] [PubMed]
- Middendorp, M.; Grünwald, F. Update on Recent Developments in the Therapy of Differentiated Thyroid Cancer. Semin. Nucl. Med. 2010, 40, 145–152. [Google Scholar] [CrossRef]
- Zulewski, H.; Giovanella, L.; Bilz, S.; Christ, E.; Haldemann, A.; Steinert, H.; Weidner, S.E.; Oertli, D.; Triponez, F.; Clerici, T. Multidisciplinary approach for risk-oriented treatment of low-risk papillary thyroid cancer in Switzerland. Swiss Med. Wkly. 2019, 149, w14700. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Clark, P.M.; Chiovato, L.; Duntas, L.; Elisei, R.; Feldt-Rasmussen, U.; Leenhardt, L.; Luster, M.; Schalin-Jäntti, C.; Schott, M. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: A clinical position paper. Eur. J. Endocrinol. 2014, 171, R33–R46. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, S.Y.; Choe, J.-H.; Kim, J.S.; Hahn, S.Y.; Kim, S.W.; Chung, J.H.; Jung, J.; Kim, T.H. Preoperative serum thyroglobulin and its correlation with the burden and extent of differentiated thyroid cancer. Cancers 2020, 12, 625. [Google Scholar] [CrossRef] [Green Version]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldt-Rasmussen, U.; Rasmussen, Å.K. Autoimmunity in differentiated thyroid cancer: Significance and related clinical problems. Hormones 2010, 9, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Latrofa, F.; Ricci, D.; Grasso, L.; Vitti, P.; Masserini, L.; Basolo, F.; Ugolini, C.; Mascia, G.; Lucacchini, A.; Pinchera, A. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J. Clin. Endocrinol. Metab. 2008, 93, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E.; Wahl, R. Thyroid autoimmunity: Role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Kim, E.S.; Lim, D.J.; Baek, K.H.; Lee, J.M.; Kim, M.K.; Kwon, H.S.; Song, K.H.; Kang, M.I.; Cha, B.Y.; Lee, K.W. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 2010, 20, 885–891. [Google Scholar] [CrossRef]
- Giera, S.; Zoeller, R.T. Effects and predicted consequences of persistent and bioactive organic pollutants on thyroid function. Eff. Persistent Bioact. Org. Pollut. Hum. Health 2013, 203–236. [Google Scholar] [CrossRef]
- Fiore, E.; Latrofa, F.; Vitti, P. Iodine, thyroid autoimmunity and cancer. Eur. Thyroid. J. 2015, 4, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, J.; Amaya-Amaya, J.; Anaya, J. Chapter 30 Thyroid Disease and Autoimmune Diseases. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Jo, K.; Lim, D.-J. Clinical implications of anti-thyroglobulin antibody measurement before surgery in thyroid cancer. Korean J. Intern. Med. 2018, 33, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.; Luthringer, D.J.; Eisen, R.N. Thyroid transcription factor-1: A review. Appl. Immunohistochem. Mol. Morphol. 2002, 10, 97–102. [Google Scholar] [CrossRef]
- Hahn, H.P.; Bundock, E.A.; Hornick, J.L. Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. Am. J. Clin. Pathol. 2006, 125, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Morris, L.; Haymart, M.; Chen, A.; Goldenberg, D.; Morris, J.; Ogilvie, J.; Terris, D.; Netterville, J.; Wong, R.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocr. Pract. 2015, 21, 686–696. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.K.; Little, M.P.; Lubin, J.H.; Brenner, A.V.; Wells, S.A., Jr.; Sigurdson, A.J.; Nikiforov, Y.E. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014, 99, E276–E285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuz, S.; Puckett, Y. Iodine-131 Uptake Study. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Müller-Gärtner, H.W.; Schneider, C. Clinical evaluation of tumor characteristics predisposing serum thyroglobulin to be undetectable in patients with differentiated thyroid cancer. Cancer 1988, 61, 976–981. [Google Scholar] [CrossRef]
- Schlumberger, M.; Hitzel, A.; Toubert, M.; Corone, C.; Troalen, F.; Schlageter, M.; Claustrat, F.; Koscielny, S.; Taieb, D.; Toubeau, M. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J. Clin. Endocrinol. Metab. 2007, 92, 2487–2495. [Google Scholar] [CrossRef]
- Spencer, C.A.; LoPresti, J.S. Technology Insight: Measuring thyroglobulin and thyroglobulin autoantibody in patients with differentiated thyroid cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.; Petrovic, I.; Fatemi, S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Bachelot, A.; Cailleux, A.F.; Klain, M.; Baudin, E.; Ricard, M.; Bellon, N.; Caillou, B.; Travagli, J.P.; Schlumberger, M. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid 2002, 12, 707–711. [Google Scholar] [CrossRef]
- Ringel, M.D.; Nabhan, F. Approach to follow-up of the patient with differentiated thyroid cancer and positive anti-thyroglobulin antibodies. J. Clin. Endocrinol. Metab. 2013, 98, 3104–3110. [Google Scholar] [CrossRef] [Green Version]
- Giovanella, L.; Avram, A.M.; Clerc, J.; Hindié, E.; Taïeb, D.; Verburg, F.A. Postoperative Serum Thyroglobulin and Neck Ultrasound to Drive Decisions about Iodine-131 Therapy in Patients with Differentiated Thyroid Carcinoma: An Evidence-Based Strategy? Springer: Berlin, Germany, 2018. [Google Scholar]
- Rosario, P.W.; Carvalho, M.; Mourao, G.F.; Calsolari, M.R. Comparison of Antithyroglobulin Antibody Concentrations Before and After Ablation with 131I as a Predictor of Structural Disease in Differentiated Thyroid Carcinoma Patients with Undetectable Basal Thyroglobulin and Negative Neck Ultrasonography. Thyroid 2016, 26, 525–531. [Google Scholar] [CrossRef]
- Xu, S.; Huang, H.; Zhang, X.; Huang, Y.; Guan, B.; Qian, J.; Wang, X.; Liu, S.; Xu, Z.; Liu, J. Predictive Value of Serum Thyroglobulin for Structural Recurrence Following Lobectomy for Papillary Thyroid Carcinoma. Thyroid 2021, 31, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Sherman, S.I.; Pak, Y.; Litofsky, D.R.; Gianoukakis, A.G. The De Novo Detection of Anti-Thyroglobulin Antibodies and Differentiated Thyroid Cancer Recurrence. Thyroid 2020, 30, 1490–1495. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Froehlich, J.; Rendl, J.; Willnich, M.; Alonso, C.; Bergmann, A.; Reiners, C. Technical evaluation of a new immunoradiometric and a new immunoluminometric assay for thyroglobulin. Clin. Chem. 2002, 48, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachelot, A.; Leboulleux, S.; Baudin, E.; Hartl, D.M.; Caillou, B.; Travagli, J.P.; Schlumberger, M. Neck recurrence from thyroid carcinoma: Serum thyroglobulin and high-dose total body scan are not reliable criteria for cure after radioiodine treatment. Clin. Endocrinol. 2005, 62, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Giovanella, L.; Pignata, S.A.; Vento, A.; Alibrandi, A.; Sturiale, L.; Laudicella, R.; Comis, A.D.; Filice, R.; Giuffrida, G. Undetectable or low (<1 ng/mL) postsurgical thyroglobulin values do not rule out metastases in early stage differentiated thyroid cancer patients. Oncotarget 2018, 9, 17491. [Google Scholar] [CrossRef] [Green Version]
- Farooq, N.; Khan, M.I.; Raziq, F.; Naeem, S. Diagnostic utility of immunohistochemistry in subtyping acute lymphoblastic leukemia: A 2 years’experience. Khyber Med Univ. J. 2020, 12, 38–42. [Google Scholar]
- Yaziji, H.; Barry, T. Diagnostic Immunohistochemistry: What can go wrong? Adv. Anat. Pathol. 2006, 13, 238–246. [Google Scholar] [CrossRef]
- O’Hurley, G.; Sjöstedt, E.; Rahman, A.; Li, B.; Kampf, C.; Pontén, F.; Gallagher, W.M.; Lindskog, C. Garbage in, garbage out: A critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol. Oncol. 2014, 8, 783–798. [Google Scholar] [CrossRef]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 2016, 50, 411. [Google Scholar] [CrossRef] [Green Version]



| Type of Thyroid Carcinoma | Number of Cases | Percentage (%) |
|---|---|---|
| Papillary carcinoma and its variants | 52 | 86.6% |
| Follicular carcinoma | 7 | 11.6% |
| Hurthle cell type | 1 | 1.6% |
| Parameters | Control (n = 40) | Cases (n = 60) | Significance |
|---|---|---|---|
| Mean (SD) | |||
| Thyroglobulin (ng/mL) | 15.07 (2.13) | 23.65 (0.36) | p < 0.001 |
| Anti-TG (IU/mL) | 58.96 (2.45) | 136.84 (6.33) | NS |
| Control | Patients | |
|---|---|---|
| TSH (Mean ± SD) (µIU/mL) | 2.3 ± 1.1 | 0.96 ± 0.15 |
| Item | Mean ± SD | p Value | |
|---|---|---|---|
| Control | Thyroid Carcinoma | ||
| ALT (u/L) | 23.76 ± 5.63 | 33.50 ± 4.93 * | p < 0.001 |
| AST (u/L) | 25.85 ± 5.39 | 36.85 ± 5.11 * | p < 0.001 |
| Hb (g/dL) | 12.27 ± 0.60 | 11.04 ±.74 * | p < 0.001 |
| Hct | 41.30 ± 60.61 | 38.41 ± 2.59 | p = 0.187 |
| RBCs (106) | 5.30 ± 6.06 | 4.31 ± 0.46 | p = 0.213 |
| Platelets (103) | 259.86 ± 57.42 | 214.93 ± 41.83 * | p < 0.001 |
| WBCs (103) | 6.26 ± 1.41 | 5.34 ± 1.02 * | p < 0.001 |
| Neutrophils | 54.00 ± 5.46 | 51.96 ± 5.23 | p = 0.065 |
| Lymphocytes | 34.00 ± 5.46 | 36.03 ± 5.23 | p = 0.076 |
| Cutoff Point | AUC (SE) p Value | AUC (95% CI) | Accuracy (95% CI) | SN (95% CI) | SP (95% CI) | PPV (95% CI) | NPP (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| TG | ≥0.350 | 0.84 ± 0.08 p = 0.002 * | (0.679–1.0) | 93.3% (93.1–93.5%) | 57.1 (20.5–93.8) | 98.1 (94.5–100) | 80 (44.9–100) | 94.5 (88.5–100) |
| TGAb | ≥105.5 | 1(0) p < 0.001 * | (1–1) | 100% (100–100%) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) |
| Comparison of area under the ROC curve between both parameters AUC1–AUC2 | difference: areaA − areaB − 0.16 SE of the difference = 0.0962 Z = −1.6625 One-tailed p value 0.048 * | |||||||
| Pathology | Thyroglobulin (ng/mL) | Anti-TG (IU/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg < 1 | Tg 1–2 | Anti-Tg 5–10 | Anti-Tg 10–20 | Anti-Tg > 20 | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | p Value | |
| Capsular invasion (N = 7/60) | 5 | 27.8 | 2 | 40 | 6 | 33.3 | 1 | 33.3 | 0 | 0 | |
| Vascular invasion (N = 10/60) | 9 | 50 | 1 | 20 | 8 | 44.4 | 1 | 33.3 | 1 | 50 | 0.98 |
| LN metastasis (N = 6/60) Total 23/60 | 4 18 | 22.2 100 | 2 5 | 40 100 | 4 18 | 22.2 100 | 1 3 | 33.3 100 | 1 2 | 50 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, H.O.; Omran, G.A.; Gewely, A.G.; Eldehn, A.F.; Abdo, W.; Elmahallawy, E.K.; Okda, T.M. Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy. Diagnostics 2021, 11, 2080. https://doi.org/10.3390/diagnostics11112080
Zahra HO, Omran GA, Gewely AG, Eldehn AF, Abdo W, Elmahallawy EK, Okda TM. Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy. Diagnostics. 2021; 11(11):2080. https://doi.org/10.3390/diagnostics11112080
Chicago/Turabian StyleZahra, Hashem O., Gamal A. Omran, Ahmed G. Gewely, Ahmed Fathy Eldehn, Walied Abdo, Ehab Kotb Elmahallawy, and Tarek M. Okda. 2021. "Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy" Diagnostics 11, no. 11: 2080. https://doi.org/10.3390/diagnostics11112080
APA StyleZahra, H. O., Omran, G. A., Gewely, A. G., Eldehn, A. F., Abdo, W., Elmahallawy, E. K., & Okda, T. M. (2021). Prognostic Value of Serum Thyroglobulin and Anti-Thyroglobulin Antibody in Thyroid Carcinoma Patients following Thyroidectomy. Diagnostics, 11(11), 2080. https://doi.org/10.3390/diagnostics11112080







