Analysis of Bifurcation and Stability in an Epidemic Model of HPV Infection and Cervical Cancer with Two Time Delays
Abstract
1. Introduction
2. Model Formulation
- ():
- HPV spreads in a population that is homogeneously mixed, the effective contact rate determines transmission, the population is fully exposed, and the bilinear incidence rate characterizes the rate of new infections.
- ():
- In order to maintain a constant total population, new susceptible people are introduced at a constant rate , and each individual has the same natural mortality rate .
- ():
- Two critical time lags from infection to progression to malignancy () and from HPV infection to recovery () were modeled.
- ():
- Because HPV is polytypic, it is considered that recovered individuals only have transient immunity rather than permanent immunity, and they may relapse at a rate .
- ():
- The highly contagious asymptomatic infection phase (I) and the complication phase (C) are distinguished clearly.
- ():
- The system focuses on transmission dynamics and does not consider additional mortality due to HPV-related diseases at this time.
3. Basic Properties and Analysis
3.1. Positivity and Boundedness of Solutions
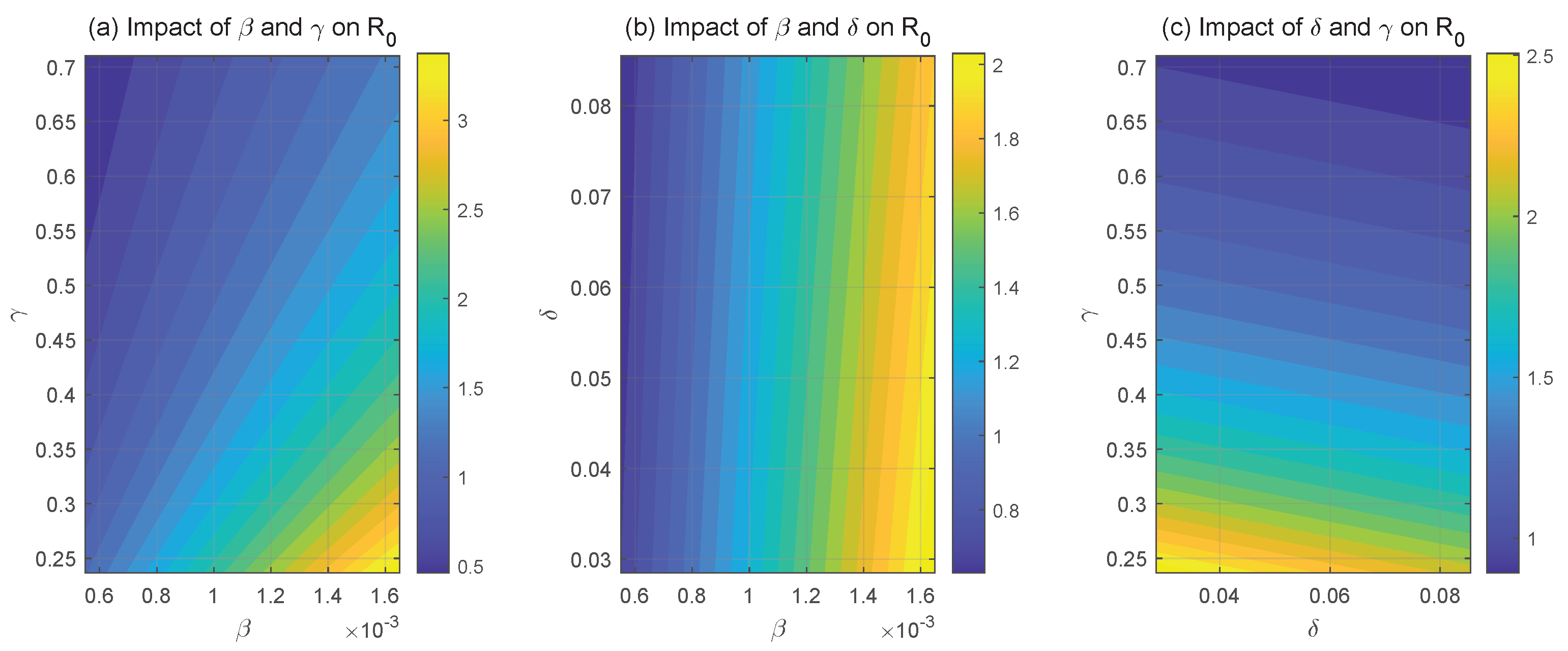
3.2. Basic Reproduction Number and Existence of Equilibria
4. Stability Analysis and Hopf Bifurcation
4.1. Stability of the Disease-Free Equilibrium
4.2. Stability of Endemic Equilibrium
4.3. Existence of Hopf Bifurcation
5. Stability and Direction of Hopf Bifurcation
- (i)
- If is negative, the bifurcation in the positive steady state is subcritical, and if it is positive, it is supercritical. When the value of τ exceeds , bifurcating periodic solutions will appear.
- (ii)
- When is negative, the periodic solutions generated by the bifurcation are stable. If they are positive, they will become unstable.
- (iii)
- A positive value of corresponds to an increase in the period, while a negative value of indicates a decrease in the period.
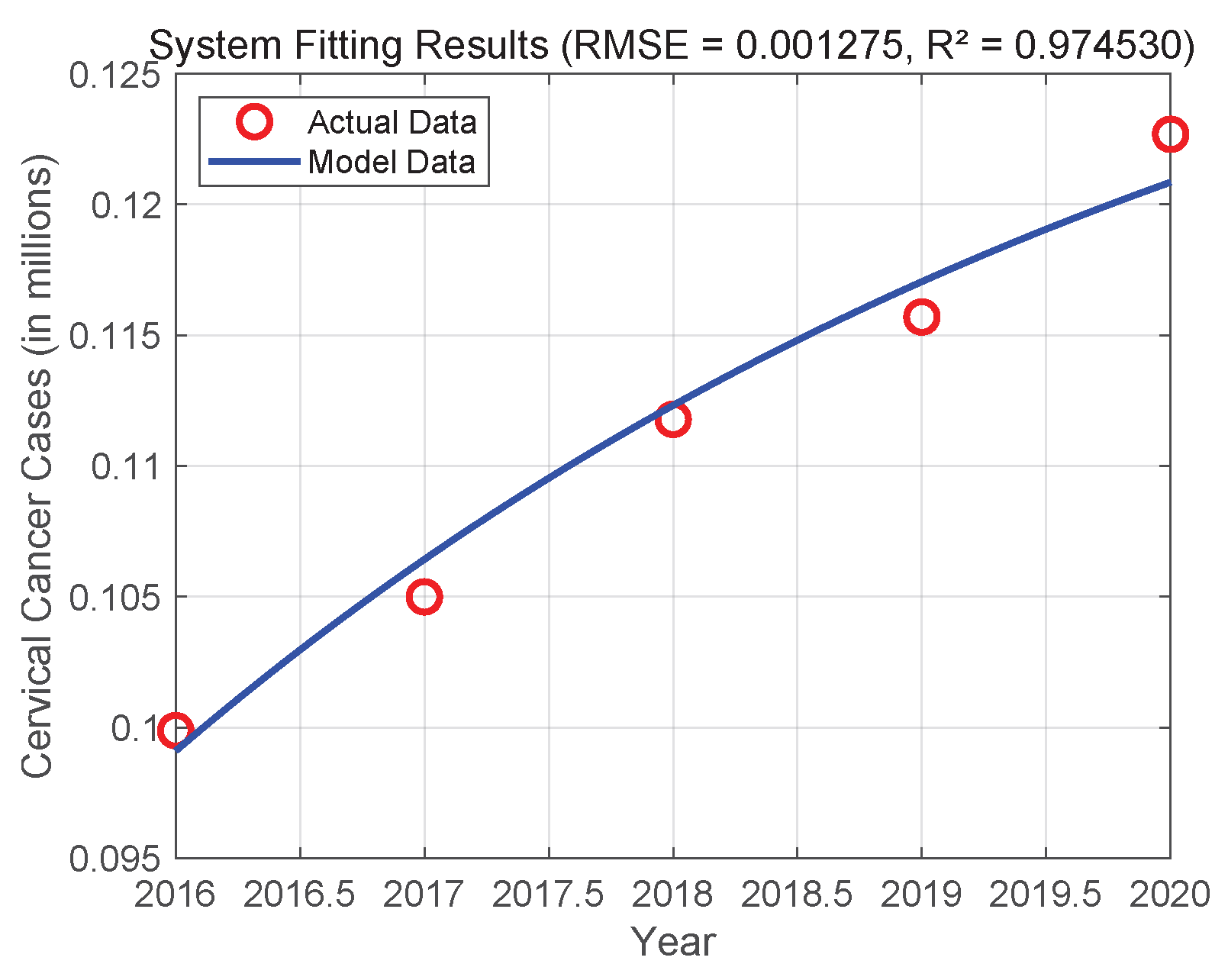
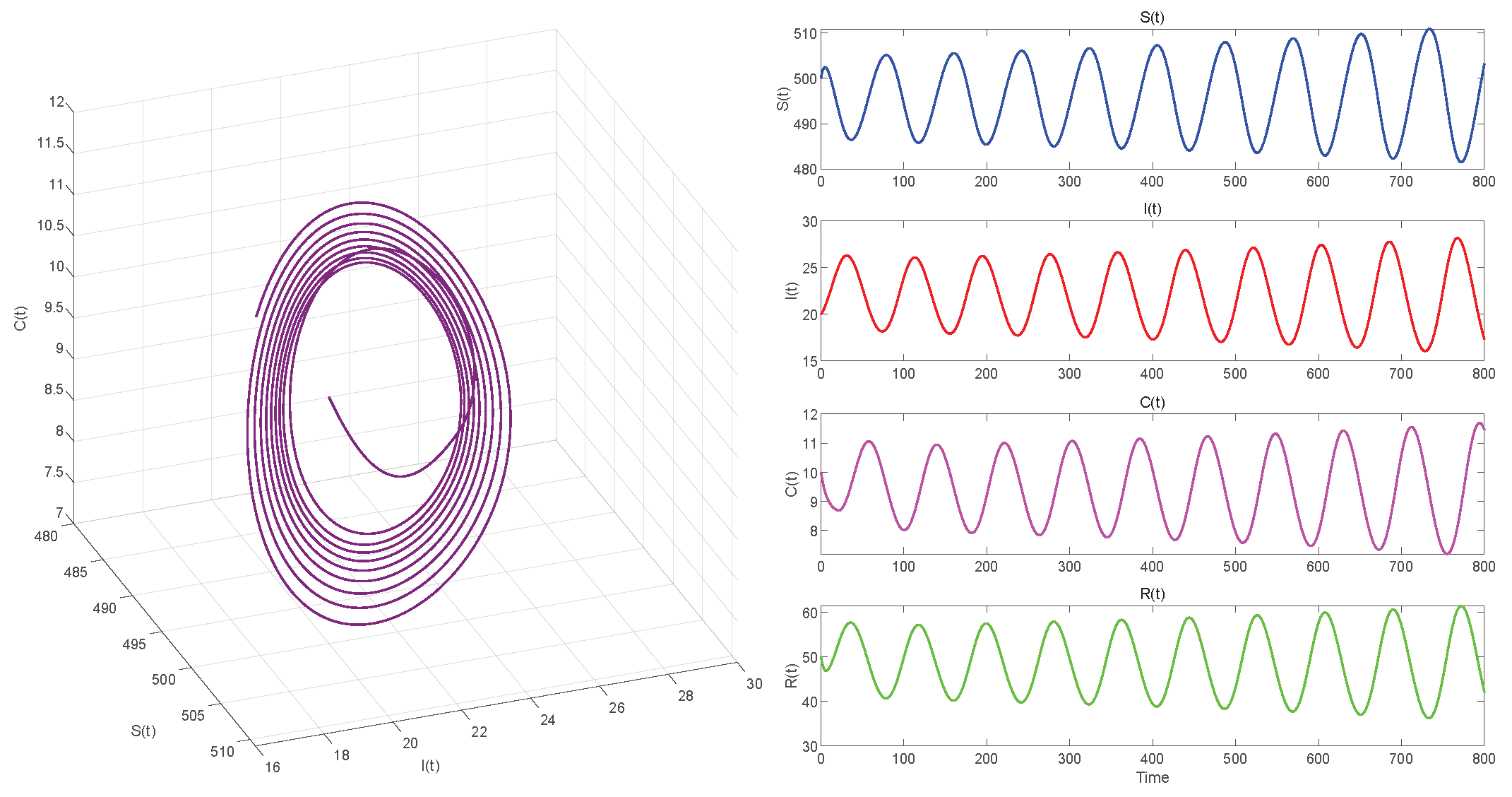
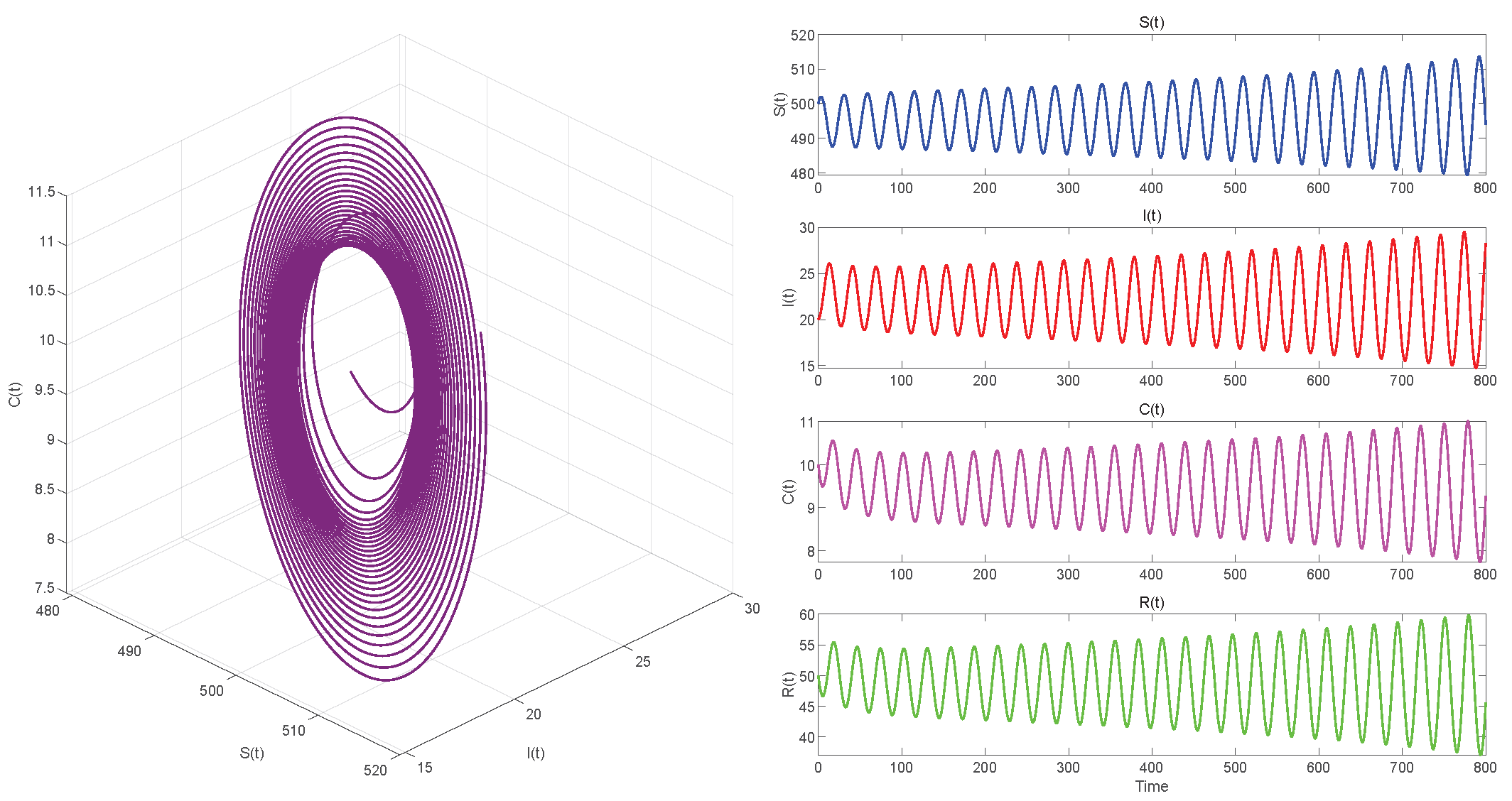
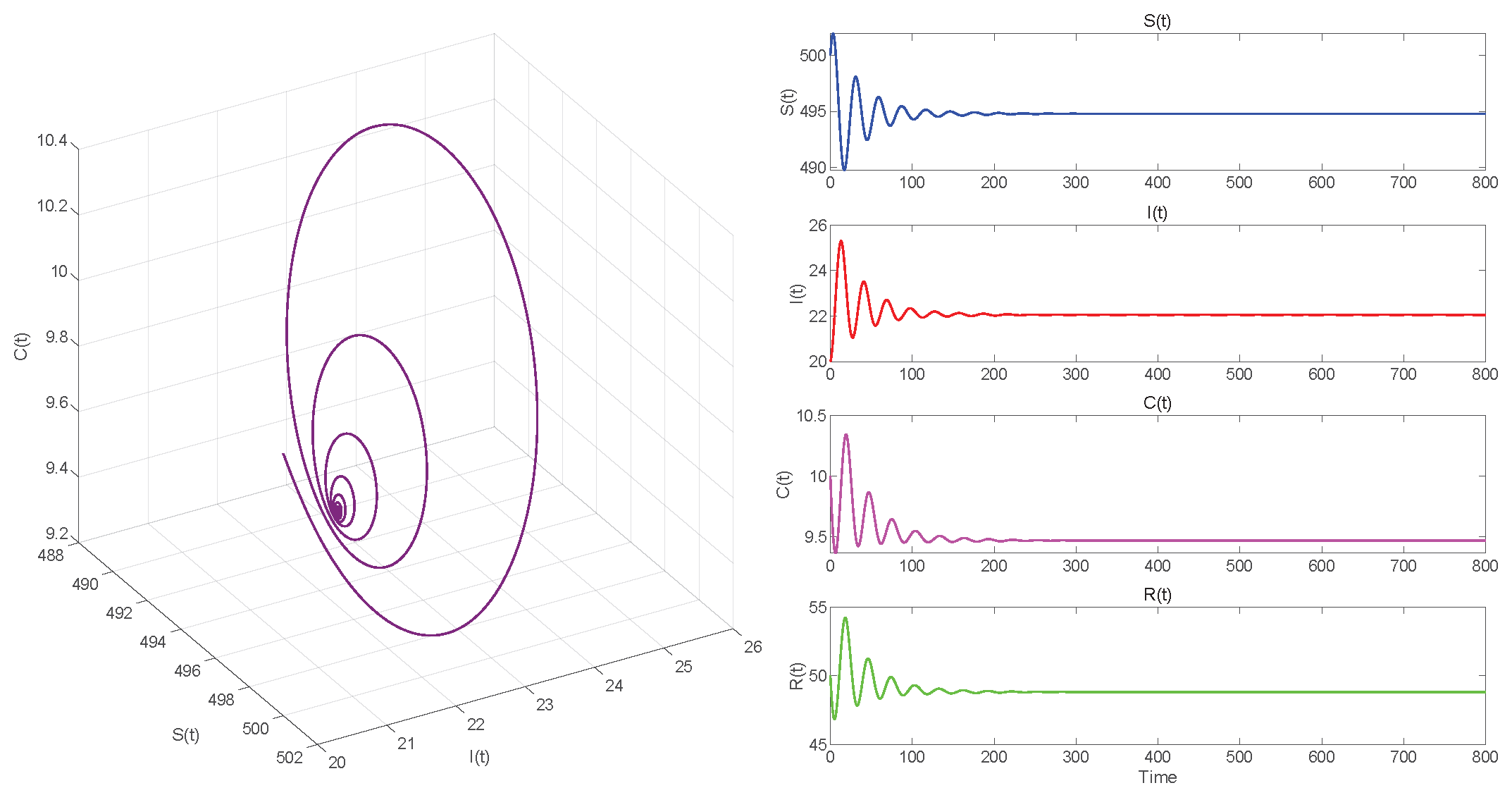
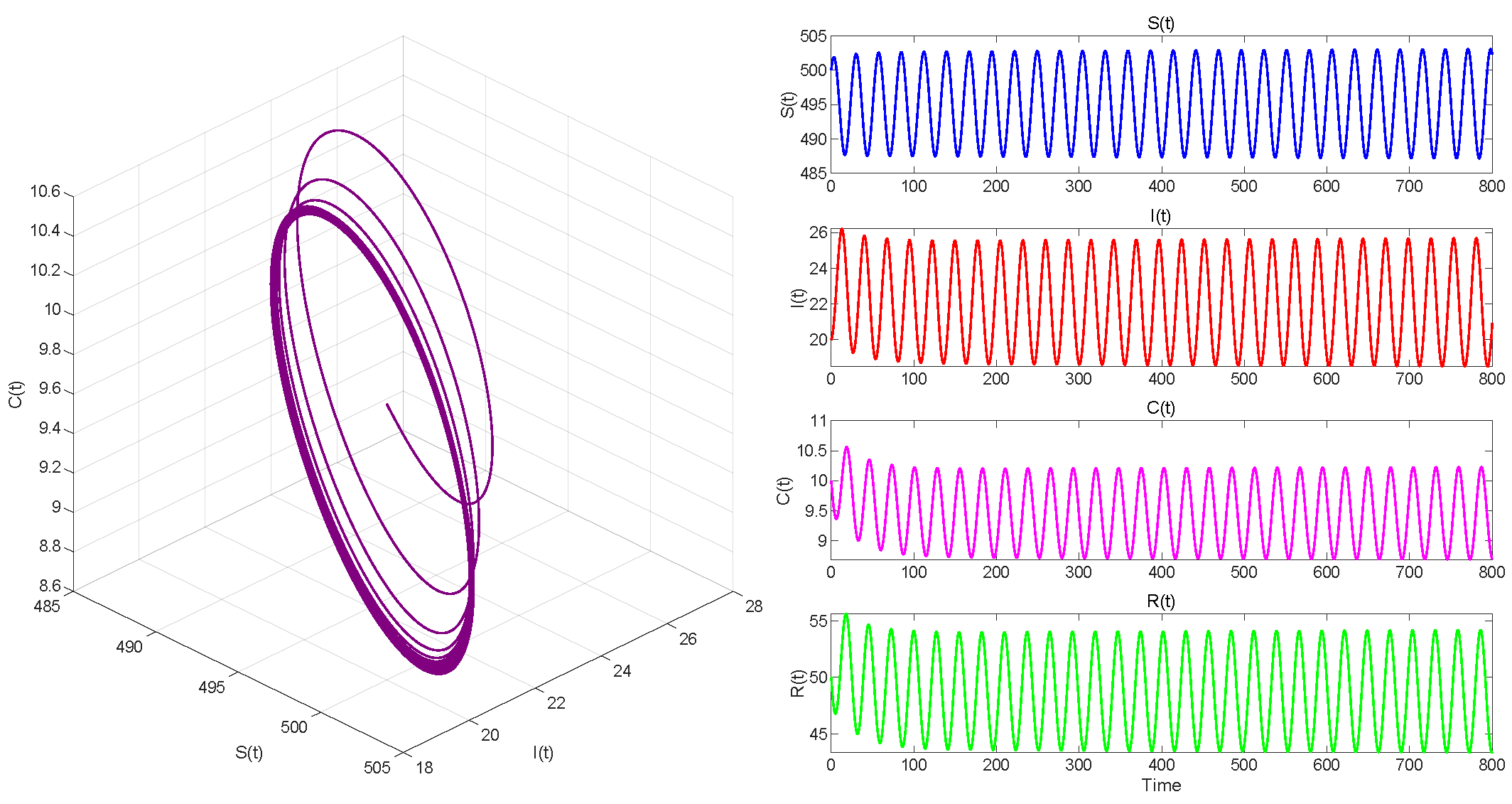
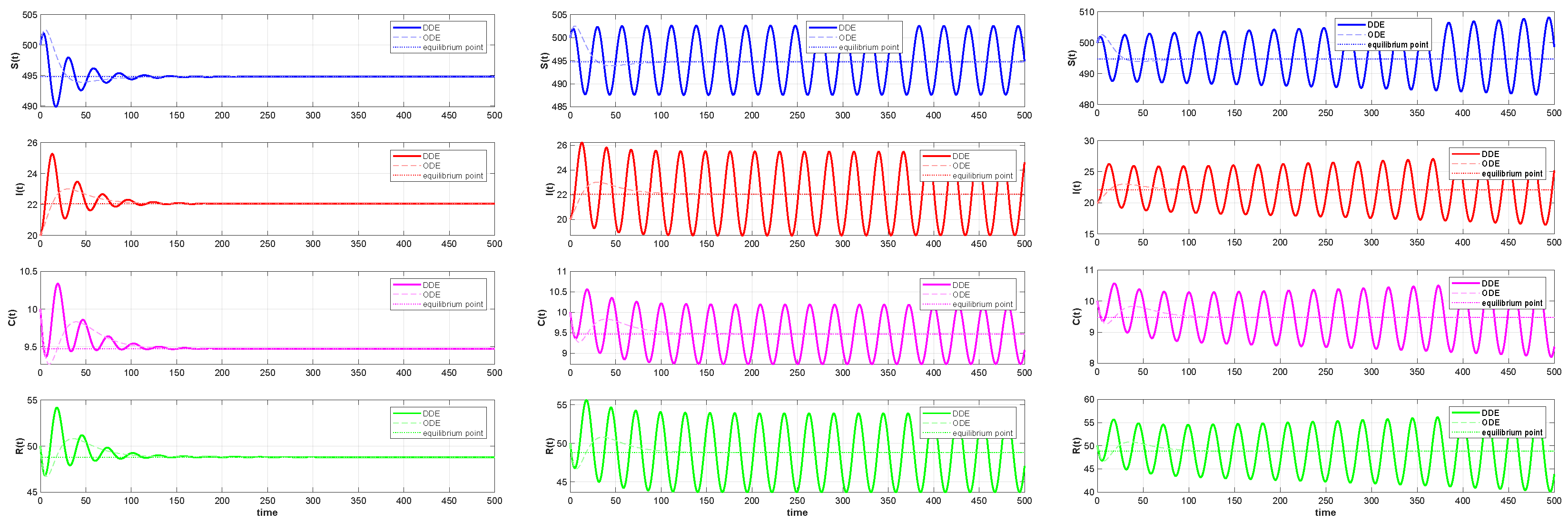
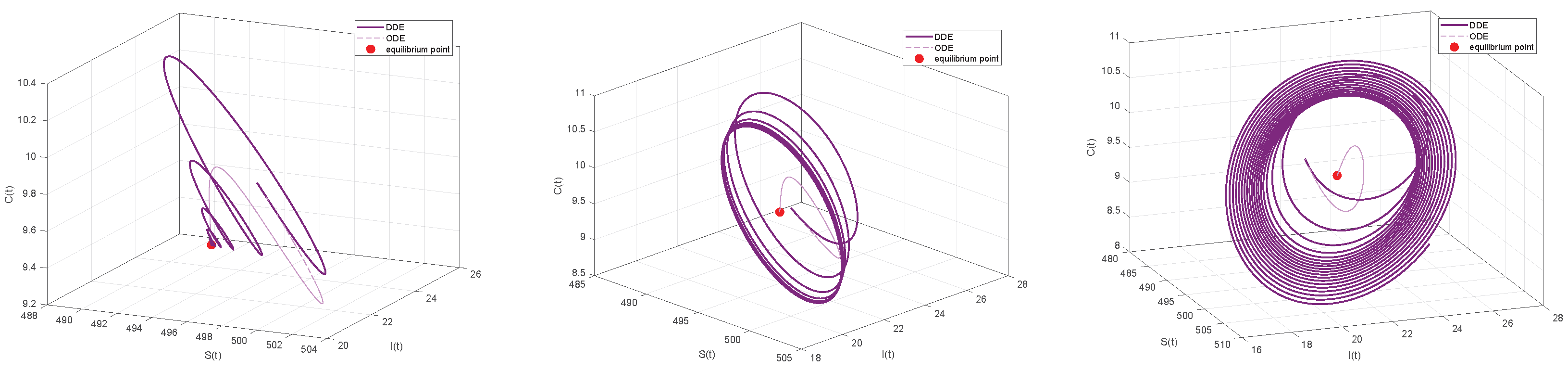
6. Numerical Simulations
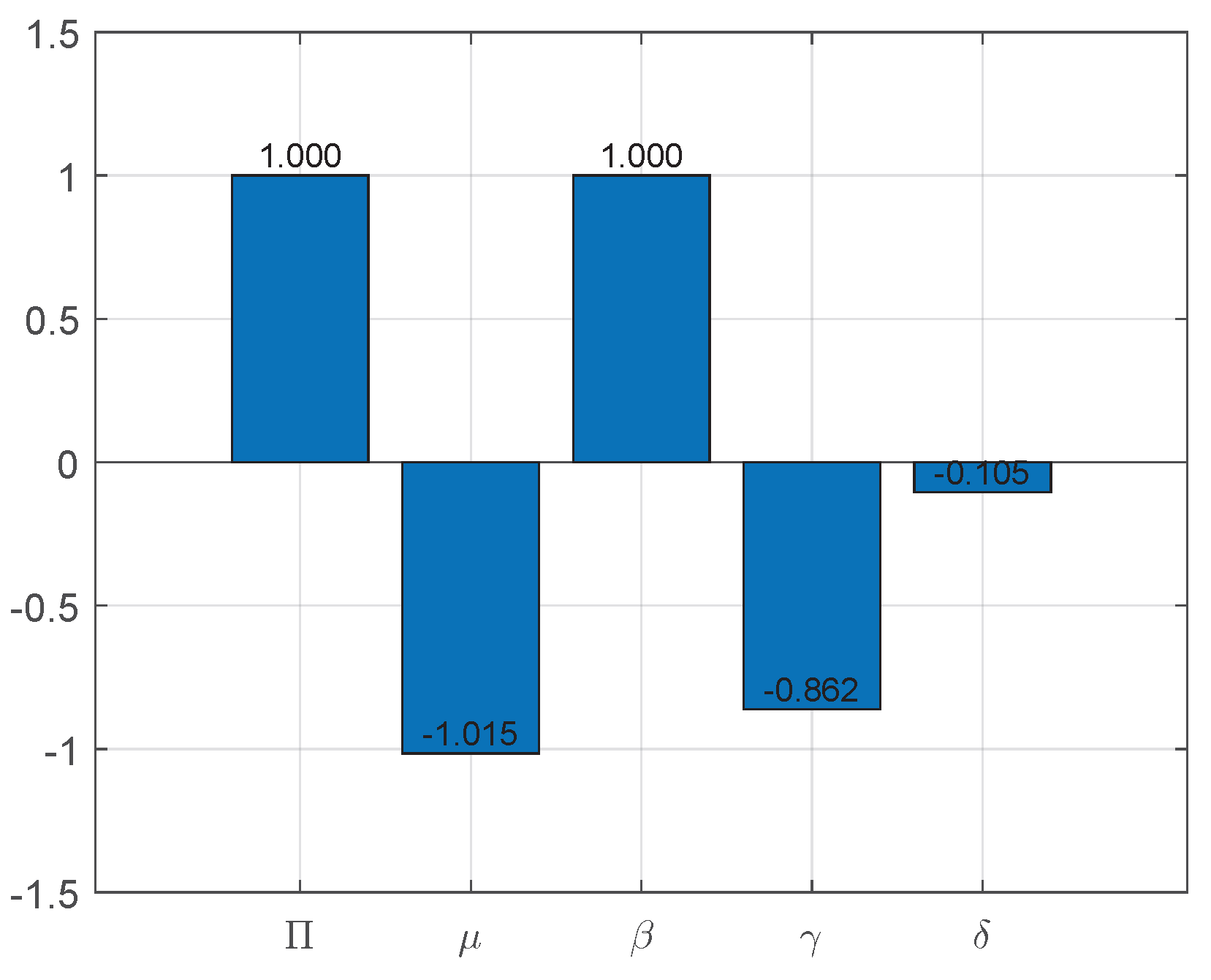
6.1. Sensitivity Analysis
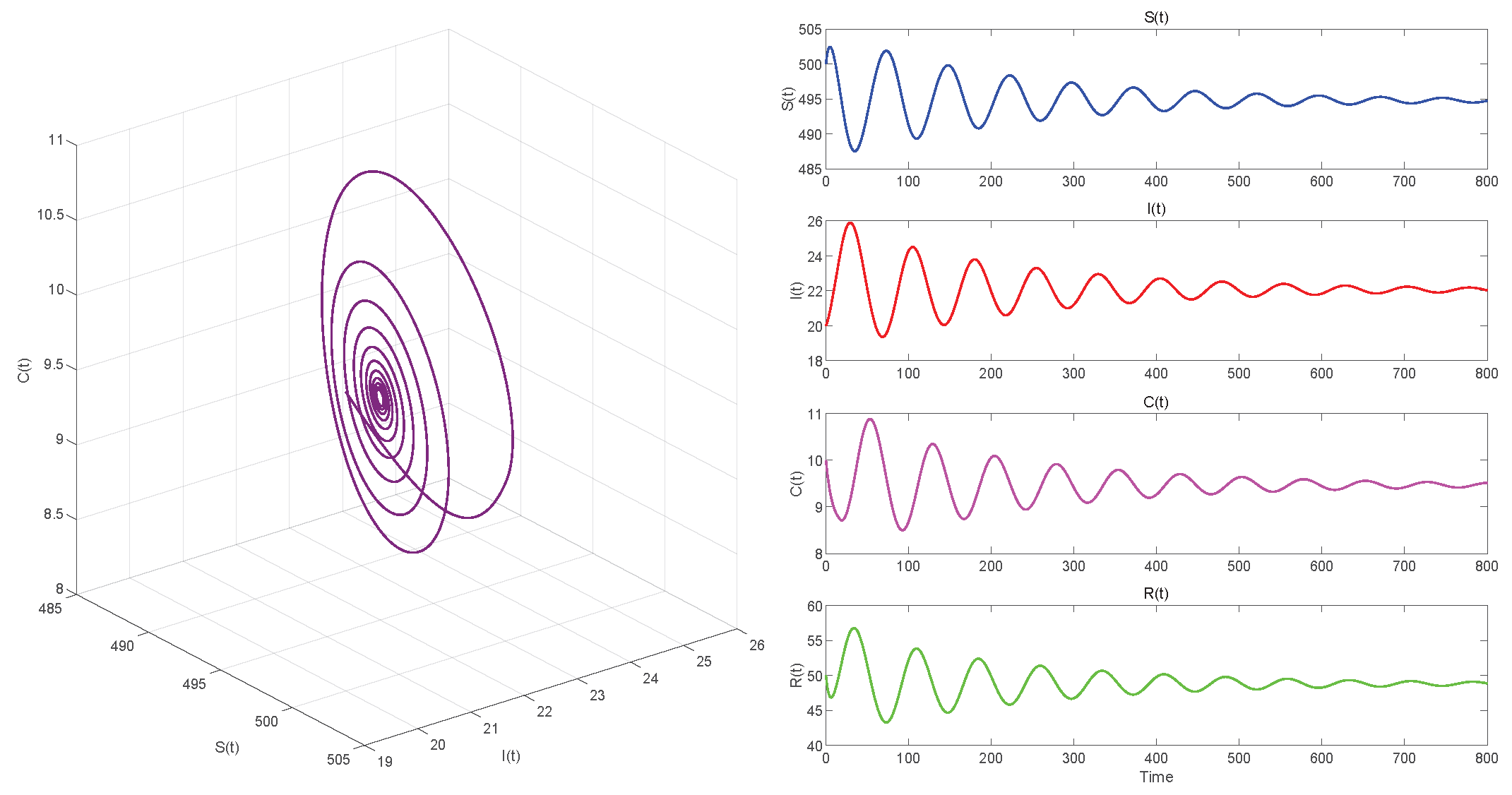
6.2. Numerical Simulations of the DDE System
6.3. Comparison with the ODE System and Discussion
7. Conclusions
- (i)
- The cancerous delay is the amount of time that passes between the start of a persistent infection with high-risk HPV strains and the final development of invasive cancer, which usually lasts 10–20 years or longer.
- (ii)
- The immune response delay is the time required from pathogen invasion to the immune system effectively eliminating the pathogen and initiating the recovery process. In clinical terms, this refers to the window of time between infection and the virus or lesions being effectively removed by cellular immunity, usually between six months and two years.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Human Papillomavirus and Cancer. 2024. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 11 April 2025).
- World Health Organization. Cervical Cancer. 2024. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/cervical-cancer (accessed on 11 April 2025).
- Tewari, K.S. Cervical cancer. N. Engl. J. Med. 2025, 392, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Snijders, P.J.; Steenbergen, R.D.; Heideman, D.A.; Meijer, C.J. HPV-mediated cervical carcinogenesis: Concepts and clinical implications. J. Pathol. 2005, 208, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ji, Y.; Pan, Q.; Wei, Y.; Ye, Y.; Liu, H. Sensitivity analysis and optimal treatment control for a mathematical model of Human Papillomavirus infection. AIMS Math. 2020, 5, 2646–2670. [Google Scholar] [CrossRef]
- Lee, S.L.; Tameru, A.M. A mathematical model of Human Papillomavirus (HPV) in the United States and its impact on cervical cancer. J. Cancer 2012, 3, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Denteh, W.; Afrifa, R.T.; Barnes, B.; Addo, K.M. Modeling the epidemiology of Human Papilloma Virus infection and Vaccination and its impact on Cervical Cancer in Ghana. J. Sci. Res. Rep. 2014, 3, 2501–2518. [Google Scholar] [CrossRef]
- Berhe, H.W.; Alarydah, M. Computational modeling of human papillomavirus with impulsive vaccination. Nonlinear Dyn. 2021, 103, 925–946. [Google Scholar] [CrossRef] [PubMed]
- Nwajeri, U.K.; Panle, A.B.; Omame, A.; Obi, M.C.; Onyenegecha, C.P. On the fractional order model for HPV and Syphilis using non–singular kernel. Results Phys. 2022, 37, 105463. [Google Scholar] [CrossRef]
- Abdeljawad, T.; Khan, N.; Abdalla, B.; Al-Jasern, A.; Alqudah, M.; Shah, K. A mathematical analysis of human papilloma virus (HPV) disease with new perspectives of fractional calculus. Alex. Eng. J. 2025, 125, 575–599. [Google Scholar] [CrossRef]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.M. HPV: The global burden. Nature 2012, 488, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Akimenko, V.V.; Adi-Kusumo, F. Age-structured delayed SIPCV epidemic model of HPV and cervical cancer cells dynamics I. Numerical method. Biomath 2021, 10, 2110027. [Google Scholar] [CrossRef]
- Goel, A.N.; Frangos, M.; Raghavan, G.; Sangar, S.; Lazaro, S.; Wang, M.B.; Long, J.L.; St. John, M.A. Survival impact of treatment delays in surgically managed oropharyngeal cancer and the role of human papillomavirus status. Head Neck 2019, 41, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, M.; Martin, E.; Frank, M.I.; Meller, L.L.T.; Haidar, Y.M. Treatment delay and HPV status on OPSCC with upfront surgery: Analysis of National Cancer Database. Otolaryngol.–Head Neck Surg. 2024, 171, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.K.; Kuppusamy, M.; Egbelowo, O.F. A mathematical model for human papillomavirus and its impact on cervical cancer in India. J. Appl. Math. Comput. 2023, 69, 753–770. [Google Scholar] [CrossRef]
- Ismail, H.; Shangerganesh, L.; Msmali, A.H.; Bourazza, S.; Meetei, M.Z. Hopf Bifurcation and Optimal Control in an Ebola Epidemic Model with Immunity Loss and Multiple Delays. Axioms 2025, 14, 313. [Google Scholar] [CrossRef]
- Hassard, B.D.; Kazarinoff, N.D.; Wan, Y.H. Theory and Applications of Hopf Bifurcation; Cambridge University Press: Cambridge, UK, 1981; Available online: https://www.google.com.tw/books/edition/Theory_and_Applications_of_Hopf_Bifurcat/3wU4AAAAIAAJ?hl=zh-CN&gbpv=0 (accessed on 15 May 2018).
- Meng, X.-Y.; Huo, H.-F.; Xiang, H. Hopf bifurcation in a three-species system with delays. J. Appl. Math. Comput. 2010, 35, 635–661. [Google Scholar] [CrossRef]
- Global Cancer Observatory: Cancer Today, International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 1 May 2025).
- Al-Arydah, M. Two-sex logistic model for human papillomavirus and optimal vaccine. Int. J. Biomath. 2021, 14, 2150011. [Google Scholar] [CrossRef]


| Parameters | Biological Meaning |
|---|---|
| Recruitment rate of susceptible women | |
| HPV recovery rate due to natural immunity | |
| HPV transmission rate contact with individuals | |
| Rate of HPV progression to CC | |
| Rate of loss of immunity | |
| The mortality rate for individuals with CC | |
| Rate of natural mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, M.; Zhang, T. Analysis of Bifurcation and Stability in an Epidemic Model of HPV Infection and Cervical Cancer with Two Time Delays. Axioms 2025, 14, 680. https://doi.org/10.3390/axioms14090680
Hua M, Zhang T. Analysis of Bifurcation and Stability in an Epidemic Model of HPV Infection and Cervical Cancer with Two Time Delays. Axioms. 2025; 14(9):680. https://doi.org/10.3390/axioms14090680
Chicago/Turabian StyleHua, Mengyuan, and Tiansi Zhang. 2025. "Analysis of Bifurcation and Stability in an Epidemic Model of HPV Infection and Cervical Cancer with Two Time Delays" Axioms 14, no. 9: 680. https://doi.org/10.3390/axioms14090680
APA StyleHua, M., & Zhang, T. (2025). Analysis of Bifurcation and Stability in an Epidemic Model of HPV Infection and Cervical Cancer with Two Time Delays. Axioms, 14(9), 680. https://doi.org/10.3390/axioms14090680






