Effect of Roller Pump Pulse in the Arterial Needle Area during Hemodialysis
Abstract
:1. Introduction
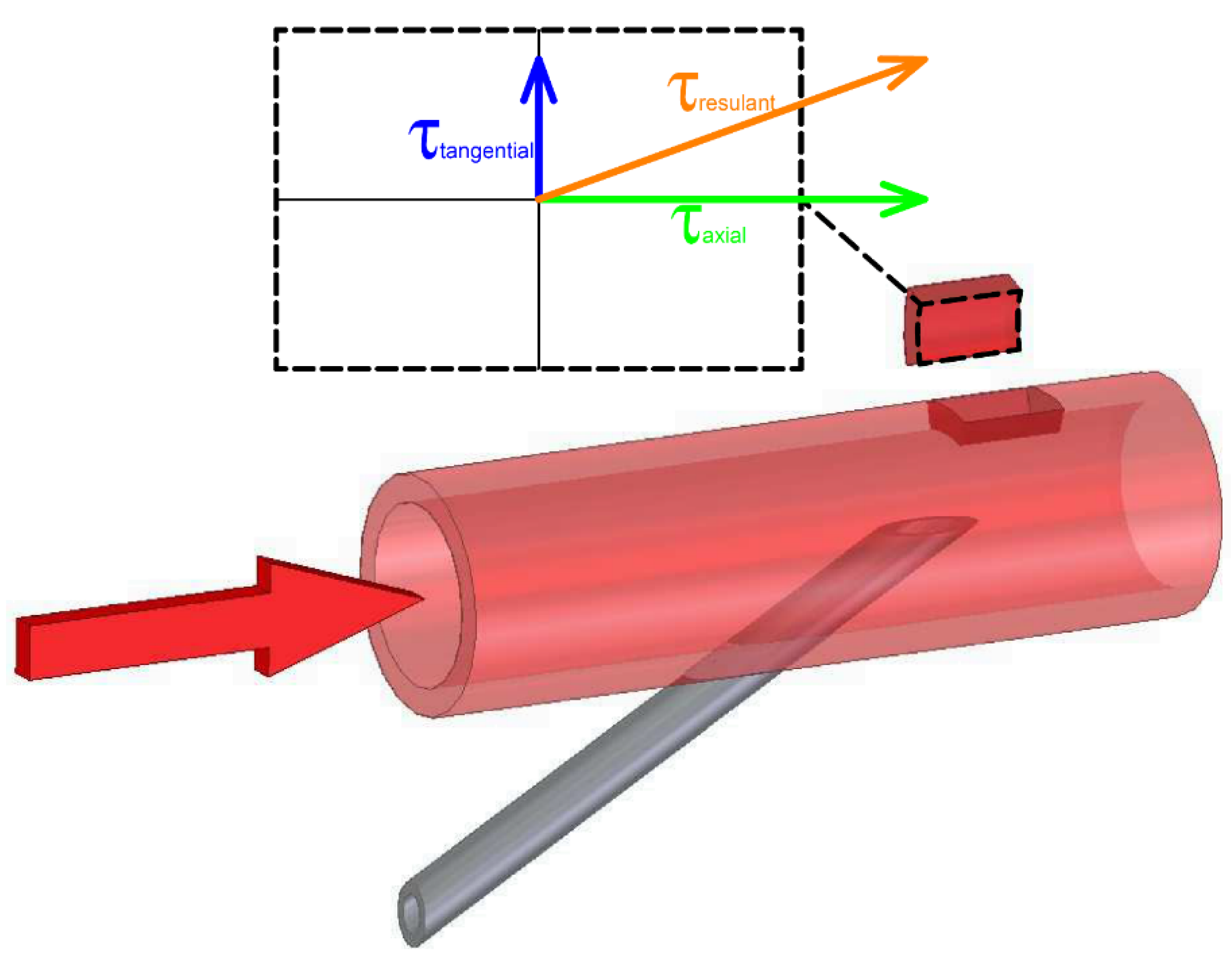
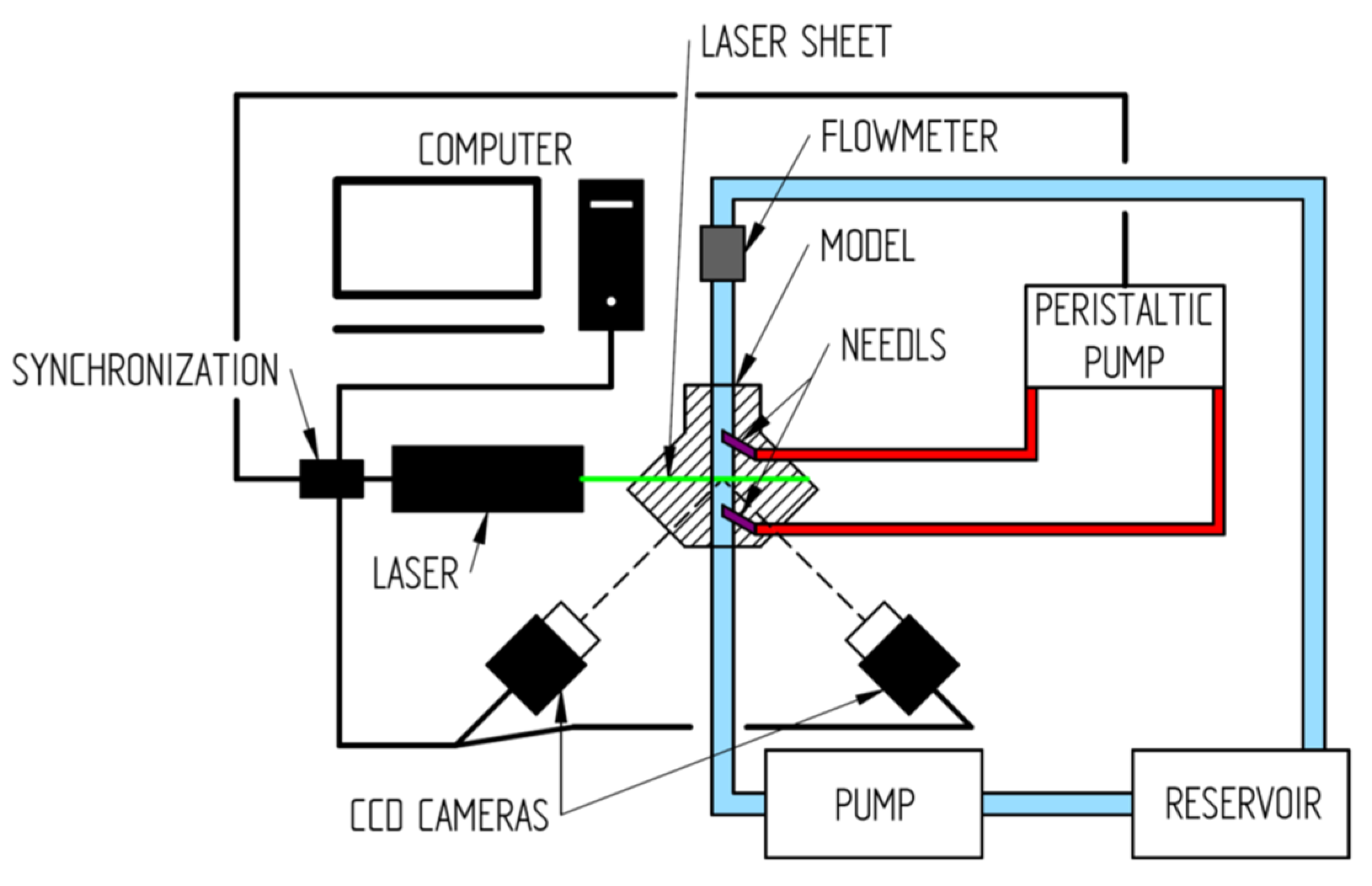
2. Materials and Methods
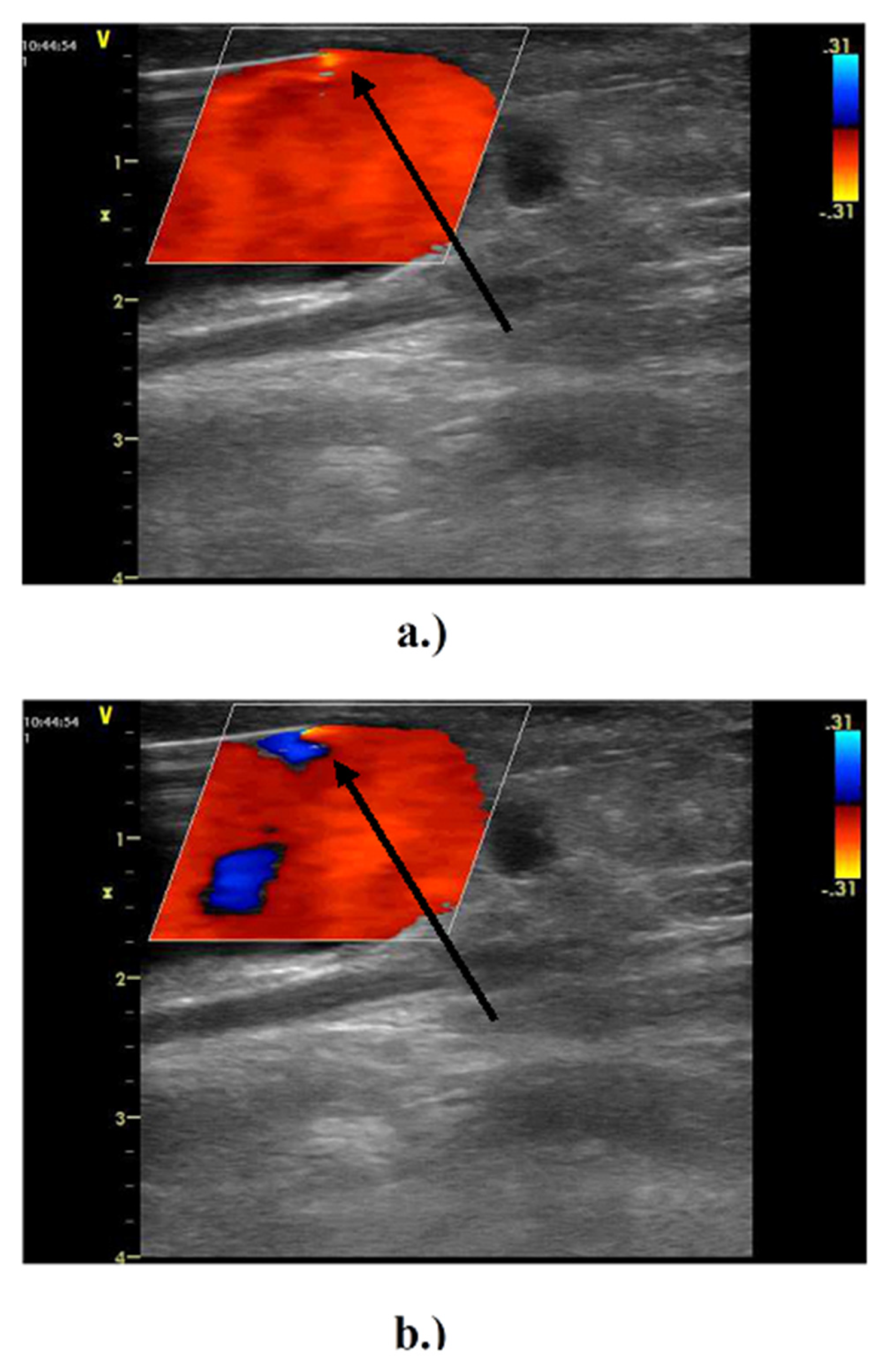
2.1. In Vivo Study
2.2. In Vitro Study
3. Results
3.1. In Vivo Experiment
3.2. In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ethier, J.; Mendelssohn, D.C.; Elder, S.J.; Hasegawa, T.; Akizawa, T.; Akiba, T.; Canaud, B.J.; Pisoni, R.L. Vascular access use and outcomes: An international perspective from the dialysis outcomes and practice patterns study. Nephrol. Dial. Transplant. 2008, 23, 3219–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Int. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- Yang, L.; Yin, A.; Liu, W. Variation of flow rate and angle of injected venous needle on influencing intimal hyperplasia at the venous anastomosis of the hemodialysis graft. Australas. Phys. Eng. Sci. Med. 2017, 40, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M. Hemo-Dynamics; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-241001-2. [Google Scholar]
- Sakariassen, K.S.; Orning, L.; Turitto, V.T. The impact of blood shear rate on arterial thrombus formation. Future Sci. OA 2015, 1, FSO30. [Google Scholar] [CrossRef] [Green Version]
- Malík, J.; Kudlicka, J.; Tuka, V.; Chytilova, E.; Adamec, J.; Ročínová, K.; Tesař, V. Common Carotid Wall Shear Stress and Carotid Atherosclerosis in End-Stage Renal Disease Patients. Physiol. Res. 2012, 61, 355–361. [Google Scholar] [CrossRef]
- Motomiya, M.; Karino, T. Flow patterns in the human carotid Artery bifurcation. Stroke 1984, 15, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Gijsen, F.J.H.; Wentzel, J.J.; Thury, A.; Mastik, F.; Schaar, J.A. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Heart Cirk. Physiol. 2008, 295, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Thim, T.; Hagensen, M.K.; Hørlyck, A.; Kim, W.Y.; Niemann, A.K.; Thrysøe, S.A.; Drouet, L.; Paaske, W.P.; Bøtker, H.E.; Falk, E. Wall shear stress and local plaque development in stenosed carotid arteries of hypercholesterolemic minipigs. J. Cardiovasc. Dis. Res. 2012, 3, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro, C.G.; Fitzgerald, J.M.; Schroter, R.C. Atheroma and Arterial Wall Shear StressObservations, Correlation and Proposal of a Shear Dependent Mass Transfer Mechanism for Atherogenesis. Proc. R. Soc. Lond. B 1971, 177, 105–159. [Google Scholar]
- Friedman, M.H.; Hutchins, G.M.; Bargeron, C.B.; Deters, O.J.; Mark, F.F. Correlation of Human Arterial Morphology with Hemodynamic Measurements in Arterial Casts. J. Biomech. Eng. 1981, 103, 204–207. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Karatzis, E.N.; Vavuranakis, M.; Lekakis, J.P.; Stefanadis, C. Assessment of wall shear stress and implications for atherosclerotic disease. Int. J. Cardiol. 2006, 113, 12–18. [Google Scholar] [CrossRef]
- Tuka, V.; Wijnen, E.; van der Sande, F.M.; Tordoir, J.H.M. Dialysis needle hemodynamics in arterio-venous fistulae: A technical report. J. Vasc. Access 2009, 10, 157–159. [Google Scholar] [CrossRef] [Green Version]
- Unnikrishnan, S.; Brott, B.C.; Ito, Y.; Cheng, G.C. Turbulent flow evaluation of the venous needle during hemodialysis. J. Biomech. Eng. 2005, 127, 1141–1146. [Google Scholar] [CrossRef]
- Fulker, D.; Kang, M.; Simmons, A.; Barber, T. The flow field near a venous needle in hemodialysis: A computational study. Hemodial. Int. 2013, 17, 602–611. [Google Scholar] [CrossRef]
- Fulker, D.; Simmons, A.; Barber, T. Computational Model of the Arterial and Venous Needle During Hemodialysis. J. Biomech. Eng. 2016, 139, 011005. [Google Scholar] [CrossRef] [PubMed]
- Fulker, D.; Ene-lordache, B.; Barber, T. High-Resolution Computational Fluid Dynamic Simulation of Haemodialysis Cannulation in a Patient-Specific Arteriovenous Fistula. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef] [PubMed]
- Novotný, J.; Nováková, L.; Machovská, I. Uncertainty Estimation Methods in Particle Image Velocimetry. In Proceedings of the EFM 19-Conference Proceedings, Franzensbad, Czech Republic, 19–22 November 2019. [Google Scholar]
- Novotný, J.; Nováková, L.; Machovská, I. Advanced metric for particle image velocimetry accuracy estimation. In Proceedings of the 19th Symposium on Application of Laser Techniques to Fluid Mechanics, Lisbon, Portugal, 16–19 July 2018; Technical University Lisboa: Lisbon, Portugal, 2018. [Google Scholar]
- Raffel, M.; Willert, C.E.; Wereley, S.T.; Kompenhans, J. Particle Image Velocimetry A Practical Guide, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; ISBN 978-3-540-72307-3. [Google Scholar]
- Kašpárek, M.; Nováková, L.; Adamec, J. UPIV methodology for small scale measurement. In Proceedings of the EFM 19-Conference, Franzensbad, Czech Republic, 19–22 November 2019. [Google Scholar]
- McEvoy, J.; Persoons, T. Aqueous Ammonium Thiosulfate as a working fluid for refractive index matched PIV applications. In Proceedings of the 9th World Conference on Experimental Heat Transfer, Fluid Mechanics and Thermodynamics, Foz do Iguaçu, Brazil, 11–15 June 2017. [Google Scholar]
- Bai, K.; Katz, J. On the refractive index of sodium iodide solutions for index matching in PIV. Exp. Fluids 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Narrow, T.L.; Yoda, M.; Abdel-Khalik, S.I. A simple model for the refractive index of sodium iodide aqueous solutions. Exp. Fluids 2000, 28, 282–283. [Google Scholar] [CrossRef]
- Yousif, M.Y.; Holdsworth, D.W.; Poepping, T.L. A blood-mimicking fluid for particle image velocimetry with silicone vascular models. Exp. Fluids 2011, 50, 769–774. [Google Scholar] [CrossRef]
- Pries, A.R.; Neuhaus, D.; Gaehtgens, P. Blood viscosity in tube flow: Dependence on diameter and hematocrit. Am. J. Physiol. Circ. Physiol. 1992, 263 Pt 2, H1770–H1778. [Google Scholar] [CrossRef] [PubMed]
- Fulker, D.; Keshavarzi, G.; Simmons, A.; Pugh, D.; Barber, T. Pulsatility Produced by Hemodialysis Roller Pump as Measured by Doppler Ultrasound. Artif. Organs 2015, 39, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, J.; Shelton, J.; Luo, X. Blood flow and damage by roller pumps during cardiopulmonary bypass. Fluids Struct. 2005, 20, 129–140. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasparek, M.; Novakova, L.; Malik, J. Effect of Roller Pump Pulse in the Arterial Needle Area during Hemodialysis. Diagnostics 2021, 11, 2010. https://doi.org/10.3390/diagnostics11112010
Kasparek M, Novakova L, Malik J. Effect of Roller Pump Pulse in the Arterial Needle Area during Hemodialysis. Diagnostics. 2021; 11(11):2010. https://doi.org/10.3390/diagnostics11112010
Chicago/Turabian StyleKasparek, Milos, Ludmila Novakova, and Jan Malik. 2021. "Effect of Roller Pump Pulse in the Arterial Needle Area during Hemodialysis" Diagnostics 11, no. 11: 2010. https://doi.org/10.3390/diagnostics11112010
APA StyleKasparek, M., Novakova, L., & Malik, J. (2021). Effect of Roller Pump Pulse in the Arterial Needle Area during Hemodialysis. Diagnostics, 11(11), 2010. https://doi.org/10.3390/diagnostics11112010






