Abstract
This paper searches for optimal strategies for the minimization of the number of high-risk latent and active tuberculosis (TB) infectious individuals using real data from Ethiopia. Optimal control theory is harnessed for investigation and analysis of the optimal combination of interventions for controlling the transmission of TB using distancing, case finding, and case holding as controls. We calculate and compare the incremental cost-effectiveness ratio (ICER) for each of the strategies to determine the most effective combination of interventions for curbing the spread of the disease. Our findings suggest that, for optimal cost-effective management of the TB disease, the government of Ethiopia must focus more on prevention strategies such as isolation of infectious people, early TB patient detection, treatment, and educational programs. The optimal strategy is quantified through simulation.
Keywords:
tuberculosis; TB detection; social distancing; isolation; incremental cost-effectiveness ratio MSC:
92D30; 34K20
1. Introduction
Tuberculosis (TB) is still a significant public health problem and is one of the top 10 causes of illness and death. Globally, in 2019, 10 million people were infected with TB, and 1.4 million died [1]. In Ethiopia, tuberculosis is still a major health problem and one of the leading causes of death [2]. Furthermore, Ethiopia is one of the 30 high-burden countries, and there were an estimated 157,000 (140 per 100,000 of the population) incident cases of TB in 2019 [1]. Therefore, effective prevention measures are needed to stop the spread of tuberculosis in Ethiopia. This study aims to identify the most cost-effective combination of interventions for curbing the spread of TB in Ethiopia.
Among the many different forms of action in the fight against TB, distancing, case finding, and case holding are the most important [3]. Distancing control is an essential strategy to curb the spread of airborne contagious diseases such as TB, influenza, COVID-19, etc., by reducing the opportunities for close contact between people. Case finding is another important controlling method of TB [4], which is the process of screening and treating latent TB patients. Finally, case holding includes the activities and techniques we can apply to help patients complete the treatment they have started.
Infectious diseases can exhibit complex nonlinear dynamics, and it is possible to examine, explain, and predict the transmission dynamics of infectious diseases using mathematical models [5,6,7,8,9,10,11]. An optimal control problem entails the identification of a feasible scheme, policy, program, strategy, or campaign to achieve the optimal possible outcome of a system [12]. Numerous scholars (for example [3,13,14,15,16,17]) have applied the optimal control theory to predict suitable control strategies and to analyze their cost-effectiveness in mitigating the TB disease. Sunhwa Choi and Eunok Jung [3] developed a mathematical model for the transmission dynamics of TB in South Korea and considered three different control strategies (distancing, case finding, and case holding efforts). The results showed that distancing control, such as isolation of infectious people, early TB patient detection, and educational programs/campaigns constitute the most effective combination of interventions for the prevention of TB transmission in South Korea. In [13] a mathematical TB model with control was developed and analysed based on the Philippines’s real data. The result of their study showed that enhancing active case finding instead of the case holding control together with distancing has significant potential for curtailing the spread of TB in the Philippines. Gao and Huang [14] analyzed a TB model that incorporates vaccination, case finding, and case holding controls. Their result revealed that the combined implementation of three controls is the most effective and less expensive among different strategies. The mathematical model [15] for the transmission dynamics of TB in Angola considered two control strategies (case finding and case holding controls). Their results showed that the combined strategy that involves both controls is preferable. Doyo Kereyu and Seleshi Demie [16] developed and analyzed a TB model for Haramaya district, Ethiopia. They considered three control strategies (distancing, case finding, and treatment efforts). The results suggested that a combination of all interventions makes for the best strategy to eradicate TB disease from the community at an optimal level with minimum cost.
All the above studies showed that the strategies we use to control the spread of TB may vary depending on the situation in the country. Therefore, each government must adopt a better and more cost-effective approach based on its realities. In this study, based on Ethiopian TB data, we propose effective methods to eliminate the disease from the country.
2. TB Model with Controls
The following model (1) of TB disease dynamics was proposed and analyzed in [11]. This model forms the basis of our investigation in the current paper, and we include it for completeness.
The total population size is partitioned into four subclasses: susceptible , high-risk latent , infectious , and low-risk latent . We aggregated the two groups, the recovered and the low-risk latent, in a class called low-risk individuals ().
with .
The recruitment rate to the susceptible population is assumed to be constant . We assume that all classes have the same natural death rate , with disease-induced mortalities occurring only in the -class at a rate . The susceptible individual acquires the TB bacteria through contact with infected individuals with a nonlinear transmission rate . It is assumed that the BCG vaccine will be administered to susceptible individuals (at a rate ). People who have been vaccinated can become infected because the vaccine is imperfect and does not completely protect against the disease. The vaccinated individuals are infected at a rate where is the loss of vaccine protection. Newly infected individuals (with a latent level) will develop active TB (at a rate ). We assume that patients at the latent stage will move to the -class with a rate of when treated. Here is the treatment coverage rate, represents the successful treatment rate for active TB infected individuals, and represents the relapse rate.
We modified the model (1) by including three control strategies, for . The controls represent the intensities of different public health interventions. The function is a distancing control associated with the effort to reduce susceptible individuals that become infected, and such effort includes an isolation policy, wearing a face mask, or public educational program. A case finding control ()) represents the effort of decreasing the number of latently infected individuals that may develop active TB. Such activities include screening and treatment of latent individuals who are at high risk of developing active TB. The third strategy is a case holding control, denoted by . It refers to efforts to prevent the failure of treatment in infectious individuals (e.g., patient supervision, including activities used to ensure the regularity of drug intake until the last treatment stage is attained).
This leads to the following system of ODEs, with all the parameters constant and in which we have suppressed the time variable:
with initial conditions .
Let
are Lebesgue integrable functions on the interval , with .
We searched for an optimal control that minimizes the objective functional
- where
In Equation (3), the values of and are taken as 0 and 20, respectively, to determine Ethiopia’s 20-year (2019–2038) effective TB control strategies. The constants , , are positive weight constants, which balance the cost factors associated with the controls and , respectively. The functions , and are the costs of the controls and , respectively. The cost terms are assumed to be nonlinear quadratic functions (as in [17,18,19]).
2.1. Existence of an Optimal Control
Theorem 1.
There exists an optimal controlthat minimizes the objective functionalsubject to the control system (2).
Proof.
Let us denote the right-hand side of the system (2) by . Then following the same procedure as in [14], we prove the existence of an optimal control . To achieve this, we must first show that the following conditions are met.
- is of class and there exists a constant such that
- ii.
- The set of all solutions to system (2) with corresponding control in is nonempty,
- iii.
- There exist functions and such that ,
- iv.
- The control set is closed, convex and compact,
- v.
- The integrand of the objective function is convex in .
To verify the first conditions, let us write
Then we can easily show that is of class and .
Moreover, we will have the following
and
Since , and are bounded, there exists a constant c such that
This shows that condition (i) is satisfied.
According to condition (i), there is a unique solution for the constant controls, which will ensure that condition (ii) is met.
Besides,
This verifies condition (). The subset of is closed and bounded, and hence compact. Thus condition (iv) is fulfilled. We proceed with verification of condition (v), the convexity of the integrand of the objective functional. We must prove that for any two values and of the control vector, and a constant the following inequality holds:
where
Further,
And
Then
Consequently, condition (v) is satisfied, and this completes the proof. □
2.2. Characterization of an Optimal Control
To find the best cost-effective strategies for reducing the number of high-risk latent and infectious we use optimal control theory. In this section, we derive the necessary conditions for the optimal control by using Pontryagin’s Maximum Principle [20,21]. We formulate the Hamiltonian
Here, are the adjoint functions.
Theorem 2.
For the optimal control () and the corresponding solutions to the variables, that minimizes the Equation (3), there exist adjoint variablesandsatisfying
with transversality conditions
Furthermore,
Proof.
By applying Pontryagin’s Maximum Principle, we obtain the adjoint system (5) as follows:
with
Evaluating the optimal control and corresponding state variables, we obtain the adjoint system (5) and the transversality conditions (6).
Finally, by applying the optimality condition
And using the bounds for the controls , we can derive the optimal control as in Equation (7). □
3. Numerical Results and Discussion
Using Matlab2019b, the optimal control system is solved by applying the forward-backward sweeping technique. According to [7], in the total population of Ethiopia, the classes and comprise 16.37% and 30% of the population, respectively. Based on these percentages we can deduce values for and .
The values of parameters and the initial values of the variables used in our simulations are presented in Table 1. The algorithm used for the solution is based on the approach proposed in [20,22].

Table 1.
Values of variables and parameters.
Studies show that applying combined strategies rather than single strategies is more effective in curbing the spread of TB [13,16]. Therefore, to examine the impact of each control on the elimination of TB, we test the following four control strategies, and we search for an optimal combination of these interventions.
Strategy: distancing and case holding controls ( and ), with
Strategy: case finding and case holding controls ( and ), with
Strategy: distancing and case finding controls ( and ), with
Strategy: Using all the control efforts (, and ).
We assume a value for the weight parameters . Since the case holding control targets active TB patients undergoing treatment, the numbers in these groups are smaller than the others. Hence it is reasonable to take as being far smaller than and and we assigned a value .
The dynamics of the total infected population () are shown in Figure 1. It can be observed that the number of infected individuals can be significantly decreased when the three control inputs ( and ) are used simultaneously. Like Strategy , Strategies and play a significant role in reducing the number of high-risk latent individuals. In contrast, Strategy has the least impact on reducing the number of patients. This shows that it is beneficial to use case finding control in combination with other strategies to prevent the disease.
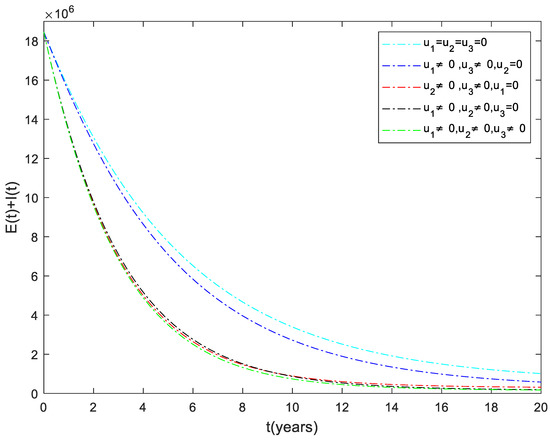
Figure 1.
The dynamics of the total infected population under different control strategies.
3.1. Strategy : Use of Distancing and Case Holding Controls
In this strategy, the distancing and the case holding controls are used to optimize the objective function while we set case finding control to zero. Figure 2a shows that the total number of infected people has a significant difference when we compare with control and without control. Specifically, when this strategy is implemented, total infected people are averted. The total cost for the combined effects of these two controls is given in Figure 2b. The simulation results in Figure 2c suggest that this strategy would require both distancing and case holding controls to be at maximum for almost the entire period of intervention.
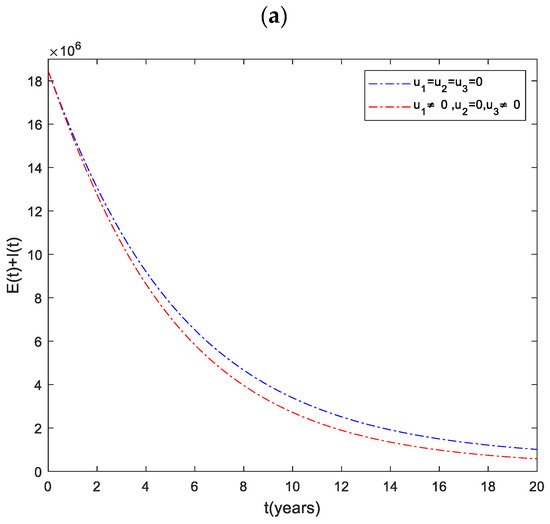
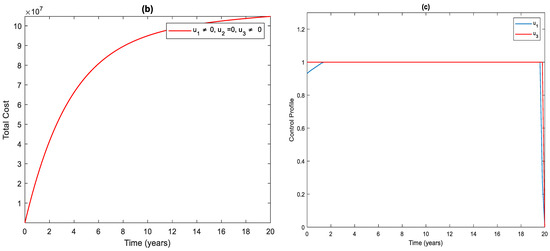
Figure 2.
(a) The impact of distancing and case holding control on the infected population. (b) Cost function. (c) Optimal controls profile.
3.2. Strategy : Control with Case Finding and Case Holding
Figure 3a shows the significant difference in the numbers of the total infected population with control and without control. More precisely, the total number of infected people with and without controls at the end of the simulation period is and , respectively. To achieve this, the control profile and should be implemented at a maximum (Figure 3c). The cost function for this strategy is shown in Figure 3b. The total cost when the strategy is implemented throughout the simulated time horizon is .
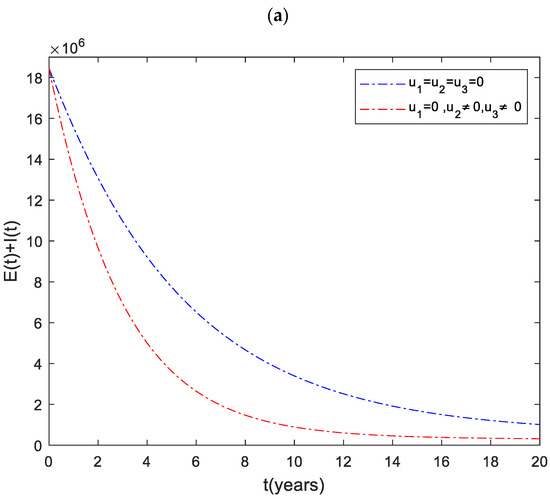
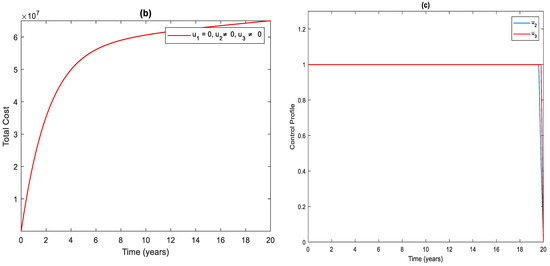
Figure 3.
(a) The impact of case finding and case holding control on the infected population. (b) Cost function. (c) Optimal controls profile.
3.3. Strategy : Use of Distancing and Case Finding Control
As shown in Figure 4a, there is a significant difference in the number of infected individuals with control and without control. By applying this strategy, infected people are averted. The cost function for this strategy is shown in Figure 4b. The simulation result in Figure 4c shows that this strategy would require that the case finding should be at maximum for almost the entire period of intervention, while distancing controls should start at and gradually increase to the maximum.
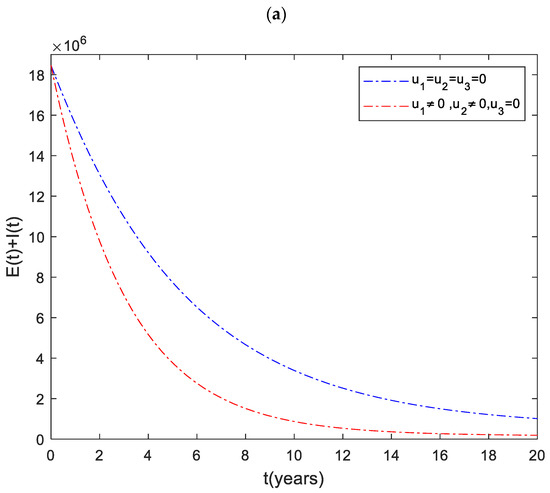
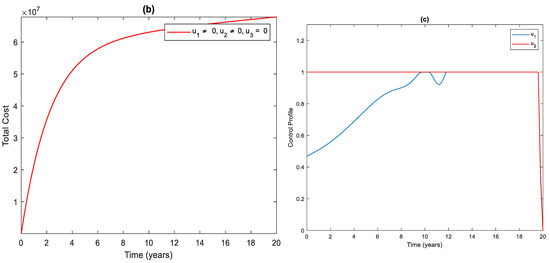
Figure 4.
(a) The impact of distancing and case finding control on the infected population. (b) Cost function. (c) Optimal controls profile.
3.4. Strategy : Using All the Controls
In this strategy, we have implemented a combination of all the three controls. This method helps us to save more people from disease than any other strategy. As we can see from Figure 5a, it averts about infected people. The cost and control functions of this strategy are displayed in Figure 5b,c.
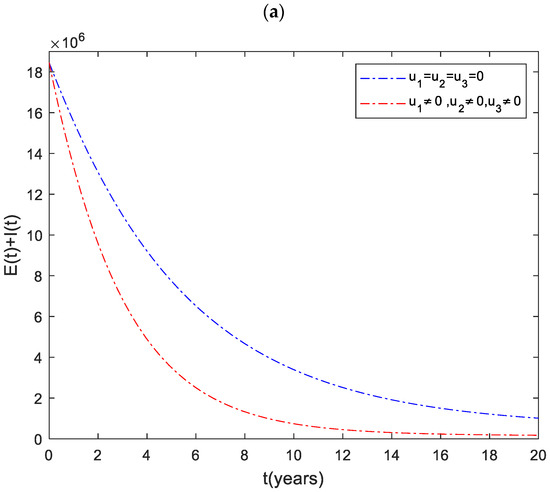
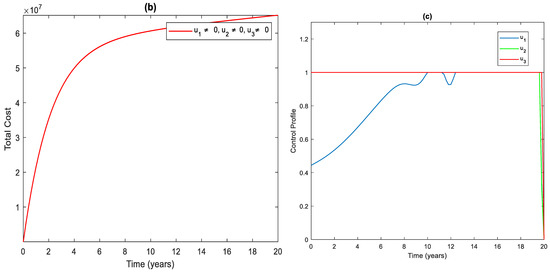
Figure 5.
(a) The impact of the combination of all controls on the infected population. (b) Cost function. (c) Optimal controls profile.
4. Cost-Effectiveness Analysis
Controlling and eliminating the spread of infectious diseases in a community requires time and money. Therefore, it is essential to identify and implement cost-effective strategies to prevent the spread of the disease. In addition, community awareness and lifestyle are critical factors determining the spread of disease. As a result, effective methods of controlling the spread of disease may vary from country to country. In this study, we identified cost-effective ways to prevent the spread of tuberculosis in Ethiopia. We used the incremental cost-effectiveness ratio () to do this. The is defined as the cost per health outcome [27], which is given by:
Table 2 calculates the total number of infections averted by each strategy and the total cost of implementing the strategy. We calculated the number of infections averted by subtracting the number of infections with control from without control. On the other hand, the total cost of each strategy was obtained using the cost function , , and .

Table 2.
Cost-effectiveness of the control strategies.
To implement the method, we first needed to rank the control strategies based on averted infection, as shown in Table 2. Based on this rank, we first compared the of strategy and strategy as follows.
Which shows that strategy is less costly than strategy . Strategy was then ignored, and the analysis continued by comparing strategy B with C as:
This indicates that Strategy is cheaper and more effective than Strategy and hence, strategy B was ignored, and the analysis continued by comparing strategy and strategy as follows:
Finally, the comparison result revealed that strategy is less costly and more effective than strategy . In conclusion, of the four strategies mentioned, strategy (combining the three controls simultaneously) is the most effective way in combating the spread of TB in Ethiopia.
5. Conclusions
The Ethiopian government is working with partners and the community to eliminate TB by 2035. Therefore, it is vital to identify and implement effective strategies to eradicate the disease. This paper has developed a mathematical model by including three control strategies (distancing, case finding, and case holding). After that, using Pontryagin’s maximum principle, the conditions for optimal control of the disease were analyzed. The optimal solution to the system was then illustrated by numerical simulations using available data from Ethiopia. From the numerical simulation result (Figure 1), one can deduce that considering the combination of distancing and case holding controls (Strategy ), does not lead to the best results in decreasing the number of TB infected individuals. On the other hand, we can understand from this analysis that the combination of all the three controls (Strategy ) is an effective way to eradicate tuberculosis from the community.
Finally, we investigated the cost-effectiveness of the control strategies by using the ICER technique. Based on the results of these analyses, we concluded that applying the combination of the three controls (distancing, case finding, and case holding) is less costly and more effective than other strategies. This suggested that intervention strategies, such as isolation of infectious people, early TB patient detection, treating high-risk latently infected individuals, educational campaigns, and preventing treatment failure of active TB patients are essential in Ethiopia to control the spread of the disease.
The paper [16] presents a theme similar to the theme of this paper. However, an essential difference is that the structure of the model and the control variables are not the same, and the model of [16] is calibrated to a single district in Ethiopia. In contrast, we consider Ethiopia as a whole in the current paper. It may be wise to consider more controls than the three we included in this paper. Accuracy of the dynamics of the disease may be improved by considering more compartments in the population, such as considering age structure or multi-group models, in which the smaller groups are more homogeneous.
Author Contributions
Conceptualization, A.K.M.; methodology, A.K.M. and P.J.W.; software, A.K.M.; validation, A.K.M. and P.J.W.; formal analysis, A.K.M. and P.J.W.; investigation, A.K.M.; resources, A.K.M. and P.J.W.; data curation, A.K.M.; writing—original draft preparation, A.K.M.; writing—review and editing, A.K.M. and P.J.W.; visualization, A.K.M.; supervision, P.J.W.; project administration, A.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
No data was generated for or during this research. All the data used are publicly available and are referenced in this manuscript.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- WHO. Global Tuberculosis Report. 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 1 February 2021).
- Centers for Disease Control and Prevention (CDC). Travelers’ Health Ethiopia. 2018. Available online: https://www.cdc.gov/globalhealth/countries/ethiopia/pdf/Ethiopia_Factsheet-p.pdf (accessed on 1 February 2021).
- Choi, S.; Jung, E. Optimal Tuberculosis Prevention and Control Strategy from a Mathematical Model Based on Real Data. Bull. Math. Biol. 2014, 76, 1566–1589. [Google Scholar] [CrossRef]
- Kim, S.; de los Reyes, A.A.V.; Jung, E. Country-specific intervention strategies for top three TB burden countries using mathematical model. PLoS ONE 2020, 15, e0230964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyambwera, S.M.; Witbooi, P. A Two-Group Model of TB in a Crowded Environment. Appl. Math. Inf. Sci. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Witbooi, P.; Vyambwera, S.M. A model of population dynamics of TB in a prison system and application to South Africa. BMC Res. Notes 2017, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengistu, A.K.; Witbooi, P.J. Modeling the Effects of Vaccination and Treatment on Tuberculosis Transmission Dynamics. J. Appl. Math. 2019, 2019, 7463167. [Google Scholar] [CrossRef] [Green Version]
- Vyambwera, S.M.; Witbooi, P. A Stochastic TB Model for a Crowded Environment. J. Appl. Math. 2018, 2018, 3420528. [Google Scholar] [CrossRef]
- Bhunu, C.P.; Mushayabasa, S.; Tchuenche, J.M. A Theoretical Assessment of the Effects of Smoking on the Transmission Dynamics of Tuberculosis. Bull. Math. Biol. 2011, 73, 1333–1357. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.S.; Obsu, L.L. Mathematical modeling for COVID-19 transmission dynamics: A case study in Ethiopia. Results Phys. 2022, 34, 105191. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, A.K.; Witbooi, P.J. Mathematical analysis of TB model with vaccination and saturated incidence rate. Abstr. Appl. Anal. 2020, 2020, 6669997. [Google Scholar] [CrossRef]
- la Torre, D.; Kunze, H.; Ruiz-Galan, M.; Malik, T.; Marsiglio, S. Optimal control: Theory and application to science, engineering, and social sciences. Abstr. Appl. Anal. 2015, 2015, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; de los Reyes, A.A.; Jung, E. Mathematical model and intervention strategies for mitigating tuberculosis in the Philippines. J. Theor. Biol. 2018, 443, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.P.; Huang, N.J. Optimal control analysis of a tuberculosis model. Appl. Math. Model. 2018, 58, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.; Torres, D.F.M. Optimal control strategies for tuberculosis treatment: A case study in Angola. Numer. Algebr. Control Optim. 2012, 2, 601–617. [Google Scholar] [CrossRef] [Green Version]
- Kereyu, D.; Demie, S. Transmission dynamics model of Tuberculosis with optimal control strategies in Haramaya district, Ethiopia. Adv. Differ. Equ. 2021, 2021, 289. [Google Scholar] [CrossRef]
- Hattaf, K.; Rachik, M.; Saadi, S.; Tabit, Y.; Yousfi, N. Optimal control of tuberculosis with exogenous reinfection. Appl. Math. Sci. 2009, 3, 231–240. [Google Scholar]
- Das, D.K.; Khajanchi, S.; Kar, T.K. The impact of the media awareness and optimal strategy on the prevalence of tuberculosis. Appl. Math. Comput. 2020, 366, 124732. [Google Scholar] [CrossRef]
- Adnaoui, K.; Elberrai, I.; Laaroussi, A.E.; Hattaf, K. A Spatiotemporal SIR Epidemic Model Two-dimensional with Problem of Optimal Control. Bol. Soc. Parana. Mat. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models, 1st ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Pontryagin, L.S. The Mathematical Theory of Optimal Processes, 1st ed.; CRC Press: New York, NY, USA, 1986; Volume 4. [Google Scholar]
- Cesari, L. Optimization—Theory and Applications: Problems with Ordinary Differential Equations, 1st ed.; Springer: New York, NY, USA, 1983. [Google Scholar]
- World Health Organisation. WHO TB Burden Estimates. Global Tuberculosis Report. 2021. Available online: https://www.who.int/teams/global-tuberculosis-programme/data (accessed on 2 January 2022).
- WHO. WHO Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary. 2020. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=ETH (accessed on 1 February 2021).
- World Health Organization. Treatment Success Data by Country. 2020. Available online: https://apps.who.int/gho/data/node.main.602?lang=en (accessed on 1 February 2021).
- WHO. Global Tuberculosis Report 2018. Available online: https://www.who.int/tb/publications/global_report/gtbr2018_main_text_28Feb2019.pdf (accessed on 3 January 2022).
- Cantor, S.B.; Ganiats, T.G. Incremental Cost-Effectiveness Analysis:The Optimal Strategy Depends on the Strategy Set. J. Clin. Epidemiol. 1999, 52, 517–522. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).









