Comparisons of Anti-dsDNA Antibody Detection Methods by Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assay in Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Measurement of Anti-dsDNA Antibody
2.3.1. CIA
2.3.2. ELISA
2.4. Clinical Parameters and Lab Data
2.5. SLEDAI
2.6. Pharmacologic Therapy
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics of Double-Positive, Double-Negative, and Inconsistent Groups
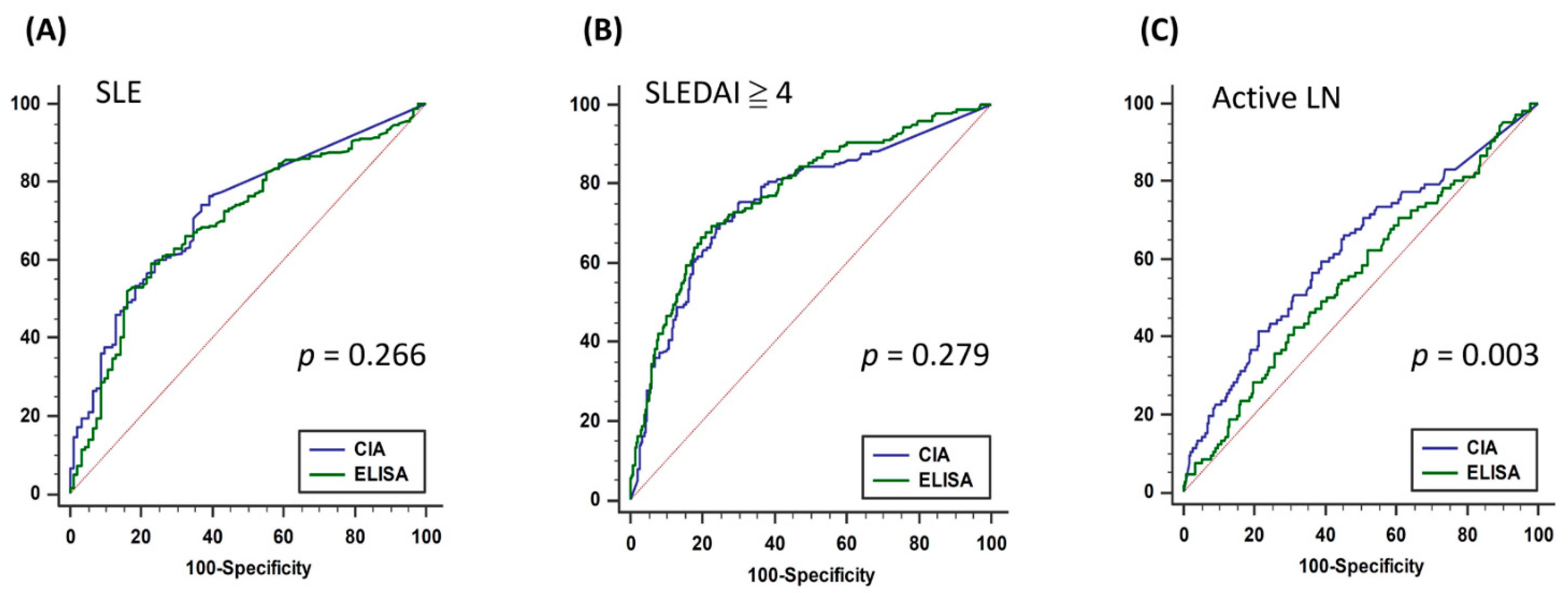
3.2. Consistency of CIA and ELISA and Diagnostic Accuracy of SLE, High Lupus Activity, and Active LN
3.3. Predictors for Inconsistent Results between CIA and ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maria, N.I.; Davidson, A. Emerging areas for therapeutic discovery in SLE. Curr. Opin. Immunol. 2018, 55, 1–8. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Mechanisms of Kidney Injury in Lupus Nephritis—The Role of Anti-dsDNA Antibodies. Front. Immunol. 2015, 6, 475. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Koffler, D.; Schur, P.H.; Kunkel, H.G. Immunological Studies Concerning the Nephritis of Systemic Lupus Erythematosus. J. Exp. Med. 1967, 126, 607–624. [Google Scholar] [CrossRef]
- Pan, N.; Amigues, I.; Lyman, S.; Duculan, R.; Aziz, F.; Crow, M.K.; Kirou, K.A. A surge in anti-dsDNA titer predicts a severe lupus flare within six months. Lupus 2014, 23, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Mummert, E.; Fritzler, M.J.; Sjöwall, C.; Bentow, C.; Mahler, M. The clinical utility of anti-double-stranded DNA antibodies and the challenges of their determination. J. Immunol. Methods 2018, 459, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Minden, P.; Anthony, B.F.; Farr, R.S. A comparison of seven procedures to detect the primary binding of antigen by antibody. J. Immunol. 1969, 102, 832–841. [Google Scholar] [PubMed]
- Venner, A.A.; Ibañez, D.; Gladman, D.D.; Urowitz, M.B.; MacKinnon, A.; Blasutig, I.M.; Yip, P.M. Comparison of three anti-dsDNA assays: Performance and correlation with systemic lupus erythematosus disease activity. Clin. Biochem. 2013, 46, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Compagno, M.; Jacobsen, S.; Rekvig, O.P.; Truedsson, L.; Heegaard, N.H.; Nossent, J.; Jönsen, A.; Jacobsen, R.S.; Eilertsen, G.Ø.; Bengtsson, A.A.; et al. Low diagnostic and predictive value of anti-dsDNA antibodies in unselected patients with recent onset of rheumatic symptoms: Results from a long-term follow-up Scandinavian multicentre study. Scand. J. Rheumatol. 2013, 42, 311–316. [Google Scholar] [CrossRef]
- Andrejevic, S.; Jeremic, I.; Sefik-Bukilica, M.; Nikolic, M.; Stojimirovic, B.; Bonaci-Nikolic, B. Immunoserological parameters in SLE: High-avidity anti-dsDNA detected by ELISA are the most closely associated with the disease activity. Clin. Rheumatol. 2013, 32, 1619–1626. [Google Scholar] [CrossRef]
- López-Hoyos, M.; Cabeza, R.; Martinez-Taboada, V.M.; Crespo, J.; SanSegundo, D.; Blanco, R.; López-Escribano, H.; Peña, M.; Rodriguez-Valverde, V. Clinical disease activity and titers of anti-dsDNA antibodies measured by an automated immunofluorescence assay in patients with systemic lupus erythematosus. Lupus 2005, 14, 505–509. [Google Scholar] [CrossRef]
- Feltkamp, T.E.; Kirkwood, T.B.; Maini, R.N.; Aarden, L.A. The first international standard for antibodies to double stranded DNA. Ann. Rheum. Dis. 1988, 47, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Enocsson, H.; Sjöwall, C.; Wirestam, L.; Dahle, C.; Kastbom, A.; Rönnelid, J.; Wetterö, J.; Skogh, T. Four Anti-dsDNA Antibody Assays in Relation to Systemic Lupus Erythematosus Disease Specificity and Activity. J. Rheumatol. 2015, 42, 817–825. [Google Scholar] [CrossRef]
- Bentow, C.; Lakos, G.; Martis, P.; Wahl, E.; Garcia, M.; Viñas, O.; Espinosa, G.; Cervera, R.; Sjöwall, C.; Mahler, M.; et al. International multi-center evaluation of a novel chemiluminescence assay for the detection of anti-dsDNA antibodies. Lupus 2016, 25, 864–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infantino, M.; Meacci, F.; Bentow, C.; Martis, P.; Benucci, M.; Afeltra, A.M.V.; Rigon, A.; Atzeni, F.; Sarzi-Puttini, P.; Manfredi, M.; et al. Clinical Comparison of QUANTA Flash dsDNA Chemiluminescent Immunoassay with Four Current Assays for the Detection of Anti-dsDNA Autoantibodies. J. Immunol. Res. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghiggeri, G.M.; D’Alessandro, M.; Bartolomeo, D.; Degl’Innocenti, M.L.; Magnasco, A.; Lugani, F.; Prunotto, M.; Bruschi, M. An Update on Antibodies to Necleosome Components as Biomarkers of Sistemic Lupus Erythematosus and of Lupus Flares. Int. J. Mol. Sci. 2019, 20, 5799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Orbai, A.-M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann-Vold, A.-M.; Gunnarsson, R.; Garen, T.; Midtvedt, Ø.; Molberg, Ø. Performance of the 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis (SSc) in Large, Well-defined Cohorts of SSc and Mixed Connective Tissue Disease. J. Rheumatol. 2015, 42, 60–63. [Google Scholar] [CrossRef]
- Xu, D.; Hou, Y.; Zheng, Y.; Zheng, Y.; Li, M.; Zeng, X. The 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis Could Classify Systemic Sclerosis Patients at Earlier Stage: Data from a Chinese EUSTAR Center. PLoS ONE 2016, 11, e0166629. [Google Scholar] [CrossRef]
- Hoogen, F.V.D.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Jr, T.A.M.; E Carreira, P.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Sag, E.; Demir, S.; Bilginer, Y.; Talim, B.; Haliloglu, G.; Ozen, S. Validation of the EULAR/ACR 2017 idiopathic inflammatory myopathy classification criteria in juvenile dermatomyositis patients. Clin. Exp. Rheumatol. 2021, 39, 688–694. [Google Scholar]
- E Lundberg, I.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; De Visser, M.; Alfredsson, L.; A Amato, A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Villalta, D.; Romelli, P.B.; Savina, C.; Bizzaro, N.; Tozzoli, R.; Tonutti, E.; Ghirardello, A.; Doria, A. Anti-dsDNA antibody avidity determination by a simple reliable ELISA method for SLE diagnosis and monitoring. Lupus 2003, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Anti-DNA antibodies-quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 2015, 12, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Seredkina, N.; Van Der Vlag, J.; Berden, J.; Mortensen, E.; Rekvig, O.P. Lupus Nephritis: Enigmas, Conflicting Models and an Emerging Concept. Mol. Med. 2013, 19, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, G.-S.; Wang, G.-H.; Li, X.-P. Anti-C1q antibody is a valuable biological marker for prediction of renal pathological characteristics in lupus nephritis. Clin. Rheumatol. 2012, 31, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Quaglini, S.; Radice, A.; Trezzi, B.; Raffiotta, F.; Messa, P.; Sinico, R.A. The Value of a Panel of Autoantibodies for Predicting the Activity of Lupus Nephritis at Time of Renal Biopsy. J. Immunol. Res. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Anders, H.J.; Saxena, R.; Zhao, M.-H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers. 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | QUANTA Lite® dsDNA | QUANTA Flash dsDNA |
|---|---|---|
| Technology | ELISA | CIA |
| Manufacturer | Inova Diagnostics | Inova Diagnostics |
| Detection | Semi-Quantitative | Quantitative |
| Assay time (minutes) | 90 | 30 |
| Analytical measuring range | 0–>370.5 WHO units/ml | 9.8–666.9 IU/mL |
| Cut-off value (range) | Negative 0–92.6 Equivocal 92.7–138.9 Moderate Positive 139–370.4 Strong Positive > 370.5 | Negative 9.8–27 Indeterminate 27–35 Positive > 35 |
| Antigen source | Calf thymus dsDNA | Synthetic dsDNA |
| Double-Negative (n = 259) | Inconsistent (n = 102) | Double-Positive (n = 141) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Age | 47.6 | (37.8–58.9) | 45.6 | (37.1–54.6) | 42.0 | (33.6–49.7) | <0.001 * |
| Gender | 0.013 * | ||||||
| Female | 214 | (82.6%) | 90 | (88.2%) | 131 | (92.9%) | |
| Male | 45 | (17.4%) | 12 | (11.8%) | 10 | (7.1%) | |
| Disease | <0.001 *#$ | ||||||
| SLE | 185 | (71.4%) | 93 | (91.2%) | 132 | (93.6%) | |
| Non-SLE | 74 | (28.6%) | 9 | (8.8%) | 9 | (6.4%) | |
| Lab data | |||||||
| Creatinine (mg/dL) | 0.375 | ||||||
| <1.4 | 220 | (85.3%) | 84 | (82.4%) | 125 | (88.7%) | |
| ≥1.4 | 38 | (14.7%) | 18 | (17.6%) | 16 | (11.3%) | |
| UPCR (mg/g) | 0.019 * | ||||||
| <500 | 167 | (78.8%) | 71 | (74.0%) | 90 | (65.2%) | |
| ≥500 | 45 | (21.2%) | 25 | (26.0%) | 48 | (34.8%) | |
| ANA | <0.001 * | ||||||
| <1:80 | 6 | (3.6%) | 3 | (4.3%) | 4 | (4.3%) | |
| 1:80–1:640 | 98 | (59.4%) | 32 | (46.4%) | 29 | (31.2%) | |
| ≥1:640 | 61 | (37.0%) | 34 | (49.3%) | 60 | (64.5%) | |
| Homogeneous (n = 281) | 78 | (43.6%) | 28 | (58.3%) | 38 | (70.4%) | 0.001 * |
| C3 (mg/dL) | <0.001 *# | ||||||
| <87 | 34 | (13.5%) | 38 | (37.6%) | 85 | (60.3%) | |
| ≥87 | 217 | (86.5%) | 63 | (62.4%) | 56 | (39.7%) | |
| C4 (mg/dL) | <0.001 *# | ||||||
| <19 | 63 | (25.2%) | 48 | (47.5%) | 94 | (66.7%) | |
| ≥19 | 187 | (74.8%) | 53 | (52.5%) | 47 | (33.3%) | |
| CIC (μg Eq/mL) | <0.001 *# | ||||||
| <10.8 | 140 | (90.3%) | 62 | (72.1%) | 66 | (53.2%) | |
| ≥10.8 | 15 | (9.7%) | 24 | (27.9%) | 58 | (46.8%) | |
| CIA (IU/mL) | 9.8 | (9.8–15.9) | 45.6 | (22.5–85.7) | 140.5 | (78.2–254.8) | <0.001 *#$ |
| ELISA (WHO units/mL) | 18.1 | (10.1–42.4) | 106.7 | (66.1–177.3) | 284.6 | (207.3–379.2) | <0.001 *#$ |
| SLEDAI | 0.0 | (0.0–2.0) | 2.0 | (0.0–4.0) | 4.0 | (4.0–8.0) | <0.001 *#$ |
| Drug | |||||||
| Glucocorticoid | 215 | (83.0%) | 93 | (91.2%) | 132 | (93.6%) | 0.004 * |
| Hydroxychloroquine | 201 | (77.6%) | 93 | (91.2%) | 133 | (94.3%) | <0.001 * |
| Cyclophosphamide | 57 | (22.0%) | 35 | (34.3%) | 42 | (29.8%) | 0.036 |
| Mycophenolic acid | 57 | (22.0%) | 57 | (40.4%) | 36 | (35.3%) | <0.001 * |
| Azathioprine | 117 | (45.2%) | 65 | (63.7%) | 106 | (75.2%) | <0.001 * |
| Methotrexate | 63 | (24.3%) | 21 | (20.6%) | 34 | (24.1%) | 0.738 |
| Cyclosporin | 47 | (18.1%) | 25 | (24.5%) | 36 | (25.5%) | 0.163 |
| CIA | Kappa Value | ||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| ELISA | 0.571 | ||||
| Negative | 259 | (51.6%) | 65 | (12.9%) | |
| Positive | 37 | (7.4%) | 141 | (28.1%) | |
| Outcome: SLE, n = 502 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | AUC | (95% CI) | p-Value | Optimal Cutoff | Sensitivity | Specificity | Accuracy | PPV | NPV |
| CIA | 0.723 | (0.682–0.762) | <0.001 | >10.1 | 76.3% | 60.9% | 73.5% | 89.7% | 36.6% |
| ELISA | 0.696 | (0.654–0.736) | <0.001 | >61.4 | 59.0% | 77.2% | 62.4% | 92.0% | 29.7% |
| Outcome: SLE & SLEDAI ≥ 4, n = 410 | |||||||||
| CIA | 0.757 | (0.712–0.797) | <0.001 | >40.3 | 70.0% | 75.2% | 72.9% | 68.9% | 76.2% |
| ELISA | 0.777 | (0.734–0.817) | <0.001 | >133.7 | 66.7% | 80.4% | 74.4% | 72.7% | 75.5% |
| Outcome: SLE & UPCR ≥ 500, n = 410 | |||||||||
| CIA | 0.620 | (0.570–0.668) | <0.001 | >28.0 | 66.0% | 54.8% | 57.8% | 34.7% | 81.6% |
| ELISA | 0.555 | (0.505–0.605) | 0.095 | >166.2 | 42. 5% | 68.8% | 61.8% | 33.1% | 76.7% |
| Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Odds Ratio | 95%CI | p-Value | Odds Ratio | 95%CI | p-Value | ||
| Age | 1.03 | (1.01–1.05) | 0.010 * | ||||
| Female | Reference | ||||||
| Male | 1.75 | (0.72–4.22) | 0.215 | ||||
| Creatine < 1.4 (mg/dL) | Reference | ||||||
| Creatine ≥ 1.4 (mg/dL) | 1.67 | (0.81–3.47) | 0.165 | ||||
| UPCR < 500 (mg/g) | Reference | ||||||
| UPCR ≥ 500 (mg/g) | 0.66 | (0.37–1.17) | 0.157 | ||||
| ANA < 1:80 | Reference | ||||||
| ANA 1:80–1:640 | 1.47 | (0.30–7.14) | 0.632 | ||||
| ANA ≥ 1:640 | 0.76 | (0.16–3.58) | 0.724 | ||||
| ANA Homogeneous (n = 281) | 0.59 | (0.26–1.34) | 0.206 | ||||
| C3 ≥ 87 (mg/dL) | Reference | Reference | |||||
| C3 < 87 (mg/dL) | 0.40 | (0.24–0.67) | 0.001 ** | 0.93 | (0.40–2.13) | 0.861 | |
| C4 ≥ 19 (mg/dL) | Reference | Reference | |||||
| C4 < 19 (mg/dL) | 0.45 | (0.27–0.77) | 0.003 ** | 1.04 | (0.43–2.51) | 0.930 | |
| CIC < 10.8 (μg Eq/mL) | Reference | Reference | |||||
| CIC ≥ 10.8 (μg Eq/mL) | 0.44 | (0.24–0.79) | 0.006 ** | 0.42 | (0.18–0.94) | 0.036 * | |
| Anti-dsDNA antibody by CIA (IU/mL) | 0.98 | (0.98–0.99) | <0.001 ** | ||||
| Anti-dsDNA antibody by ELISA (WHO units/mL) | 0.98 | (0.98–0.99) | <0.001 ** | 0.98 | (0.98–0.99) | <0.001 ** | |
| SLEDAI < 4 | Reference | Reference | |||||
| SLEDAI ≥ 4 | 0.22 | (0.12–0.39) | <0.001 ** | 0.33 | (0.14–0.79) | 0.013 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-C.; Wu, Y.-C.; Chen, J.-P.; Wu, Y.-D.; Huang, W.-N.; Chen, Y.-H.; Chen, Y.-M. Comparisons of Anti-dsDNA Antibody Detection Methods by Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assay in Systemic Lupus Erythematosus. Diagnostics 2021, 11, 1940. https://doi.org/10.3390/diagnostics11111940
Chang H-C, Wu Y-C, Chen J-P, Wu Y-D, Huang W-N, Chen Y-H, Chen Y-M. Comparisons of Anti-dsDNA Antibody Detection Methods by Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assay in Systemic Lupus Erythematosus. Diagnostics. 2021; 11(11):1940. https://doi.org/10.3390/diagnostics11111940
Chicago/Turabian StyleChang, Huang-Chen, Yen-Ching Wu, Jun-Peng Chen, Yi-Da Wu, Wen-Nan Huang, Yi-Hsing Chen, and Yi-Ming Chen. 2021. "Comparisons of Anti-dsDNA Antibody Detection Methods by Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assay in Systemic Lupus Erythematosus" Diagnostics 11, no. 11: 1940. https://doi.org/10.3390/diagnostics11111940
APA StyleChang, H.-C., Wu, Y.-C., Chen, J.-P., Wu, Y.-D., Huang, W.-N., Chen, Y.-H., & Chen, Y.-M. (2021). Comparisons of Anti-dsDNA Antibody Detection Methods by Chemiluminescent Immunoassay and Enzyme-Linked Immunosorbent Assay in Systemic Lupus Erythematosus. Diagnostics, 11(11), 1940. https://doi.org/10.3390/diagnostics11111940









