Margin Status Post Cervical Conization Predicts Residual Adenocarcinoma In Situ (AIS) and Occult Adenocarcinoma in a Predominantly Hispanic Population
Abstract
:1. Introduction
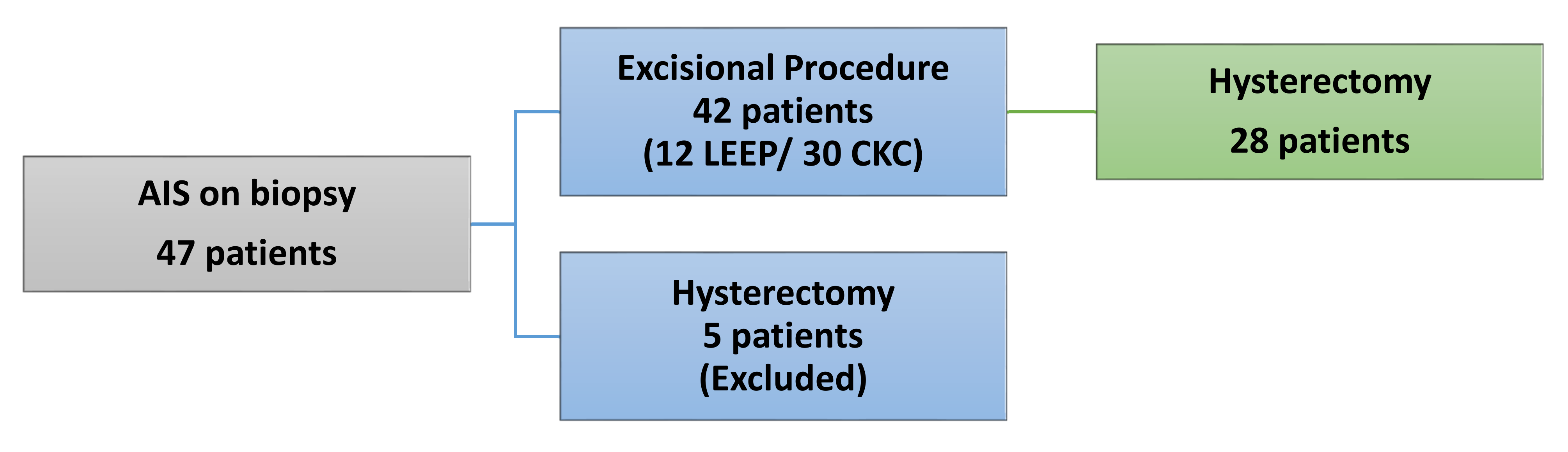
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Concurrent Invasive Adenocarcinoma upon Initial Presentation
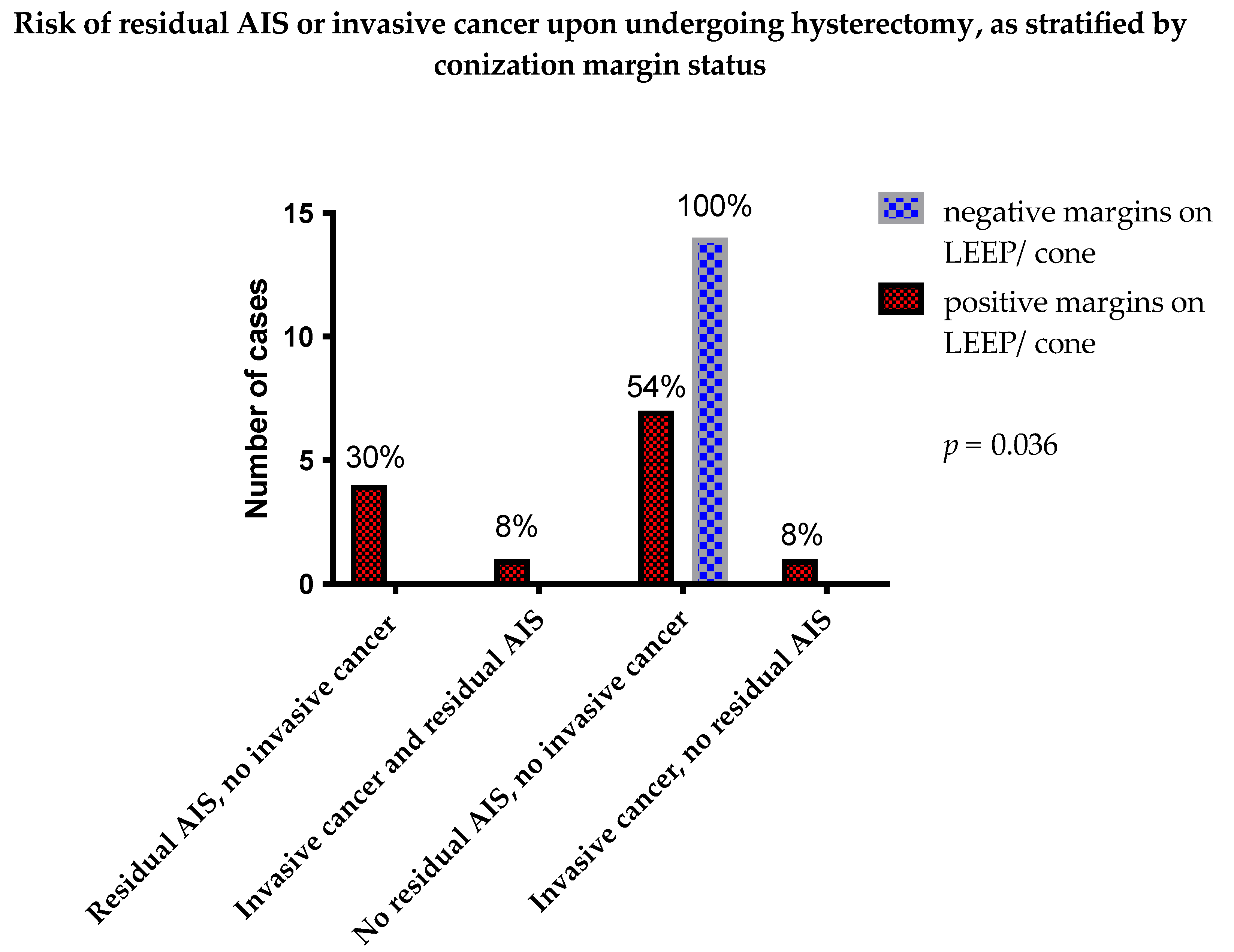
3.3. Positive Margins and Residual AIS on Subsequent Hysterectomy
3.4. Positive Margins and Post Excision Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satterwhite, C.L.; Torrone, E.; Meites, E.; Dunne, E.F.; Mahajan, R.; Ocfemia, M.C.B.; Su, J.; Fujie, X.; Weinstock, H. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm. Dis. 2013, 40, 187–193. [Google Scholar] [CrossRef]
- Bosch, F.X.; de Sanjose, S. Human papillomavirus in cervical cancer. Curr. Oncol. Rep. 2002, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegoke, O.; Kulasingam, S.; Virnig, B. Cervical Cancer Trends in the United States: A 35-Year Population-Based Analysis. J. Women’s Health 2012, 21, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Saraiya, M.; Benard, V.; Coughlin, S.S.; Flowers, L.; Cokkinides, V.; Schwenn, M.; Huang, Y.; Giuliano, A. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008, 113, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Flynn, C. Early invasive adenocarcinoma of the cervix. Cancer 2000, 89, 1048–1055. [Google Scholar] [CrossRef]
- Munro, A.; Codde, J.; Spilsbury, K.; Steel, N.; Stewart, C.J.; Salfinger, S.G.; Tan, J.; Mohan, G.R.; Leung, Y.; Semmens, J.B.; et al. Risk of persistent and recurrent cervical neoplasia following incidentally detected adenocarcinoma in situ. Am. J. Obstet. Gynecol. 2017, 216, 272.e1–272.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.O.; Tiffany, M.F.; Qualls, C.R.; Key, C.R. The Rising Incidence of Adenocarcinoma Relative to Squamous Cell Carcinoma of the Uterine Cervix in the United States—A 24-Year Population-Based Study. Gynecol. Oncol. 2000, 78, 97–105. [Google Scholar] [CrossRef]
- Wang, S.S.; Sherman, M.E.; Hildesheim, A.; Lacey, J.V.; Devesa, S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 2004, 100, 1035–1044. [Google Scholar] [CrossRef]
- Dunton, C.J. Management of Atypical Glandular Cells and Adenocarcinoma in Situ. Obstet. Gynecol. Clin. N. Am. 2008, 35, 623–632. [Google Scholar] [CrossRef]
- Shin, C.H.; Schorge, J.O.; Lee, K.R.; Sheets, E.E. Conservative Management of Adenocarcinoma in Situ of the Cervix. Gynecol. Oncol. 2000, 79, 6–10. [Google Scholar] [CrossRef]
- Denehy, T.R.; Gregori, C.A.; Breen, J.L. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet. Gynecol. 1997, 90, 1–6. [Google Scholar] [CrossRef]
- Plaxe, S.C.; Saltzstein, S.L. Estimation of the Duration of the Preclinical Phase of Cervical Adenocarcinoma Suggests That There Is Ample Opportunity for Screening. Gynecol. Oncol. 1999, 75, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Salani, R.; Puri, I.; Bristow, R.E. Adenocarcinoma in situ of the uterine cervix: A metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am. J. Obstet. Gynecol. 2009, 200, 182.e1–182.e5. [Google Scholar] [CrossRef] [PubMed]
- Teoh, D.; Musa, F.; Salani, R.; Huh, W.; Jimenez, E. Diagnosis and Management of Adenocarcinoma in Situ: A Society of Gynecologic Oncology Evidence-Based Review and Recommendations. Obstet. Gynecol. 2020, 135, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleveland, A.A.; Gargano, J.W.; Park, I.U.; Griffin, M.R.; Niccolai, L.M.; Powell, M.; Bennett, N.M.; Saadeh, K.; Pemmaraju, M.; Higgins, K.; et al. Cervical adenocarcinoma in situ: Human papillomavirus types and incidence trends in five states, 2008–2015. Int. J. Cancer 2020, 146, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hariri, S.; Steinau, M.; Rinas, A.; Gargano, J.W.; Ludema, C.; Unger, E.R.; Carter, A.L.; Grant, K.L.; Bamberg, M.; McDermott, J.E.; et al. HPV Genotypes in High Grade Cervical Lesions and Invasive Cervical Carcinoma as Detected by Two Commercial DNA Assays, North Carolina, 2001–2006. PLoS ONE 2012, 7, e34044. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef] [Green Version]
- Ostör, A.G. Early Invasive Adenocarcinoma of the Uterine Cervix. Int. J. Gynecol. Pathol. 2000, 19, 29–38. [Google Scholar] [CrossRef]
- Östör, A.G.; Duncan, A.; Quinn, M.; Rome, R. Adenocarcinoma in Situ of the Uterine Cervix: An Experience with 100 Cases. Gynecol. Oncol. 2000, 79, 207–210. [Google Scholar] [CrossRef]
- Krivak, T.C.; Rose, G.S.; McBroom, J.W.; Carlson, J.W.; Winter, W.E.; Kost, E.R. Cervical Adenocarcinoma in Situ: A Systematic Review of Therapeutic Options and Predictors of Persistent or Recurrent Disease. Obstet. Gynecol. Surv. 2001, 56, 567–575. [Google Scholar] [CrossRef]
- Soutter, W.P.; Haidopoulos, D.; Gornall, R.J.; McIndoe, G.A.; Fox, J.; Mason, W.P.; Flangan, A.; Nicholas, N.; Baker, F.; Abrahams, J.; et al. Is conservative treatment for adenocarcinoma in situ of the cervix safe? BJOG 2001, 108, 1184–1189. [Google Scholar]
- Costales, A.B.; Milbourne, A.M.; Rhodes, H.E.; Munsell, M.F.; Wallbillich, J.J.; Brown, J.; Frumovitz, M.; Ramondetta, L.M.; Schmeler, K.M. Risk of residual disease and invasive carcinoma in women treated for adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2013, 129, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.H.; Malloy, M.J.; Thangamani, R.; Gertig, D.; Drennan, K.T.; Wrede, C.D.; Saville, M.; Quinn, M. Management and long-term outcomes of women with adenocarcinoma in situ of the cervix: A retrospective study. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Srisomboon, J.; Kietpeerakool, C.; Suprasert, P.; Siriaunkgul, S.; Khunamornpong, S.; Prompittayarat, W. Factors affecting residual lesion in women with cervical adenocarcinoma in situ after cone excisional biopsy. Asian Pac. J. Cancer Prev. 2007, 8, 225–228. [Google Scholar] [PubMed]
- Tierney, K.E.; Lin, P.S.; Amezcua, C.; Matsuo, K.; Ye, W.; Felix, J.C.; Roman, L.D. Cervical conization of adenocarcinoma in situ: A predicting model of residual disease. Am. J. Obstet. Gynecol. 2014, 210, 366–e1. [Google Scholar] [CrossRef]
- Young, J.L.; Jazaeri, A.A.; Lachance, J.A.; Stoler, M.H.; Irvin, W.P.; Rice, L.W.; Andersen, W.A.; Modesitt, S.C. Cervical adenocarcinoma in situ: The predictive value of conization margin status. Am. J. Obstet. Gynecol. 2007, 197, 195–e1. [Google Scholar] [CrossRef] [PubMed]
- Baalbergen, A.; Helmerhorst, T.J. Adenocarcinoma in Situ of the Uterine Cervix—A Systematic Review. Int. J. Gynecol. Cancer 2014, 24, 1543–1548. [Google Scholar] [CrossRef]
- Bull-Phelps, S.L.; Garner, E.I.; Walsh, C.S.; Gehrig, P.A.; Miller, D.; Schorge, J.O. Fertility-sparing surgery in 101 women with adenocarcinoma in situ of the cervix. Gynecol. Oncol. 2007, 107, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Codde, E.; Munro, A.; Stewart, C.J.R.; Spilsbury, K.; Bowen, S.; Codde, J.; Steel, N.; Leung, Y.; Salfinger, S.G.; Mohan, G.R.; et al. Risk of persistent or recurrent cervical neoplasia in patients with ‘pure’ adenocarcinoma-in-situ (AIS) or mixed AIS and high-grade cervical squamous neoplasia (cervical intra-epithelial neoplasia grades 2 and 3 (CIN 2/3)): A population-based study. BJOG 2018, 125, 74–79. [Google Scholar] [CrossRef] [Green Version]
- US Census Bureau. Quick Facts; San Bernadino County: San Bernadino, CA, USA, 2020. [Google Scholar]
- DeSimone, C.P.; Day, M.; Dietrich, C.S.; Tovar, M.M.; Modesitt, S.C. Risk for residual adenocarcinoma in situ or cervical adenocarcinoma in women undergoing loop electrosurgical excision procedure/conization for adenocarcinoma in situ. J. Reprod. Med. 2011, 56, 376–380. [Google Scholar] [PubMed]
- Im, D.D.; Duska, L.R.; Rosenshein, N.B. Adequacy of conization margins in adenocarcinoma in situ of the cervix as a predictor of residual disease. Gynecol. Oncol. 1995, 59, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.S.; Mani, A. The Status and Distance of Cone Biopsy Margins as a Predictor of Excision Adequacy for Endocervical Adenocarcinoma In Situ. Am. J. Clin. Pathol. 1998, 109, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Kietpeerakool, C.; Khunamornpong, S.; Srisomboon, J.; Kasunan, A.; Sribanditmongkol, N.; Siriaungkul, S. Predictive value of negative cone margin status for risk of residual disease among women with cervical adenocarcinoma in situ. Int. J. Gynecol. Obstet. 2012, 119, 266–269. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) Median: 37 (23–71) | Race (n = 47) | High Risk HPV Status (n = 47) | Parity (n = 46) | ||||
|---|---|---|---|---|---|---|---|
| 23–34 | 19 (40%) | Hispanic | 23 (49%) | Positive | 21 (45%) | 0 | 9 (19%) |
| 35–44 | 14 (30%) | White | 21 (45%) | Negative | 2 (4%) | 1 | 9 (19%) |
| 45–64 | 12 (26%) | Asian | 2 (4%) | Unknown | 24 (51%) | 2 | 17 (36%) |
| 65+ | 2 (4%) | Black | 1 (2%) | 3+ | 11 (23%) | ||
| Hysterectomy Specimens | Conization Specimens | Total | |
|---|---|---|---|
| Positive Margins on LEEP/Cone | Negative Margins on LEEP/Cone | ||
| Residual AIS | 4 (30%) | 0 (0%) | |
| Residual AIS and residual invasive cancer | 1 (8%) | 0 (0%) | 5 (19%) |
| No residual AIS | 7 (54%) | 14 (100%) | |
| Invasive cancer, no residual AIS | 1 (8%) | 0 (0%) | 22 (81%) |
| Total | 13 (100%) | 14 (100%) | 27 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.J.; Huynh, S.; Kim, J.; Denham, L.; Momeni, M.; Ioffe, Y.J.M. Margin Status Post Cervical Conization Predicts Residual Adenocarcinoma In Situ (AIS) and Occult Adenocarcinoma in a Predominantly Hispanic Population. Diagnostics 2021, 11, 1889. https://doi.org/10.3390/diagnostics11101889
Hong LJ, Huynh S, Kim J, Denham L, Momeni M, Ioffe YJM. Margin Status Post Cervical Conization Predicts Residual Adenocarcinoma In Situ (AIS) and Occult Adenocarcinoma in a Predominantly Hispanic Population. Diagnostics. 2021; 11(10):1889. https://doi.org/10.3390/diagnostics11101889
Chicago/Turabian StyleHong, Linda J., Sandy Huynh, Joy Kim, Laura Denham, Mazdak Momeni, and Yevgeniya J. M. Ioffe. 2021. "Margin Status Post Cervical Conization Predicts Residual Adenocarcinoma In Situ (AIS) and Occult Adenocarcinoma in a Predominantly Hispanic Population" Diagnostics 11, no. 10: 1889. https://doi.org/10.3390/diagnostics11101889
APA StyleHong, L. J., Huynh, S., Kim, J., Denham, L., Momeni, M., & Ioffe, Y. J. M. (2021). Margin Status Post Cervical Conization Predicts Residual Adenocarcinoma In Situ (AIS) and Occult Adenocarcinoma in a Predominantly Hispanic Population. Diagnostics, 11(10), 1889. https://doi.org/10.3390/diagnostics11101889





