Evaluation of CT-Guided Ultra-Low-Dose Protocol for Injection Guidance in Preparation of MR-Arthrography of the Shoulder and Hip Joints in Comparison to Conventional and Low-Dose Protocols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
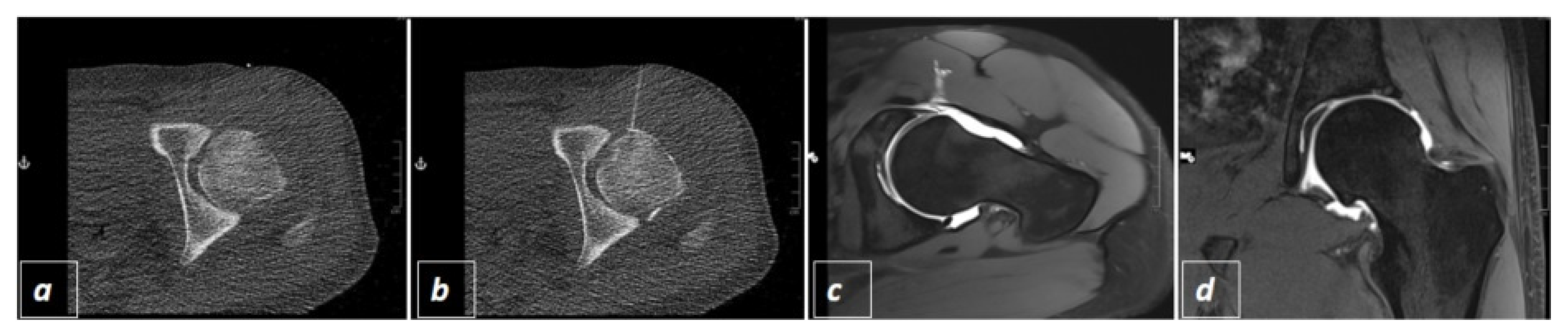
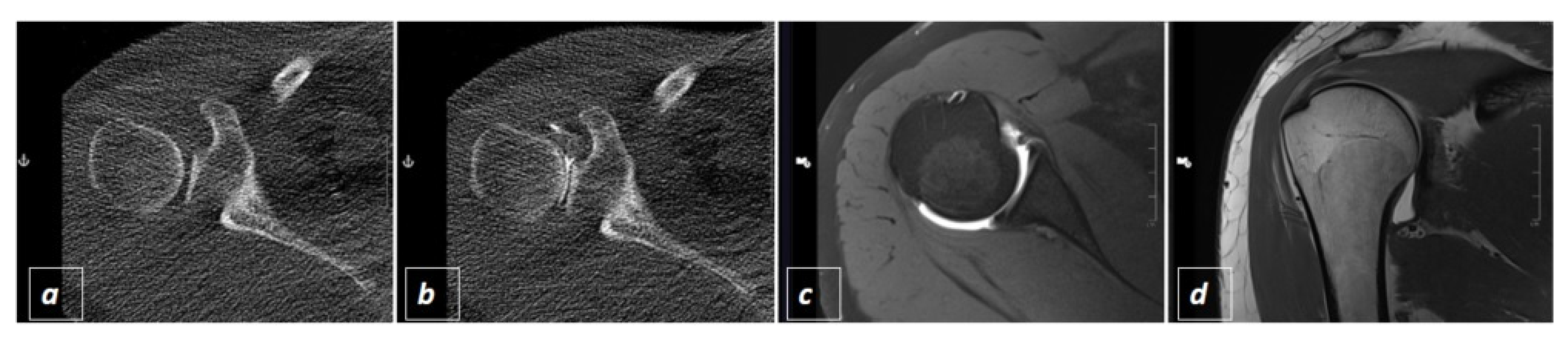
2.2. CT-Guided Joint Injections
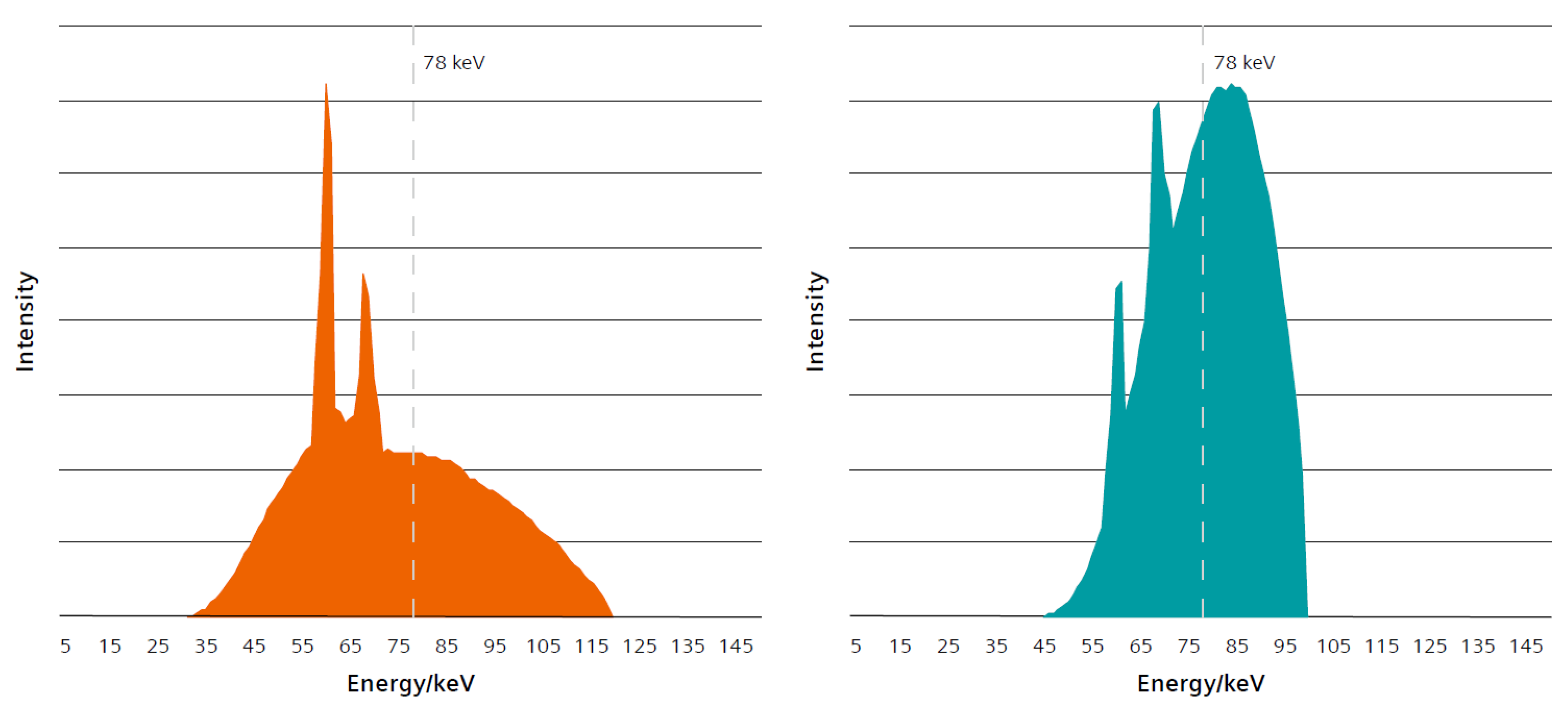
2.3. The Three Different Examination Protocols
2.4. Radiation Exposure and Interventional Success
2.5. Statistical Analysis
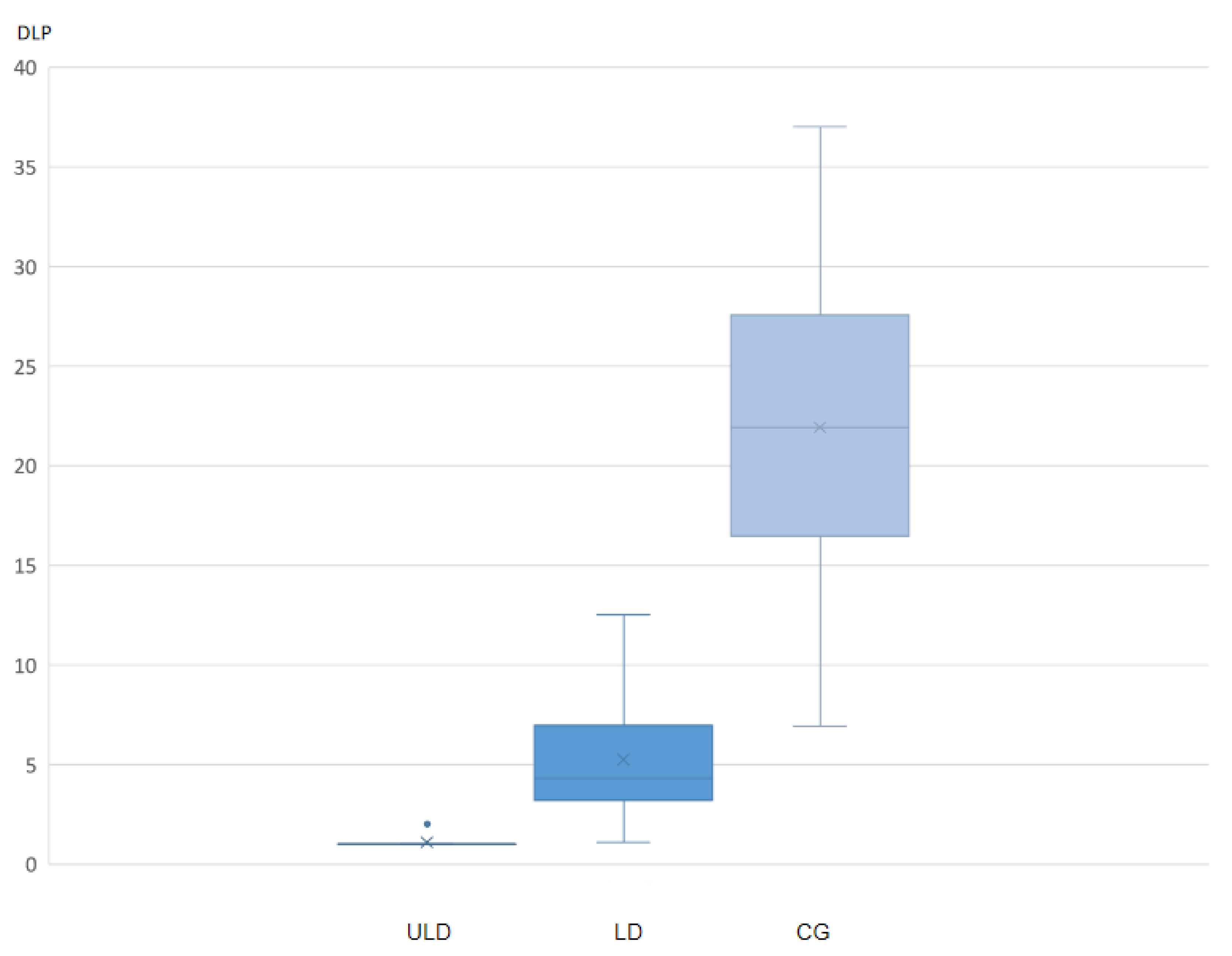
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Symanski, J.S.; Subhas, N.; Babb, J.; Nicholson, J.; Gyftopoulos, S. Diagnosis of superior labrum anterior-to-posterior tears by using Mr imaging and Mr arthrography: A systematic review and meta-analysis. Radiology 2017, 285, 101–113. [Google Scholar] [CrossRef]
- Sutter, R.; Zubler, V.; Hoffmann, A.; Mamisch-Saupe, N.; Dora, C.; Kalberer, F.; Zanetti, M.; Hodler, J.; Pfirrmann, C.W. Hip MRI: How useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am. J. Roentgenol. 2014, 202, 160–169. [Google Scholar] [CrossRef]
- Agten, C.A.; Sutter, R.; Buck, F.M.; Pfirrmann, C.W. Hip imaging in athletes: Sports imaging series. Radiology 2016, 280, 351–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sconfienza, L.M.; Albano, D.; Messina, C.; Silvestri, E.; Tagliafico, A.S. How, when, why in magnetic resonance arthrography: An international survey by the european society of musculoskeletal radiology (ESSR). Eur Radiol. 2018, 28, 2356–2368. [Google Scholar] [CrossRef]
- Mulligan, M.E. CT-guided shoulder arthrography at the rotator cuff interval. AJR Am. J. Roentgenol. 2008, 191, W58–W61. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.; Akhan, O. CIRSE guidelines on percutaneous needle biopsy (PNB). Cardiovasc. Interv. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Binkert, C.A.; Verdun, F.R.; Zanetti, M.; Pfirrmann, C.W.; Hodler, J. CT arthrography of the glenohumeral joint: CT fluoroscopy versus conventional CT and fluoroscopy--comparison of image-guidance techniques. Radiology 2003, 229, 153–158. [Google Scholar] [CrossRef]
- Miller, D.L.; Klein, L.W.; Balter, S.; Norbash, A.; Haines, D.; Fairobent, L.; Goldstein, J.A. Occupational health hazards in the interventional laboratory: Progress report of the multispecialty occupational health group. J. Vasc. Interv. Radiol. 2010, 21, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Hur, J.; Lee, S.M.; Lee, H.-J.; Hong, Y.J.; Nam, J.E.; Kim, H.S.; Kim, Y.J.; Choi, B.W.; Kim, T.H.; et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur. Radiol. 2011, 21, 232–239. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Aguet, J.; Becce, F.; Dunet, V.; Vlassenbroek, A.; Coche, E.E.; Omoumi, P. Optimizing radiation dose parameters in MDCT arthrography of the shoulder: Illustration of basic concepts in a cadaveric study. Skelet. Radiol. 2019, 48, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Choo, H.J.; Lee, S.J.; Kim, D.W.; Han, J.Y.; Kim, D.S. Computed tomography arthrography of the shoulder with tin filter-based spectral shaping at 100 kV and 140 kV. Acta Radiol. 2020, 284185120965551. [Google Scholar] [CrossRef]
- Guggenberger, R.; Ulbrich, E.J.; Dietrich, T.J.; Scholz, R.; Kaelin, P.; Köhler, C.; Elsässer, T.; Le Corroller, T.; Pfammatter, T.; Alkadhi, H.; et al. C-arm flat-panel CT arthrography of the shoulder: Radiation dose considerations and preliminary data on diagnostic performance. Eur. Radiol. 2017, 27, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.; Brehm, M. Shaping the Beam: Versatile Filtration for Unique Diagnostic Potential within Siemens Healthineers. Available online: https://www.ct-meeting.org/data/ct_whitepaper_tin-filter-shaping-the-beam_hood05162003024776_152114222.pdf (accessed on 21 September 2021).
- May, M.S.; Brand, M.; Lell, M.M.; Sedlmair, M.; Allmendinger, T.; Uder, M.; Wuest, W. Radiation dose reduction in parasinus CT by spectral shaping. Neuroradiology 2017, 59, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, G.G.S.J.; Golding, S.J.; Jurik, A.G.; Leonardi, M.; Van Persijn Van Meerten, E.; Rodríguez, R.; Schneider, K.; Calzado, A.; Geleijns, J.; Jessen, K.A.; et al. European Guidelines for Multislice Computed Tomography; European Commission: Brussels, Belgium; Luxembourg City, Luxembourg, 2004. [Google Scholar]
- Christner, J.A.; Kofler, J.M.; McCollough, C.H. Estimating effective dose for CT using dose-length product compared with using organ doses: Consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am. J. Roentgenol. 2010, 194, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sealedenvelope.com/power/continuous-superiority/ (accessed on 3 August 2021).
- Available online: https://odlinfo.bfs.de/EN/themen/was-ist-odl/strahlenbelastung-vergleich.html (accessed on 3 August 2021).
- Seuss, H.; Janka, R.; Hammon, M.; Cavallaro, A.; Uder, M.; Dankerl, P. Virtual computed tomography colonography: Evaluation of 2D and virtual 3D image quality of sub-mSv examinations enabled by third-generation dual source scanner featuring tin filtering. Acad. Radiol. 2018, 25, 1046–1051. [Google Scholar] [CrossRef]
- Wetzl, M.; May, M.S.; Weinmann, D.; Hammon, M.; Kopp, M.; Ruppel, R.; Trollmann, R.; Woelfle, J.; Uder, M.; Rompel, O. Potential for radiation dose reduction in dual-source computed tomography of the lung in the pediatric and adolescent population compared to digital radiography. Diagnostics 2021, 11, 270. [Google Scholar] [CrossRef]
- Stern, C.; Sommer, S.; Germann, C.; Galley, J.; Pfirrmann, C.W.A.; Fritz, B.; Sutter, R. Pelvic bone CT: Can tin-filtered ultra-low-dose CT and virtual radiographs be used as alternative for standard CT and digital radiographs? Eur. Radiol. 2021, 31, 6793–6801. [Google Scholar] [CrossRef]
- Vahlensieck, M.; Sommer, T.; Textor, J.; Pauleit, D.; Lang, P.; Genant, H.K.; Schild, H.H. Indirect MR arthrography: Techniques and applications. Eur. Radiol. 1998, 8, 232–235. [Google Scholar] [CrossRef]
- Herold, T.; Hente, R.; Zorger, N.; Finkenzeller, T.; Feuerbach, S.; Lenhart, M.; Paetzel, C. Indirect MR-arthrography of the shoulder-value in the detection of SLAP-lesions. RoFo 2003, 175, 1508–1514. [Google Scholar] [CrossRef]
- Allmann, K.H.; Schäfer, O.; Hauer, M.; Winterer, J.; Laubenberger, J.; Reichelt, A.; Uhl, M. Indirect MR arthrography of the unexercised glenohumeral joint in patients with rotator cuff tears. Investig. Radiol. 1999, 34, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Yagci, B.; Manisali, M.; Yilmaz, E.; Ozkan, M.; Ekin, A.; Ozaksoy, D.; Kovanlıkaya, I. Indirect MR arthrography of the shoulder in detection of rotator cuff ruptures. Eur. Radiol. 2001, 11, 258–262. [Google Scholar] [CrossRef]
- Schweitzer, M.E.; Natale, P.; Winalski, C.S.; Culp, R. Indirect wrist MR arthrography: The effects of passive motion versus active exercise. Skelet. Radiol. 2000, 29, 10–14. [Google Scholar] [CrossRef]
- Steinbach, L.S.; Palmer, W.E.; Schweitzer, M.E. Special focus session. MR arthrography. Radiographics 2002, 22, 1223–1246. [Google Scholar] [CrossRef]
- Morrison, W.B. Indirect MR arthrography: Concepts and controversies. Semin. Musculoskelet. Radiol. 2005, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cheng, X.; Dong, J.; Zhou, D.; Han, S.; Yang, Y. Comparison of MRI and MRA for the diagnosis of rotator cuff tears: A meta-analysis. Medicine (Baltimore) 2020, 99, e19579. [Google Scholar] [CrossRef]
- Winkelmeyer, E.M.; Schock, J.; Wollschläger, L.M.; Schad, P.; Huppertz, M.S.; Kotowski, N.; Prescher, A.; Kuhl, C.; Truhn, D.; Nebelung, S. Seeing beyond morphology-standardized stress MRI to assess human knee joint instability. Diagnostics 2021, 11, 1035. [Google Scholar] [CrossRef]
- Schmaranzer, F.; Lerch, T.D.; Strasser, U.; Vavron, P.; Schmaranzer, E.; Tannast, M. Usefulness of MR arthrography of the Hip with and without leg traction in detection of intra-articular bodies. Acad. Radiol. 2019, 26, e252–e259. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.J.; Rahim, M.S.M.; Salim, N.; Mohammed, M.A.; Garcia-Zapirain, B.; Abdulkareem, K.H. Efficient detection of knee anterior cruciate ligament from magnetic resonance imaging using deep learning approach. Diagnostics 2021, 11, 105. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goeller, A.; Pogarell, T.; May, M.S.; Uder, M.; Dankerl, P. Evaluation of CT-Guided Ultra-Low-Dose Protocol for Injection Guidance in Preparation of MR-Arthrography of the Shoulder and Hip Joints in Comparison to Conventional and Low-Dose Protocols. Diagnostics 2021, 11, 1835. https://doi.org/10.3390/diagnostics11101835
Goeller A, Pogarell T, May MS, Uder M, Dankerl P. Evaluation of CT-Guided Ultra-Low-Dose Protocol for Injection Guidance in Preparation of MR-Arthrography of the Shoulder and Hip Joints in Comparison to Conventional and Low-Dose Protocols. Diagnostics. 2021; 11(10):1835. https://doi.org/10.3390/diagnostics11101835
Chicago/Turabian StyleGoeller, Anja, Tobias Pogarell, Matthias Stefan May, Michael Uder, and Peter Dankerl. 2021. "Evaluation of CT-Guided Ultra-Low-Dose Protocol for Injection Guidance in Preparation of MR-Arthrography of the Shoulder and Hip Joints in Comparison to Conventional and Low-Dose Protocols" Diagnostics 11, no. 10: 1835. https://doi.org/10.3390/diagnostics11101835
APA StyleGoeller, A., Pogarell, T., May, M. S., Uder, M., & Dankerl, P. (2021). Evaluation of CT-Guided Ultra-Low-Dose Protocol for Injection Guidance in Preparation of MR-Arthrography of the Shoulder and Hip Joints in Comparison to Conventional and Low-Dose Protocols. Diagnostics, 11(10), 1835. https://doi.org/10.3390/diagnostics11101835






