Abstract
Predators impact prey populations directly through consumption and indirectly via trait-mediated effects like predator-induced emigration (PIE), where prey alter movement due to predation risk. While PIE can significantly influence prey dynamics, its combined effect with direct predation in fragmented habitats is underexplored. Habitat fragmentation reduces viable habitats and isolates populations, necessitating an understanding of these interactions for conservation. In this paper, we present a reaction–diffusion model to investigate prey persistence under both direct predation and PIE in fragmented landscapes. The model considers prey growing logistically within a bounded habitat patch surrounded by a hostile matrix. Prey move via unbiased random walks internally but exhibit biased movement at habitat boundaries influenced by predation risk. Predators are assumed constant, operating on a different timescale. We examine three predation functional responses—constant yield, Holling Type I, and Holling Type III—and three emigration patterns: density-independent, positive density-dependent, and negative density-dependent emigration. Using the method of sub- and supersolutions, we establish conditions for the existence and multiplicity of positive steady-state solutions. Numerical simulations in one-dimensional habitats further elucidate the structure of these solutions. Our findings demonstrate that the interplay between direct predation and PIE crucially affects prey persistence in fragmented habitats. Depending on the functional response and emigration pattern, PIE can either mitigate or amplify the impact of direct predation. This underscores the importance of incorporating both direct and indirect predation effects in ecological models to better predict species dynamics and inform conservation strategies in fragmented landscapes.
Keywords:
boundary value problems; population dynamics; harvesting models; reaction diffusion; nonlinear boundary conditions; predator induced emigration MSC:
35J15; 35J25; 35J30; 35J60; 35J66
1. Introduction
Studies have shown that predators have both direct (density-mediated) and indirect (trait-mediated indirect) effects on prey populations (see [1,2,3,4]). In fact, it is widely accepted that ecological communities are replete with trait-mediated indirect effects arising from phenotypic plasticity, and these effects are important to community dynamics (see [1,3,5]). Trait-mediated behavioral responses to predators have the potential to greatly affect the dynamics of a population (see [1,3,4]). One such effect is trait-mediated emigration, wherein the prey changes its emigration patterns due to the presence of a predator [2,6,7,8]. This can, in turn, modify population dynamics and species interactions (see [9], for example). Few empirical studies have considered this effect in the predator–prey context, where increased predation risk was shown to increase the emigration rates of prey. For example, ref. [2] found evidence of predator-induced emigration (PIE) in a spider (predator) and planthopper (prey) system, and concluded that at high predator density, the predator had a greater impact on prey density through induced emigration than consumption (also see [6,7,8]).
Human-dominated habitat fragmentation continues at unprecedented levels, giving rise to the need for a better understanding of the consequences of density dependence and its role in conservation efforts [10,11,12,13,14]. Habitat fragmentation reduces viable habitat or patch size and also separates populations among much smaller residual patches which are surrounded by a human-modified “matrix” with varying degrees of hostility [11]. The modeling of theoretical populations has seen great success in predicting patch- and even landscape-level patterns in response to habitat fragmentation. The reaction diffusion framework has been particularly successful at providing better understanding of the coupling of density dependent growth mechanisms with density dependent movement or dispersal (see [15]). The framework’s ability to handle space explicitly at the landscape level, including modeling animal movement behavior differences when a patch boundary is reached, has been well demonstrated [16,17,18,19].
The modeling of predator–prey population dynamics dates back to the classic works of [20,21]. A key component of those models is the predator functional response, the relationship between prey consumption per predator per unit of time as prey density increases [22,23]. In the original formulation of the Lotka–Volterra model, predators and prey were assumed to encounter each other at random, resulting in a constant rate of prey consumption as the prey density increased. Subsequent derivations of the functional response have included more realistic predator behaviors such as handling time constraints and predator satiation that result in a decreasing rate of prey consumption as the prey density increases (Holling’s type II functional response), or learning or switching to more abundant or profitable prey that results in an increasing, then decreasing, rate of prey consumption as the prey density increases (Holling’s type III functional response) (see [22,24,25]). Over the years, many studies have expanded on Holling’s seminal work and investigated the biological implications of a wide range of predator functional responses (e.g., see [26,27,28,29,30]).
In this paper, we employ a model based on the reaction diffusion framework to study the persistence of a prey species that is experiencing both direct (density-mediated) and indirect (predator-induced emigration) effects while facing habitat fragmentation. This framework was derived in [19] (but also see [31]) and connects assumptions regarding movement behavior at the individual level to the patch- and landscape levels. To our knowledge, no other study has examined the linkage between predator presence, the prey’s density–emigration relationship and prey population dynamics in a landscape context. Here, we envision a prey species which inhabits a patch which is a bounded domain in ; with smooth boundary or that is surrounded by a hostile matrix. In the framework, prey grow logistically and move according to an unbiased random walk inside the patch and in the matrix but follow a biased random walk at the patch/matrix interface, making an emigration decision based upon the presence of a predator. We assume that the predator is acting on a different timescale than the prey and thus assume a constant predator population. The nondimensionalized steady-state equation for the model is then given by
where is the prey population density for , is a composite parameter that is proportional to the patch size squared, is a parameter that quantifies the matrix hostility, and is the outward normal derivative of u. Here, , where is the probability of the organism staying inside upon reaching the boundary and represents emigration patterns such as density-independent emigration (DIE) when is a constant, positive density-dependent emigration (+DDE) when is increasing, and negative density-dependent emigration (−DDE) where is decreasing.
- Here, represents the predation functional response, and we consider the following forms:
- (i)
- Constant-yield predation (CYP): .
- (ii)
- Constant-effort predation: Holling Type I functional response: .
- (iii)
- Prey switching predation: Holling Type III functional response: ; .
For the emigration form g, we consider the following:
- (i)
- Density-independent emigration (DIE):
- (ii)
- Positive density-dependent emigration (+DDE):
- (iii)
- Negative density-dependent emigration (−DDE):
Where is a composite parameter that measures the prey’s sensitivity to the presence of the predator (we will also denote this as the strength of the PIE relationship) and is a composite parameter that dictates the strength of the DDE form (see [32]). For example, a −DDE form with is very similar to DIE, while one with shows a strong negative relationship between density and emigration.
Recently, several studies have either considered models similar to (1) but with only direct effects of predation or indirect effects such as PIE (see [29,33,34,35,36,37,38]).
To date, few, if any, studies have considered the combined effects of both the direct and indirect effects of the predation of a prey in the presence of habitat fragmentation. Our motivation here is to compare the structure of the positive solutions for (1) when predation does not affect the emigration rate () to the case when predation does influence the emigration rate (). We establish several existence, non-existence, and multiplicity results via the method of sub- and supersolutions and then numerically explore the structure of positive steady-state solutions in the one-dimensional case where more complete results and biological interpretation can be obtained.
To present our main results, we first describe a useful eigenvalue problem. Given and , let be the principal eigenvalue of
with corresponding eigenfunction . Further, let for , and be the positive falling zero of . Throughout this paper, to ensure the existence of positive solutions for (1), we assume that is a fixed number small enough so that this exists and , where . Now, we state our main results.
- Results for Case A: Constant-Yield Predation
Notice that for , has two distinct zeros, with . We now state the results for this case with an expected bifurcation curve presented in Figure 1.
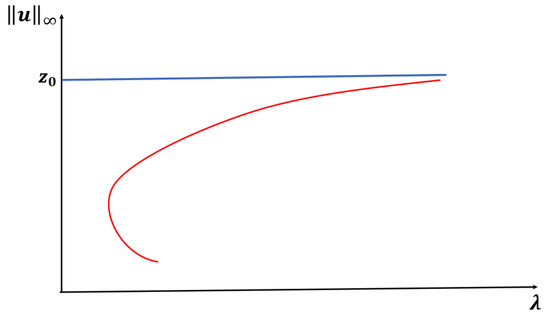
Figure 1.
An expected bifurcation diagram for Case A constant-yield predation.
Theorem 1.
For the next two cases ( and ), we also focus on exploring the existence of a patch-level Allee effect (PAE). In this scenario, the trivial solution and at least one other positive solution of (1) are both stable, giving rise to a population density threshold that must be maintained to ensure the persistence of the population. A sufficient condition for a PAE is the existence of a range of to the left of where at least one positive solution exists (see Figure 2 and [32]). Here, when and when . Furthermore, when , numerical evidence shows that there is also a range of with multiple positive solutions. However, this is a well-known open problem to establish analytically (see the literature on semi-positone problems).
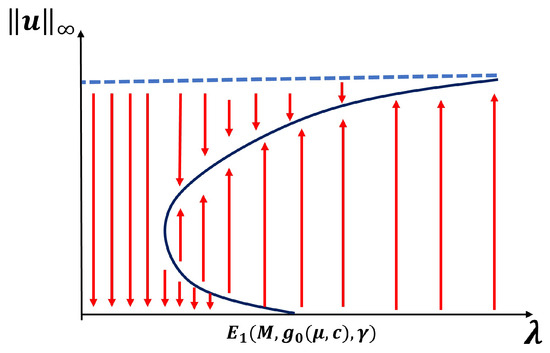
Figure 2.
Bifurcation diagram exhibiting a patch level Allee effect.
- Results for Case B: Constant-Effort Predation:
- In this case, the roots of are zero and . We now state the results for this case.
Theorem 2.
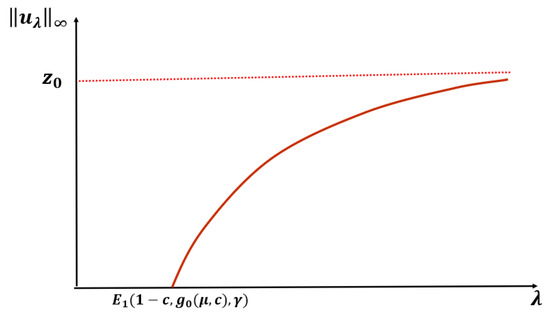
Let or and be fixed. Then, (1) has a unique positive solution for and no positive solution for . Further, as and as (see Figure 3).

Figure 3.
An expected bifurcation diagram for Case B constant-effort predation with (DIE) or (+DDE).
Remark 1.
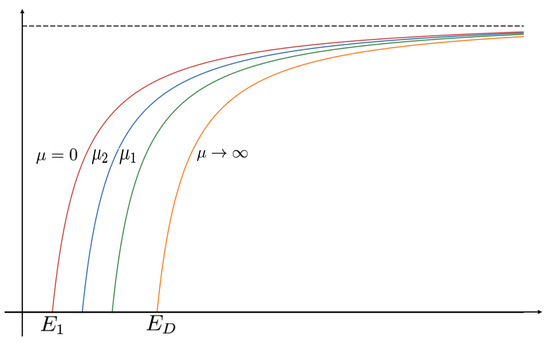
Let or , , , and be the unique positive solutions of (1) when and , respectively, then (see Figure 4).

Figure 4.
Variation of bifurcation diagrams when varies for Case B constant-effort predation, where .
Before we state our next theorem, we define
Theorem 3.
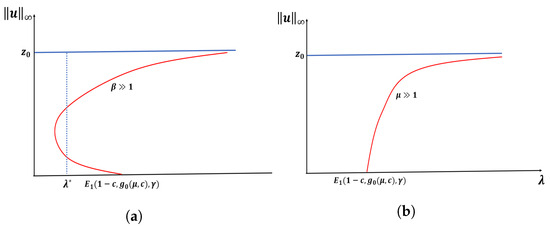
Let . Then, for fixed and , there exists such that a PAE occurs for for . Further, if and are fixed, then there exists such that (1) has no positive solution for when , where is the principal eigenvalue of
(see Figure 5).

Figure 5.
Expected bifurcation diagrams for Case B constant-effort predation when (−DDE) with in (a) and in (b).
Next, we state the following conjecture based on our numerical results (see Section 2).
Conjecture: For any and fixed , when , (1) has no positive solution for , and (1) has a unique positive solution for such that as and as (see Figure 6).
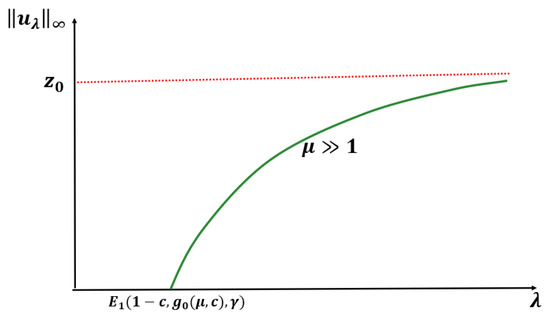
Figure 6.
Expected bifurcation diagram for Case B constant-effort predation for any DDE and when .
- Results for Case C: Prey Switching Predation:
Here, we state two hypotheses before we state our results. Let be the radius of the largest ball that can be inscribed inside the domain , , , and given a , denote as the unique solution of
with and .
Now, we state two hypotheses regarding f:
- (H1):
- there exist such that and .
- (H2):
- there exist and such that f is non-decreasing in .
Define
Theorem 4.
Let . Then, for and fixed , there exists such that a PAE occurs for for . Further, if and are fixed, there exists such that (1) has no positive solution for when .
Theorem 5.
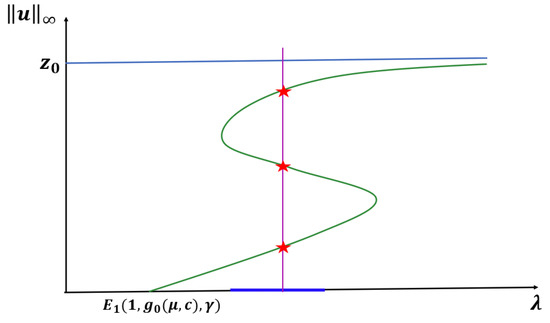
Let , , or with β be fixed. Let and hold. Then (1) has at least three positive solutions for (see Figure 7).

Figure 7.
Occurrence of an S-shaped bifurcation diagram for Case C prey switching predation for any DDE form.
Remark 2.
In Section 4, when Ω is a ball of radius R, we prove that f and g satisfy the hypothesis of Theorem 5 for certain parameter values when μ is large.
Remark 3.
In Section 2, we numerically compute complete bifurcation diagrams of the positive solutions in the one-dimensional case where and discuss their biological relevance. We provide some mathematical preliminaries in Section 3, and in Section 4 we construct several sub- and supersolutions that we will use to establish our analytical results. Finally, we prove Theorems 1–6 and Remark 1 in Section 5.
2. One-Dimensional Results and Biological Conclusions
In this section, we present computationally generated results for the one-dimensional case when . In this way, we obtain more detailed bifurcation diagrams and provide some biological conclusions of these results. Namely, we consider the steady-state model:
and study the structure of positive solutions via bifurcation curves ( vs. curves) in order to contrast model predictions of the cases: (when predation does not affect emigration probability) and (when predation does influence the emigration probability). Here, we denote as the maximum density of the positive solution of (7). We obtain complete bifurcation diagrams via a quadrature method (presented in Lemma 3 of Section 3 and numerical computation using Mathematica (Wolfram Research Inc., version 14.0). See [32,39], and [40] for extensions of the original quadrature method developed in [41] for the case of Dirichlet boundary conditions. For completeness, we prove the extension of the quadrature method employed here in Appendix A. For simplicity of presentation, we choose for and , and and for . We note that a full exposition of the parameter space is outside the scope of this current work. Rather, we choose certain parameter ranges to provide prototypical model predictions. We also highlight several cases where predator-induced emigration and the strength of this response as measured in plays a significant role on model predictions.
2.1. Results for Case A: Constant-Yield Predation
In this subsection, we present results for the constant-yield predation case, where we have fixed and .
We note that when , our model has a semi-positone structure (see [29]), and for Lemma 3 to hold, the -value must be between and , where is the first positive zero of F and is the falling positive zero of f (see Figure 8).
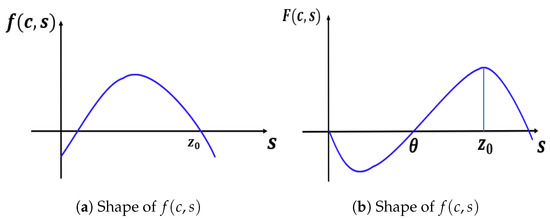
Figure 8.
Shapes of and in the constant-yield predation case: .
Before we state our results, we denote the -value associated with the minimum patch size as , namely, is proportional to the minimum size of the habitat that will allow unconditional population persistence.
2.1.1. Bifurcation Diagrams for DIE:
The evolution of bifurcation curves with respect to PIE strength is shown in Figure 9, while Table 1 shows the evolution of , the uniqueness region, and the multiplicity region. Notice that for all , the smaller root of is a supersolution of (7), implying that the time-dependent problem would predict extinction for any size patch if the initial density distribution is too small. Here, corresponds to the case where predation does not influence the emigration probability. We observe that the bifurcation curves shift from left to the right, the multiplicity region increases, and the uniqueness region decreases as increases. The bifurcation curves are approaching the bifurcation curve corresponding to a case of a completely lethal matrix (Dirichlet boundary condition) as the PIE strength . For a given -value, we see that the number of positive steady states also varies as changes. To highlight changes in model predictions with respect to the PIE strength, consider a patch with fixed matrix hostility and patch size yielding a as shown in Figure 9. In the absence of PIE (), there is a unique positive solution of (7), and show two positive solutions, and no positive solution is possible (extinction) when for some . Notice also that the red curve () and the black curve () define an envelope of possible bifurcation curves, where PIE would have an effect on the model predictions of the population’s persistence. In other words, only populations with patch sizes having corresponding -values lying in this envelope could be adversely affected by the presence of PIE.
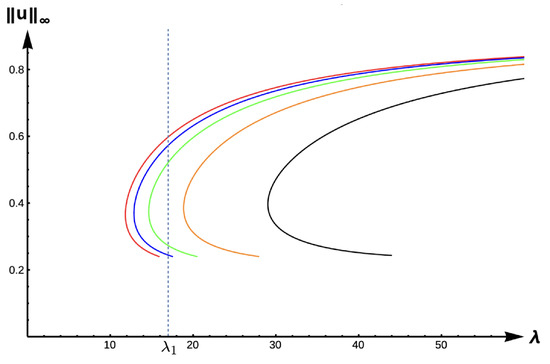
Figure 9.
Bifurcation diagram of positive solutions for (7) with (DIE). Note that the dashed line is for , red represents , blue , green , orange , and black .

Table 1.
.
2.1.2. Bifurcation Diagrams for +DDE
In the case of +DDE with strength measured in the nondimensional parameter , the bifurcation diagram given in Figure 10 is very similar to that of the DIE case (Figure 9), albeit shifted further to the right. The , uniqueness region, and multiplicity region are given in Table 2 for , and in Table 3 for (a stronger +DDE response). Model predictions are identical for this case as the previous one, including our ability to identify -values where the number of positive solutions changes from one to two, and then to none, as increases in the respective envelope.
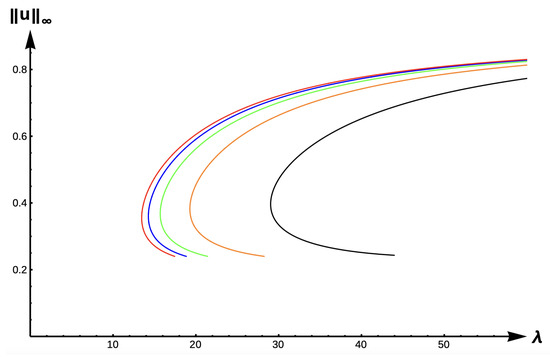
Figure 10.
Bifurcation diagram of positive solutions for (7) with (+DDE with ). Note that red represents , blue , green , orange , and black .

Table 2.
(with ).

Table 3.
(with ).
2.1.3. Bifurcation Diagrams for −DDE:
Figure 11 shows the evolution of bifurcation curves with respect to , the −DDE case with . Again, the bifurcation diagram is very similar to that of the DIE case (Figure 9), albeit shifted further to the left. The , uniqueness region, and multiplicity region are given in Table 4 for , and in Table 5 for (a stronger +DDE response). Model predictions are identical for this case as the previous one, including our ability to identify -values where the number of positive solutions changes from one to two, and then to none, as increases in the respective envelope.
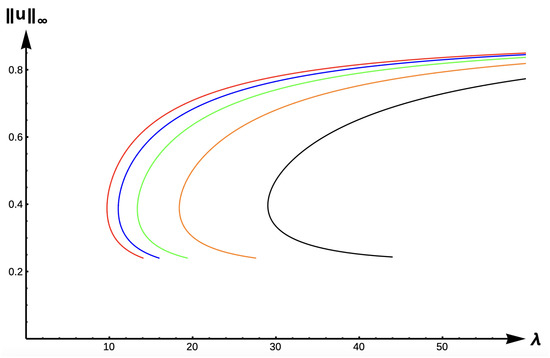
Figure 11.
Bifurcation diagram of positive solutions for (7) with (−DDE). Note that red represents , blue , green , orange , and black .

Table 4.
(with ).

Table 5.
(with ).
2.2. Results for Case B: Constant-Effort Predation:
In this subsection, we present results for the constant-effort predation case, where we have fixed and . We note that has two zeros (0 and 0.9) when . Here, Lemma 3 holds for and the shapes of and are as in Figure 12.
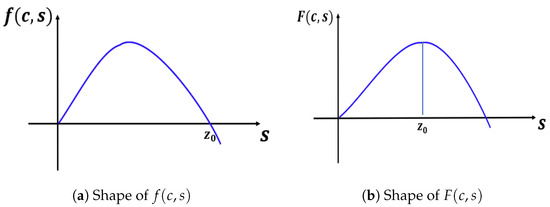
Figure 12.
Shapes of and in the case of constant-effort predation ().
2.2.1. Bifurcation Diagrams for DIE:
For the DIE case, Figure 13 displays the bifurcation diagram of the positive solutions for (7), and Table 6 gives the corresponding -values for various -values. We observe that the solution to (7) is always unique with a prediction of extinction for and unconditional persistence for . The bifurcations curve translate to the right, and hence -values increase as increases (see Table 6). The bifurcation curves are again approaching the bifurcation curve corresponding to the completely lethal matrix case (Dirichlet boundary condition) as . We again highlight that model predictions are drastically affected by PIE strength as measured in for patch sizes with a corresponding -value inside the envelope determined by the red () and black () curves. For example, for a patch size with corresponding , Figure 13 shows model predictions of unconditional persistence when , and extinction when for some .
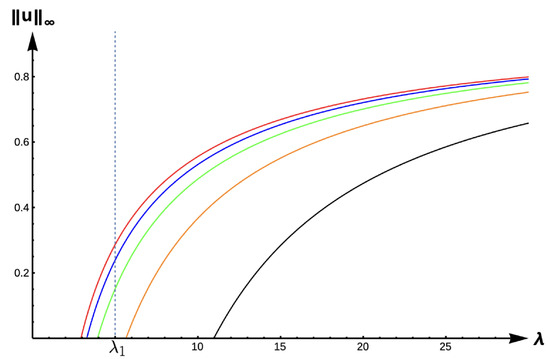
Figure 13.
Bifurcation diagram of positive solutions for (7) with (DIE). Note that the dashed line is for , red represents , blue , green , orange , and black .

Table 6.
.
2.2.2. Bifurcation Diagrams for +DDE:
Figure 14 shows evolution of the bifurcation curves, and Table 7 gives the corresponding -values for the +DDE case with various values. The bifurcation curves translate to the right and approach the bifurcation curve corresponding to the completely lethal matrix case (Dirichlet boundary condition) as increases to ∞. Model predictions are identical to that of the DIE case.
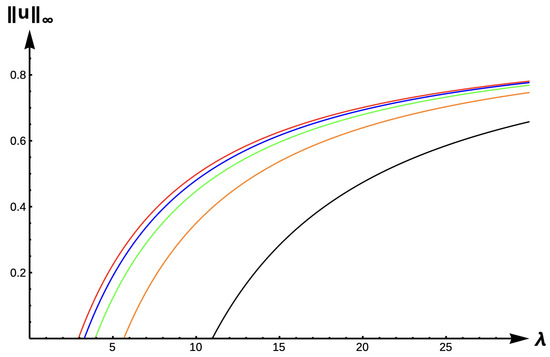
Figure 14.
Bifurcation diagram of positive solutions for (7) with (+DDE with ). Note that red represents , blue , green , orange , and black .

Table 7.
(with ).
2.2.3. Bifurcation Diagrams for −DDE:
Figure 15 and Figure 16 show the bifurcation curves for the −DDE case with strength and , respectively, for various PIE strength values . Table 8 and Table 9 give -values, uniqueness regions, and multiplicity regions for various values of for and , respectively. First, we observe that for any fixed DDE strength , the bifurcation curves translate to the right as increases, approaching the bifurcation curve of the completely hostile matrix case (Dirichlet boundary condition) when . For , model predictions are again similar to those of the previous DIE and +DDE cases. However, when the DDE strength is sufficiently high (as in Figure 16 with ), model predictions become much more interesting. For small PIE strength (, red, blue, green, and orange in Figure 16), there is a range of patch sizes where a patch-level Allee effect (PAE) is predicted by the model. For a patch with size corresponding to a -value in this range, the model predicts that the population will need to remain above a certain threshold in order to persist. Since the growth term is logistic, this PAE arises solely from the −DDE relationship. Similar cases of PAE have been noted by previous authors for the case when (see [18,32,37,42]). However, for higher PIE strength (, black in Figure 16), no such PAE is present and unconditional persistence is predicted by the model for patch sizes corresponding to .
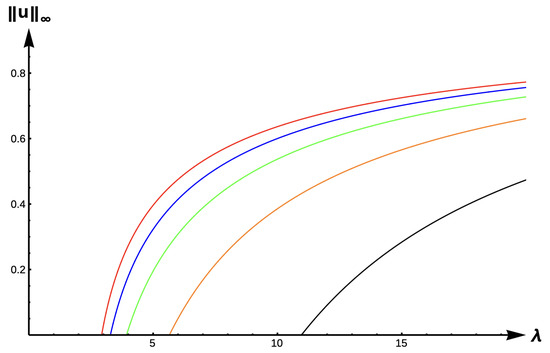
Figure 15.
Bifurcation diagram of positive solutions for (7) with (−DDE with ). Note that red represents , blue , green , orange , and black .
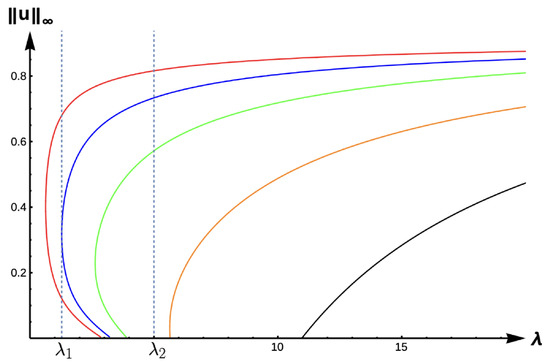
Figure 16.
Bifurcation diagram of positive solutions for (7) with (−DDE with ). Note that the dashed lines represent and , respectively, red represents , blue , green , orange , and black .

Table 8.
(with ).

Table 9.
(with ).
Notice the PIE envelope between the red and black curves in Figure 16 provides a varied range of predictions crucially dependent upon the PIE strength. As an example, a patch with size corresponding to would have predictions of a PAE for and extinction for . For a patch corresponding to , the model predicts the population is not as sensitive to PIE strength with unconditional persistence predicted for low PIE strength, a PAE for medium levels of PIE, and extinction predicted for high sensitivity to predation. For patches with corresponding outside of this envelope, predictions of unconditional persistence for and extinction for are unaffected by PIE altogether.
2.3. Results for Case C: Prey Switching Predation:
In this subsection, we present the results for the prey switching predation (Holling Type III) case, where we have fixed and . We note that has two zeros (0 and 0.849) when and . Here, Lemma 3 holds when and , have similar shapes to those in Figure 12.
2.3.1. Bifurcation Diagrams for DIE:
For these results, we fix the effective matrix hostility at . Figure 17 shows the bifurcation curves for the DIE case for various -values. Regardless of the PIE strength, the model predicts one of four outcomes: (1) extinction for , (2) unconditional persistence at a low maximum density level, (3) multiple positive steady states (one at low max density and one at high density), or (4) unconditional persistence at a high maximum density level. Table 10 shows the evolution of the uniqueness region as varies and Table 11 gives multiplicity regions. We also observe that there are three solutions for a certain interval of between the uniqueness regions. The interval where the multiplicity occurs translates to the right as increases. The variation in -values is given in Table 12 and we observe that the bifurcation curves translate to the right, approaching the bifurcation curve of the completely lethal matrix case (Dirichlet boundary condition) when .
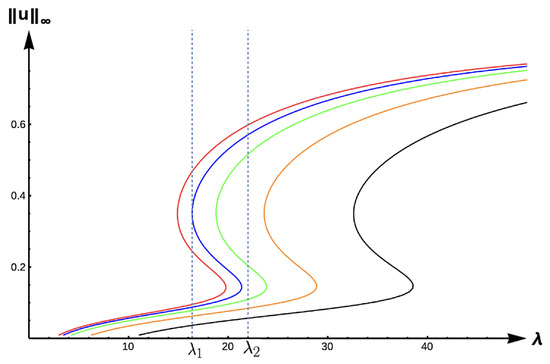
Figure 17.
Bifurcation diagram of positive solutions for (7) with (DIE). Note that the dashed lines represent and , respectively, red represents , blue , green , orange , and black .

Table 10.
Uniqueness regions.

Table 11.
Multiplicity regions.

Table 12.
Variation in .
As in previous cases, the envelope of -values for which model predictions are especially sensitive to PIE strength is given between the red and black curves in Figure 17. To highlight the effects of PIE, we first consider the example of a patch with size corresponding to in Figure 17. For a small PIE strength (), there is a non-Allee type bi-stability as denoted in case (3) above; however, for large PIE strength, unconditional persistence at a low density level (as in case (2) above) is predicted. For a larger patch with size corresponding to , cases (2)–(4) from above are possible as the PIE strength increases. Again, the sensitivity of the predation–emigration relationship causes vast population dynamical outcomes, even for the same patch size and matrix quality.
2.3.2. Bifurcation Diagrams for +DDE: ,
For the +DDE case, we again fix . Figure 18 shows how the bifurcation curves translate to the right as the PIE strength increases. Corresponding -values are given in Table 12, while Table 10 shows the variation in the uniqueness regions as changes. The -interval where the multiplicity occurs translates to the right as increases. Model predictions are identical to those of the DIE case.
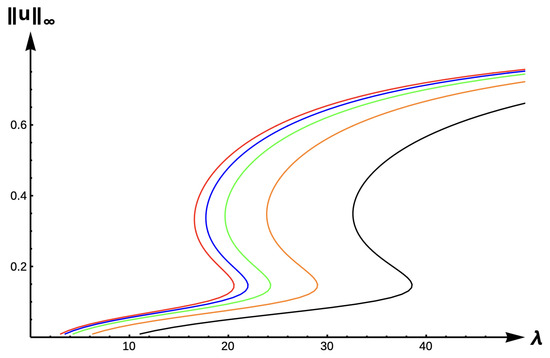
Figure 18.
Bifurcation diagram of positive solutions for (7) with (+DDE with ). Note that red represents , blue , green , orange , and black .
2.3.3. Bifurcation Diagrams for −DDE:
For the final case of −DDE, Figure 19, Figure 20 and Figure 21 show the evolution of bifurcation curves for , respectively, as varies. Table 13 gives uniqueness regions, Table 14 gives multiplicity regions, Table 15 gives patch level Alllee effect regions, and Table 16 provide uniqueness regions and -values, respectively, as varies. The case of low DDE strength () gives a scenario that is identical to the DIE and +DDE cases but with a leftward translation in the curves as illustrated in Figure 19. However, the envelope created by the red and black curves in Figure 20 and Figure 21 show a myriad of possible model predictions.
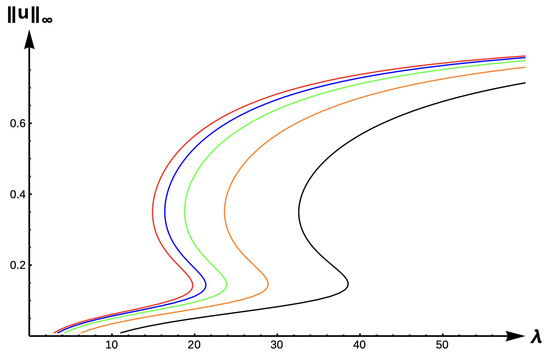
Figure 19.
Bifurcation diagram of positive solutions for (7) with (−DDE with ). Note that red represents , blue , green , orange , and black .
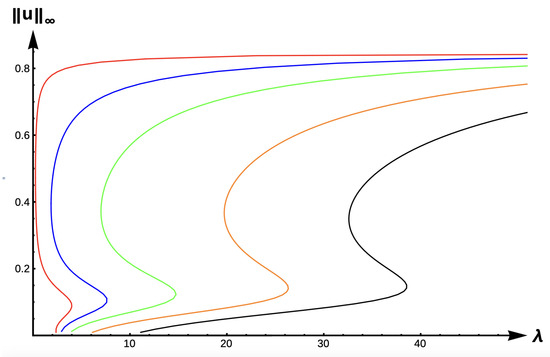
Figure 20.
Bifurcation diagram of positive solutions for (7) with (−DDE with ). Note that red represents , blue , green , orange , and black .
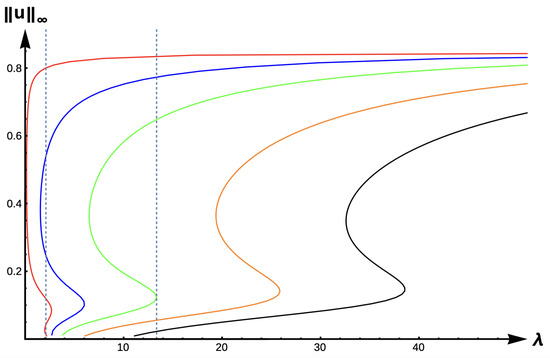
Figure 21.
Bifurcation diagram of positive solutions for (7) with (−DDE with ). Note that the dashed lines represent & , respectively, and red represents , blue , green , orange , and black .

Table 13.
, uniqueness regions.

Table 14.
, multiplicity region.

Table 15.
, Patch Allee effect region.

Table 16.
, .
In the case of medium DDE strength ( in Figure 20), potential model predictions include (1) extinction for , (2) a PAE, (3) unconditional persistence at a low maximum density level, (4) non-Allee effect type bi-stability (one positive steady state at low max density and one at high density), or (5) unconditional persistence at a high maximum density level. For the high DDE strength case (), all five of these dynamical outcomes are possible with the addition of a scenario as illustrated at in the red curve of Figure 21. For this -value, a PAE occurs and persistence is possible at either a high maximum density or a small maximum density level. Increasing the PIE strength for this patch size will change model prediction to that of a PAE, followed by extinction for sufficiently high PIE strength. However, for a patch with size corresponding to , outcomes (2)–(5) above are possible as the PIE sensitivity increases.
Next, we explore the effects of matrix hostility by considering the case with high DDE strength () and high matrix hostility (). Figure 22 illustrates the effect that extreme matrix hostility has on the model predictions, showing a new dynamical scenario at . At this value, there are five positive solutions to the steady-state problem, showing a very complex situation. Here, persistence is possible but with a variety of different maximum density levels. Figure 23 shows the evolution of bifurcation curves for as the PIE strength increases.
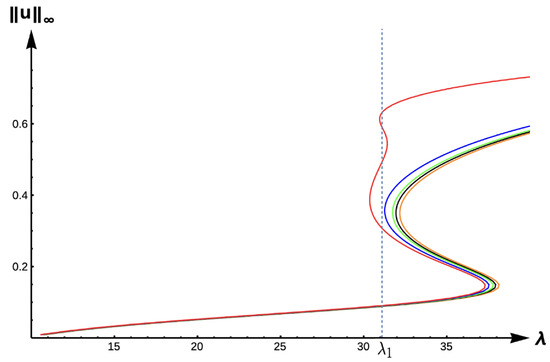
Figure 22.
Bifurcation diagram of positive solutions for (7) with (−DDE with ) and . Note that the dashed line represents , red represents , blue , green , orange , and black .
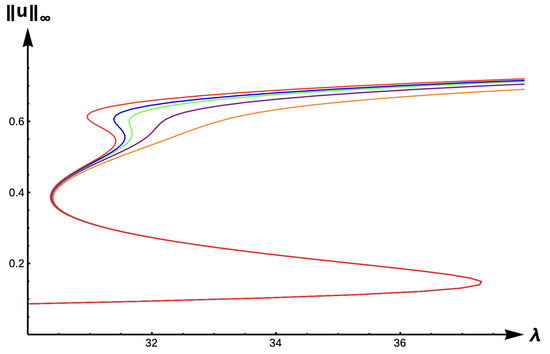
Figure 23.
Bifurcation diagram of positive solutions for (7) with (−DDE with ) and . Note that red represents , blue , green , purple , and orange .
3. Preliminaries
In this section, we introduce definitions of the (strict) subsolution and (strict) supersolution of (1), state a sub-supersolution theorem that is used to prove the existence and multiplicity results of positive solutions, and state a lemma which we employ to numerically generate bifurcation curves.
By a subsolution of (1), we mean that satisfies
By a supersolution of (1), we mean that satisfies
By a strict subsolution (supersolution) of (1), we mean a subsolution (supersolution) which is not a solution. Then, the following results hold (see [43,44,45]):
Lemma 1.
Lemma 2.
Let u be a positive solution of (7) when . Since is increasing for all it follows that u must be symmetric about and has the shape as in Figure 24 below (see [32]). Let and .
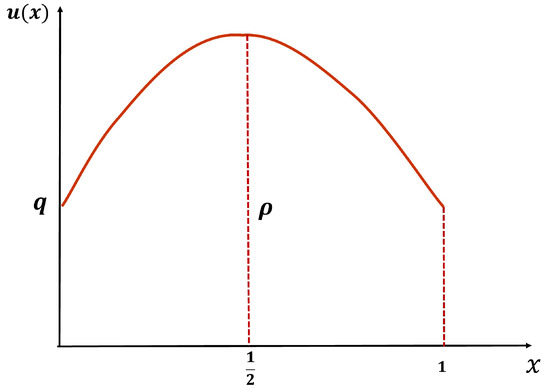
Figure 24.
The shape of the symmetric positive solutions of (7).
Then the following result holds:
Lemma 3.
(See [32]). For (7) has a positive solution u such that , , with if and only if λ, ρ, and q satisfy
and
where and .
Remark 4.
We provide the proof of Lemma 3 in the appendix for the convenience of the reader.
Next, we briefly explain how we obtain bifurcation curves using (8)–(9). Let be the unique positive falling zero of f and for some , where is the smallest positive zero of F when and when or . Letting , we numerically solve the Equation (9) for q using the FindRoot command in Mathematica. The values of q and are substituted into (8) to find the corresponding value of . Repeating this procedure for , , we obtain points for the bifurcation diagram.
We note that, in our study, when , the branch of positive solutions bifurcates from at , where is the principal (first positive) eigenvalue of
with an eigenfunction . Here, when and when .
4. Construction of Subsolutions and Supersolutions to Prove Theorems 1–6
Here, we state a couple of eigenvalue problems which are crucial to our proofs and recall some properties of their respective principal eigenvalues. For , let be the principal eigenvalue, be the corresponding normalized eigenfunction of
and let be the principal eigenvalue and be the corresponding normalized eigenfunction of
Note that the existence of both principle eigenvalues is standard (see [15,46]). For simplicity of notation, we denote with corresponding normalized eigenfunction for .
The following lemma gives several useful properties of and (see [15,46,47]).
Lemma 4.
Let , denote the principal eigenvalue of (11), the principal eigenvalue of (12), and the principal eigenvalue of (2). Then, we have the following for :
- (1)
- for .
- (2)
- for .
- (3)
- is decreasing in M and increasing in b and γ.
- (4)
- .
- (5)
- is decreasing in M and increasing in b and γ.
Now, we present the construction of several crucial sub- and supersolutions for (2).
- Construction of a subsolution when for , or and (DIE), (+DDE) or (−DDE), where when and when .
For a fixed , recall that is the principal eigenvalue and the corresponding normalized eigenfunction of (12). We note that for . Let for and Then, we have and since . Therefore, . This implies that
for . We also have
for since and for Hence, is a subsolution of (1) for .
- Construction of a subsolution when such that as for , and (DIE) or (+DDE).
Consider the following problem:
Let be the unique positive solution of (13) for (see [15]), where is the principal eigenvalue of (4).
We note that as Let . Then, we have Also,
by the Hopf maximum principle. Therefore, is a subsolution of (1) for such that as .
- Construction of a strict subsolution in when , , and when for some
Let where I is as in (3). Choose and in (11). Next, for a fixed , let be the principal eigenvalue and be the corresponding normalized eigenfunction of (11). We note that when . Define for . Then, and since , , and . This implies that for . Let be such that
Next, we define where . Observe that , and implies that Let and define From (14), we have
Next, since , we have
This implies that
Let be such that
This implies that Observe that and
for Now, for , we have
by (15). Hence, is a strict subsolution of (1) for and
- Construction of a strict subsolution when & hold for .
Let be such that is non-decreasing on on and on Then, the following boundary value problem
has a solution such that for provided and are satisfied (see [33]). Let Since on and on by the Hopf maximum principle, it is easy to show that is a strict subsolution of (1) for .
- Construction of a strict supersolution for , , , , or , and any form of g.
- is a global supersolution of (1) for all .
- Construction of a strict supersolution for , for , or and any form of g where when and when .
For a fixed , recall is the principal eigenvalue and the corresponding normalized eigenfunction of (12). We note that for (see Lemma 4). Let and Since and , we have and giving that for . This implies that
and
for since Hence, is a strict supersolution of (1) for and .
- Construction of a small supersolution for and when and (DIE) or (+DDE).
For a fixed , recall is the principal eigenvalue and is the corresponding normalized eigenfunction of (11) (here, and ). We note that for (see Lemma 4). Define , where . Then, we have
Also, we have
since when (DIE) or (+DDE). This implies that is a supersolution of (1) when . Since as , as and hence, as .
- Construction of a strict supersolution when & hold for
Hence, is a strict supersolution of (1) with .
5. Proofs of Theorems 1–4
In this section, we provide proofs of our main results.
Proof of Theorem 1.
We note that the problem
has a positive solution for such that as (see [48]). Let . Then, we have Also,
by the Hopf maximum principle. Therefore, is a subsolution of (1) for . Further, is a global supersolution of (1). Then, by Lemma 1, it follows that (1) has at least one positive solution in for This implies that (1) has at least one positive solution for such that as since as . □
Proof of Theorem 2.
We first prove here the non-existence of a positive solution for . Let be the principal eigenvalue and be the corresponding normalized eigenfunction of (11). We note that when (see Lemma 4). Suppose is a positive solution of (1) for . Then, by Green’s Second Identity, we have
On the other hand, we have
since and when . This is a contradiction. Thus, (1) has no positive solution for
We prove the existence of a positive solution, , for such that as
Recall the subsolution for and the supersolution Since , by Lemma 1, it follows that (1) has a positive solution in for Also, recall the subsolution for Then, by Lemma 1, it follows that (1) has a positive solution in for This implies that (1) has a positive solution for such that as since as .
Next, we prove that as . Recall the subsolution and supersolution and choose small enough such that . Then, by Lemma 1, (1) has a positive solution such that as since as . But, the uniqueness of positive solutions of (1) proved above implies that . Hence, we have as
Now, we prove the uniqueness of the positive solution for . Suppose that (1) has two distinct positive solutions, , for . Since is a global supersolution, it follows that (1) has a maximal solution. Thus, without loss of generality, we may assume that Then, by Green’s Second Identity, we have
since g is non-decreasing and . We note that is decreasing, and hence
since . This is a contradiction. Hence, (1) has at most one positive solution for . □
Proof of Remark 1.
Let , , , and be the unique positive solutions of (1) when and , respectively. Then, we have
It is clear that is a strict subsolution of
Proof of Theorem 3.
We note that is a solution and hence a subsolution of (1). Recall the strict subsolution for when , strict supersolution (with ) for , and supersolution for We can also choose to be small enough such that . By Lemma 2, (1) has at least two positive solutions, and , for . Since is a solution, Lemma 2 can only guarantee the existence of at least two positive solutions. Hence, there is a PAE for .
We now show that if and are fixed, then there exists such that (1) has no positive solution for when . Choose in (11) such that (see Lemma 4). For a given , recall that is the principal eigenvalue, and is the corresponding normalized eigenfunction of (11). We note that for (see Lemma 4). Now, suppose that (1) has a positive solution , for . Then, we have . This implies that
Proof of Theorem 4.
The proof of this Theorem follows from the proof of Theorem 3. □
Proof of Theorem 5.
Recall the subsolution for (here, ), strict subsolution for , supersolution for and strict supersolution for Since , we have By construction, we have Choosing , we have . Then, by Lemma 2, the result follows. □
Justification of the Remark 3:
Here, we prove that our growth term f and emigration term g satisfy the conditions in Theorem 5 when , and is a ball of radius R.
Recall that . For simplicity of demonstration, we let . Note that
and has three positive solutions , and such that (see Figure 25). It can be shown that , and . Note that increases on and decreases on with respect to s. Hence and are the local maximum and is a local minimum.
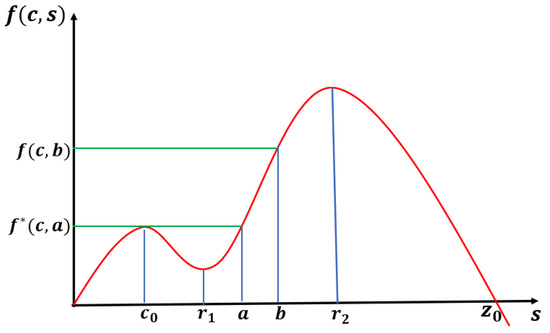
Figure 25.
The shape of f along with the values of , and .
We choose such that , and . Now, we will show that which will imply for . By choosing , we have . This implies that for since is an increasing function of N. Then, we have
We note that the solution of (5) is given by
and as since as . Hence, we have
by (20) for . Hence, when is large, we have
Thus, we have when is large for .
We note that the principal eigenvalue (of (4)) when is a ball of radius R is given by
where is the first zero of the Bessel function of order n. From [33], we have for , and when . Then, and
for . On the other hand, we have
This implies that when is large for since as . Thus, we have and
holds when . This completes the justification.
Proof of Theorem 6.
Here, we prove the non-existence of a positive solution for
Note that for all . Let be the principal eigenvalue and be the corresponding normalized eigenfunction of (11). We note that when (see Lemma 4). Suppose is a positive solution of (1) for . Then, by Green’s Second Identity, we have
On the other hand, we have
since and for . This is a contradiction. Thus, (1) has no positive solution for . □
Proof of Remark 3.
Here, we prove non-existence for in the Cases A and B. Recall that for . Assume is a positive solution of (1) for . Then, by Green’s Second Identity, we have
since . On the other hand, we have
This is a contradiction. Thus, (1) has no positive solution for . This completes the proof. □
Author Contributions
Conceptualization, J.T.C., N.F., Jerome Goddard II, R.S. and X.X.; Methodology, J.T.C., N.F., J.G.II, R.S. and X.X.; Formal analysis, J.T.C., N.F., J.G.II, R.S. and X.X.; Writing—original draft, J.T.C., N.F., J.G.II, R.S. and X.X.; Writing—review & editing, J.T.C., N.F., J.G.II, R.S. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by NSF grants Cronin (DMS-2150945 & DMS-2246724), Goddard (DMS-2150946 & DMS-2246725), and Shivaji (DMS-2150947 & DMS-2246723).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the NSF for their support via grants: Cronin (DMS-2150945 & DMS-2246724), Goddard (DMS-2150946 & DMS-2246725), and Shivaji (DMS-2150947 & DMS-2246723).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1. Proof of Lemma 3
Integrating both sides gives us
Now using the fact that , we obtain
and
Since the solution is symmetric about , it is enough to consider the case when . Then, integrating both sides, we obtain
Using the substitution , we have
By letting , we obtain
This implies that
Conversely, assume that satisfy the equations
and
Define by
and .
We will now show that is a positive solution of (7). The function
is a differentiable function of u which is strictly increasing from 0 to as u increases from q to . Thus, for each , there is a unique such that
Moreover, by the Implicit Function Theorem, is differentiable with respect to x. Differentiating (A9) gives
Through a similar argument, is a differentiable, decreasing function of x for with
These imply
Differentiating again, we have
Thus, satisfies Equation (7). Now, we show that satisfies the boundary conditions in (7). Since q is a solution of (A6), we have
Substituting into (A10) gives
A similar argument shows that
Hence, satisfies (7), and the proof is complete.
References
- Werner, E.E.; Peacor, S.D. A review of trait-mediated indirect interactions in ecological communities. Ecology 2003, 84, 1083–1100. [Google Scholar] [CrossRef]
- Cronin, J.T.; Haynes, K.J.; Dillemuth, F. Spider effects on planthopper mortality, dispersal, and spatial population dynamics. Ecology 2004, 85, 2134–2143. [Google Scholar] [CrossRef]
- Holt, R.D.; Barfield, M. Trait-mediated effects, density dependence and the dynamic stability of ecological systems. In Trait-Mediated Indirect Interactions: Ecological and Evolutionary Perspectives; Cambridge University Press: Cambridge, UK/New York, NY, USA, 2012. [Google Scholar]
- Ohgushi, T.; Schmitz, O.; Holt, R.D. Trait-Mediated Indirect Interactions: Ecological and Evolutionary Perspectives; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Schmitz, O.J. Top predator control of plant biodiversity and productivity in an old-field ecosystem. Ecol. Lett. 2003, 6, 156–163. [Google Scholar] [CrossRef]
- Sih, A.; Kats, L.B.; Moore, R.D. Effects of predatory sunfish on the density, drift, and refuge use of stream salamander larvae. Ecology 1992, 73, 1418–1430. [Google Scholar] [CrossRef]
- Peckarsky, B.L. Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ecology 1996, 77, 1888–1905. [Google Scholar] [CrossRef]
- Hakkarainen, H.; Ilmonen, P.; Koivunen, V.; Korpimäki, E. Experimental increase of predation risk induces breeding dispersal of Tengmalm’s owl. Oecologia 2001, 126, 355–359. [Google Scholar] [CrossRef]
- Cantrell, R.S.; Cosner, C. Effects of harvesting mediated by dispersal traits. Nat. Resour. Model. 2018, 31, e12168. [Google Scholar] [CrossRef]
- Fahrig, L. How much habitat is enough? Biol. Conserv. 2001, 100, 65–74. [Google Scholar] [CrossRef]
- Ricketts, T.H. The matrix matters: Effective isolation in fragmented landscapes. Am. Nat. 2001, 158, 87–99. [Google Scholar] [CrossRef]
- Heilman, G.E.; Strittholt, J.R.; Slosser, N.C.; Dellasala, D.A. Forest fragmentation of the conterminous United States: Assessing forest intactness through road density and spatial characteristics: Forest fragmentation can be measured and monitored in a powerful new way by combining remote sensing, geographic information systems, and analytical software. BioScience 2002, 52, 411–422. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K.; Pearse, W.D.; Lefebvre, V.; Rosa, I.M.D.; Carreiras, J.M.B.; Lucas, R.M.; Reuman, D.C. Using landscape history to predict biodiversity patterns in fragmented landscapes. Ecol. Lett. 2013, 16, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Cantrell, R.S.; Cosner, C. Spatial Ecology via Reaction-Diffusion Equations; Mathematical and Computational Biology; Wiley: Chichester, UK, 2003; p. 411. [Google Scholar]
- Haynes, K.J.; Cronin, J.T. Matrix composition affects the spatial ecology of a prairie planthopper. Ecology 2003, 84, 2856–2866. [Google Scholar] [CrossRef]
- Fonseka, N.; Goddard, J., II; Morris, Q.; Shivaji, R.; Son, B. On the effects of the exterior matrix hostility and a U-shaped density dependent dispersal on a diffusive logistic growth model. Discret. Contin. Dyn. Syst.-Ser. S 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Goddard, J., II; Morris, Q.; Payne, C.; Shivaji, R. A diffusive logistic equation with U-shaped density dependent dispersal on the boundary. Topol. Methods Nonlinear Anal. 2019, 53, 335–349. [Google Scholar] [CrossRef]
- Cronin, J.T.; Goddard, J., II; Shivaji, R. Effects of patch–matrix composition and individual movement response on population persistence at the patch level. Bull. Math. Biol. 2019, 81, 3933–3975. [Google Scholar] [CrossRef] [PubMed]
- Lotka, A. Elements of Physical Biology; Williams and Wilkins Company: Baltimore, MA, USA, 1925. [Google Scholar]
- Volterra, V. Variations and fluctuations of the number of individuals in animal species living together. J. Cons. Cons. Int. Pour L’Explor. Mer 1928, 3, 2–51. [Google Scholar] [CrossRef]
- Holling, C.S. The Functional Response of Predators to Prey Density and its Role in Mimicry and Population Regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef]
- Hassell, M.P. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Oaten, A. Predation and Population Stability. In Advances in Ecological Research; MacFadyen, A., Ed.; Academic Press: Cambridge, MA, USA, 1975; Volume 9, pp. 1–131. [Google Scholar] [CrossRef]
- Real, L.A. Ecological Determinants of Functional Response. Ecology 1979, 60, 481–485. [Google Scholar] [CrossRef]
- Matson, P.; Berryman, A. Ratio-dependent predator-prey theory. Ecology 1992, 73, 1529–1566. [Google Scholar] [CrossRef]
- Oruganti, S.; Shi, J.; Shivaji, R. Diffusive logistic equation with constant yield harvesting. Trans. Am. Math. Soc. 2002, 354, 3601–3619. [Google Scholar] [CrossRef]
- Shang, Z.; Qiao, Y.; Duan, L.; Miao, J. Bifurcation analysis in a predator–prey system with an increasing functional response and constant-yield prey harvesting. Math. Comput. Simul. 2021, 190, 976–1002. [Google Scholar] [CrossRef]
- Maciel, G.A.; Lutscher, F. How individual movement response to habitat edges affects population persistence and spatial spread. Am. Nat. 2013, 182, 42–52. [Google Scholar] [CrossRef]
- Cronin, J.T.; Fonseka, N.; Goddard, J., II; Leonard, J.; Shivaji, R. Modeling the effects of density dependent emigration, weak Allee effects, and matrix hostility on patch-level population persistence. Math. Biosci. Eng. 2020, 17, 1718. [Google Scholar] [CrossRef]
- Lee, E.; Sasi, S.; Shivaji, R. S-shaped bifurcation curves in ecosystems. J. Math. Anal. Appl. 2011, 381, 732–741. [Google Scholar] [CrossRef]
- Lee, E.; Sasi, S.; Shivaji, R. An ecological model with a ∑-shaped bifurcation curve. Nonlinear Anal. Ser. B Real World Appl. 2012, 13, 634–642. [Google Scholar] [CrossRef]
- Fonseka, N.; Shivaji, R.; Son, B.; Spetzer, K. Classes of reaction diffusion equations where a parameter influences the equation as well as the boundary condition. J. Math. Anal. Appl. 2019, 476, 480–494. [Google Scholar] [CrossRef]
- Goddard, J.; Shivaji, R. Diffusive logistic equation with constant yield harvesting and negative density dependent emigration on the boundary. J. Math. Anal. Appl. 2014, 414, 561–573. [Google Scholar] [CrossRef]
- Cronin, J.T.; Goddard, J., II; Muthunayake, A.; Quiroa, J.; Shivaji, R. Predators-induced prey dispersal can cause hump-shaped density-area relationships in prey populations. J. Math. Biol. 2024, 88, 1–31. [Google Scholar] [CrossRef]
- Cantrell, R.S.; Cosner, C.; Fagan, W.F. How Predator Incursions Affect Critical Patch Size: The Role of the Functional Response. Am. Nat. 2001, 158, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Fonseka, N.; Goddard, J., II; Henderson, A.; Shivaji, R. On the effects of density-dependent emigration on ecological models with logistic and weak Allee type growth terms. Discret. Contin. Dyn. Syst. Ser. B 2024, 29, 1501–1524. [Google Scholar] [CrossRef]
- Goddard, J., II; Morris, Q.; Shivaji, R.; Son, B. Bifurcation curves for singular and nonsingular problems with nonlinear boundary conditions. Electron. J. Differ. Equ. 2018, 2018, 1–12. [Google Scholar]
- Laetsch, T. The number of solutions of a nonlinear two point boundary value problem. Indiana Univ. Math. J. 1970/1971, 20, 1–13. [Google Scholar] [CrossRef]
- Harman, R.; Goddard, J., II; Shivaji, R.; Cronin, J.T. Frequency of occurrence and population-dynamic consequences of different forms of density-dependent emigration. Am. Nat. 2020, 195, 851–867. [Google Scholar] [CrossRef]
- Amann, H. Fixed point equations and nonlinear eigenvalue problems in ordered Banach spaces. SIAM Rev. 1976, 18, 620–709. [Google Scholar] [CrossRef]
- Inkmann, F. Existence and multiplicity theorems for semilinear elliptic equations with nonlinear boundary conditions. Indiana Univ. Math. J. 1982, 31, 213–221. [Google Scholar] [CrossRef]
- Shivaji, R. A remark on the existence of three solutions via sub-super solutions. Nonlinear Anal. Appl. Lect. Notes Pure Appl. Math. 1987, 109, 561–566. [Google Scholar]
- Amann, H. Nonlinear elliptic equations with nonlinear boundary conditions. In North-Holland Mathematics Studies; North-Holland: Amsterdam, The Netherlands, 1976; Volume 21, pp. 43–63. [Google Scholar] [CrossRef]
- Goddard, J., II; Morris, Q.; Robinson, S.; Shivaji, R. An exact bifurcation diagram for a reaction diffusion equation arising in population dynamics. Bound. Value Probl. 2018, 170, 1–17. [Google Scholar] [CrossRef]
- Clement, P.; Sweers, G. Existence and multiplicity results for a semilinear elliptic eigenvalue problem. Ann. Della Sc. Norm. Super. Pisa—Cl. Sci. Sér. 4 1987, 14, 97–121. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).