Preparation of a Thermally Modified Diatomite and a Removal Mechanism for 1-Naphthol from Solution
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Characterization
2.3. Adsorption Experiments
2.4. Measurement of 1-NAP
2.5. Adsorption Equilibrium Isotherm
2.6. Kinetic Equation
2.7. Thermodynamic Parameters
3. Results and Discussion
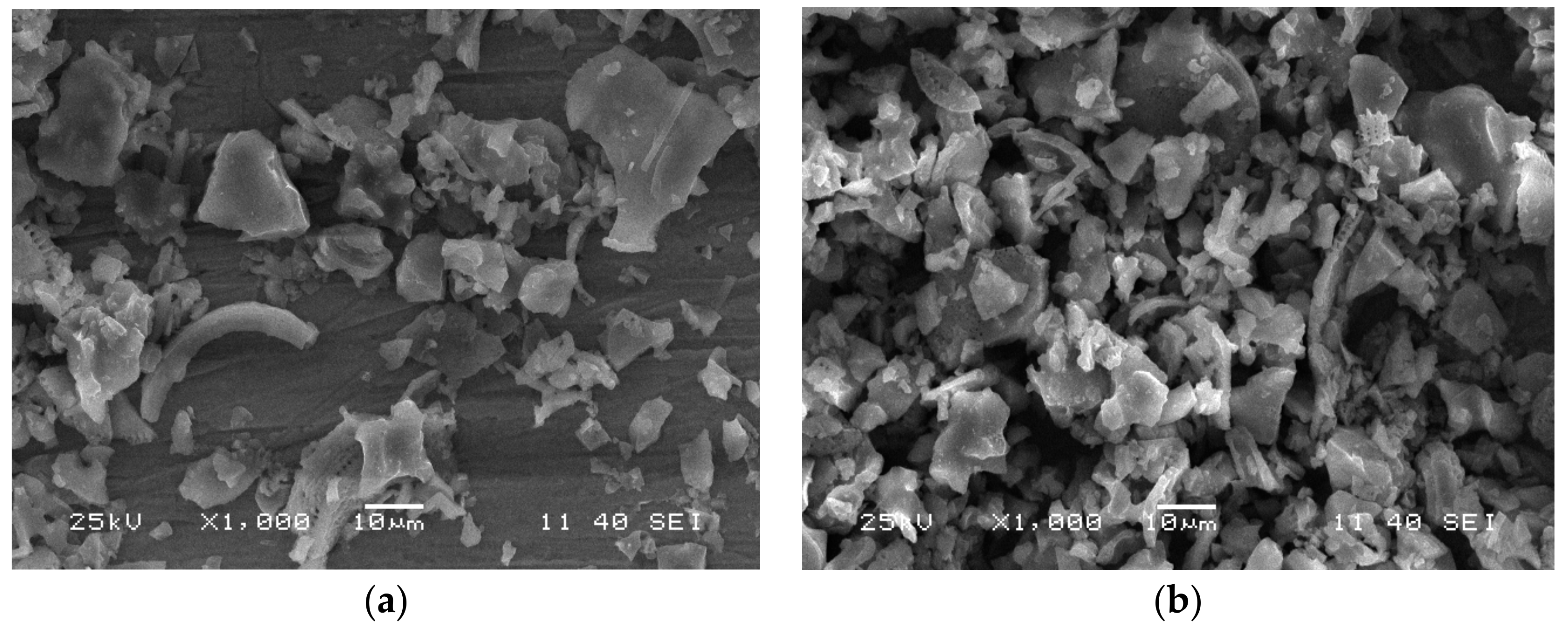
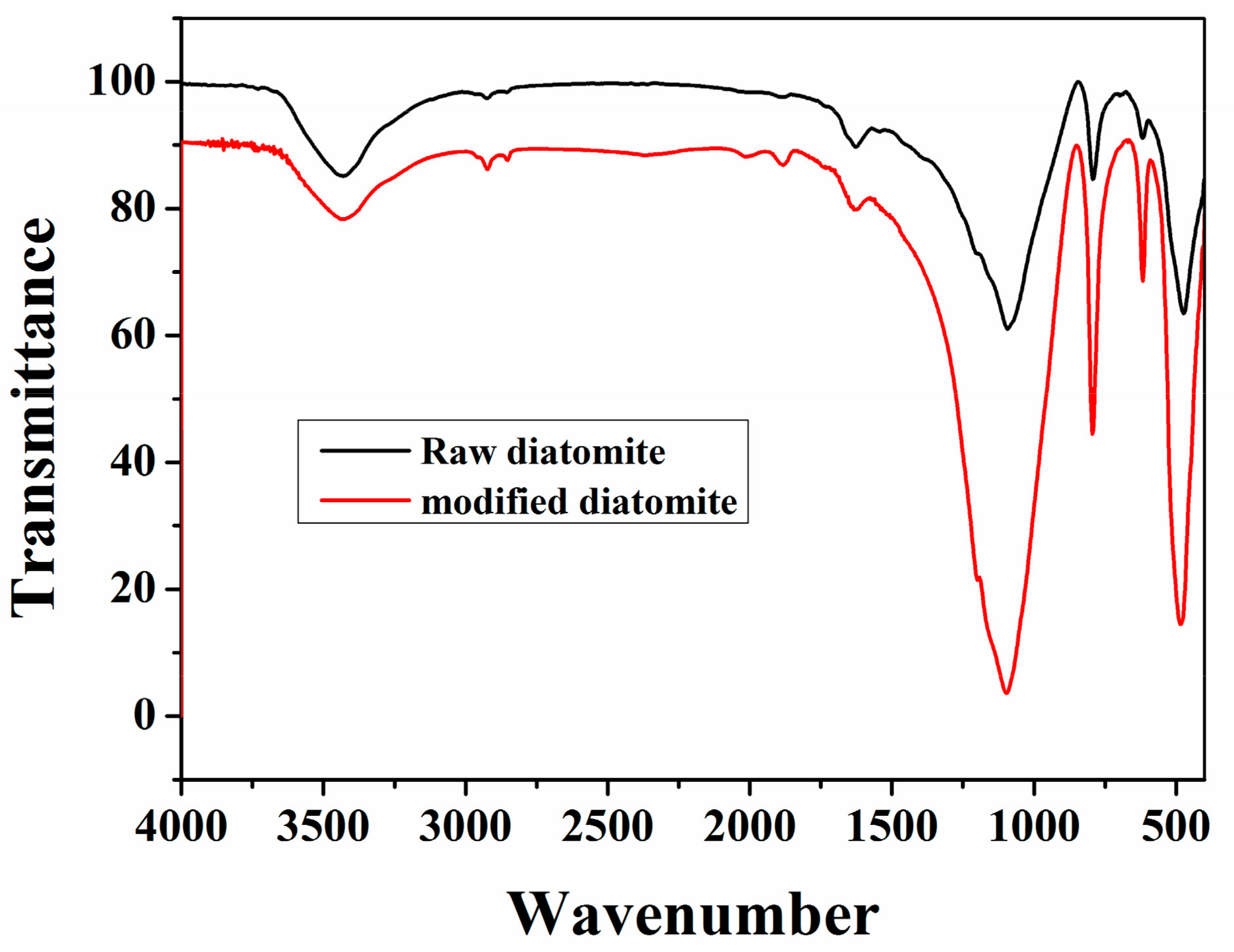
3.1. Characterisation of Diatomite
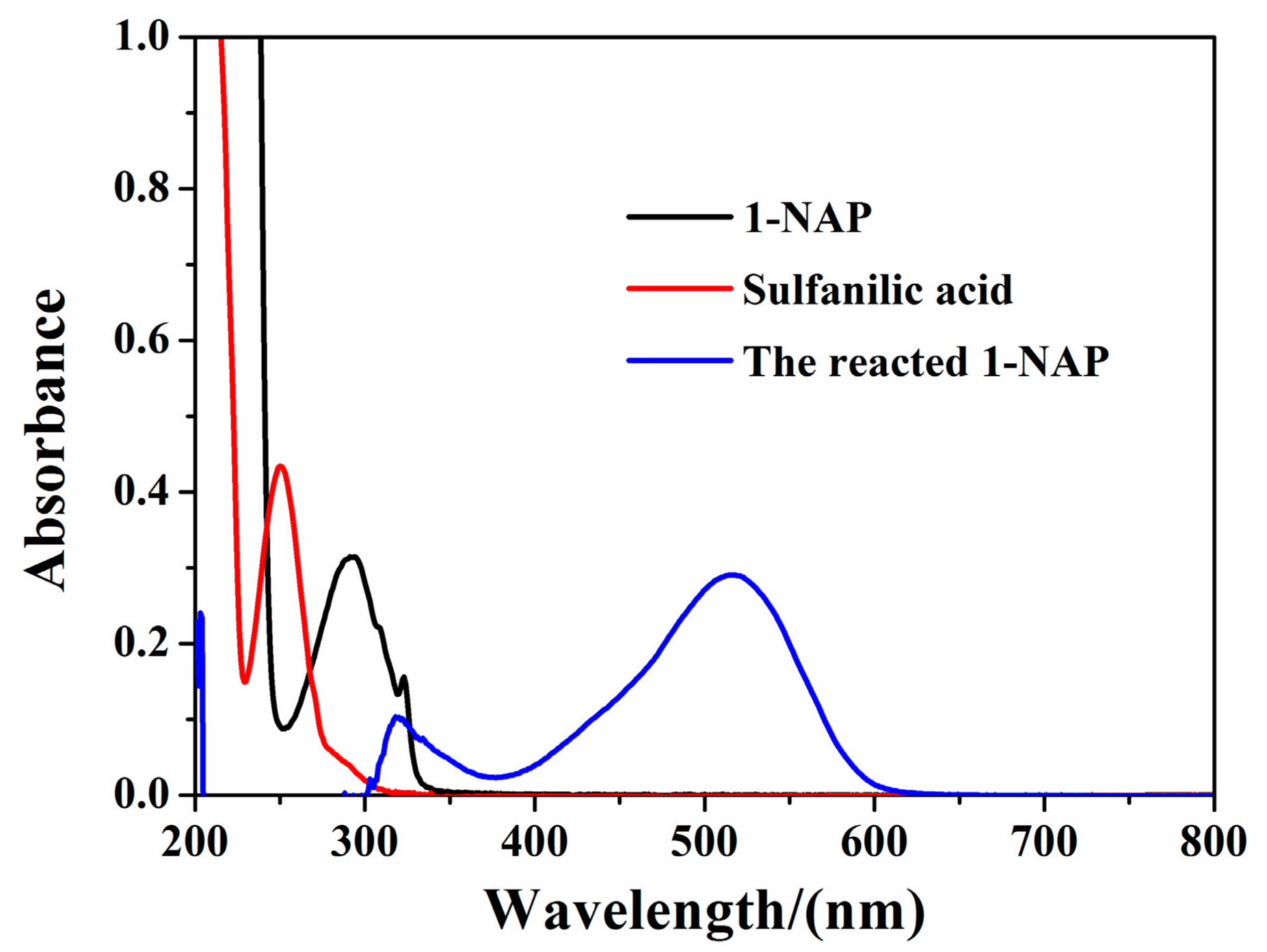
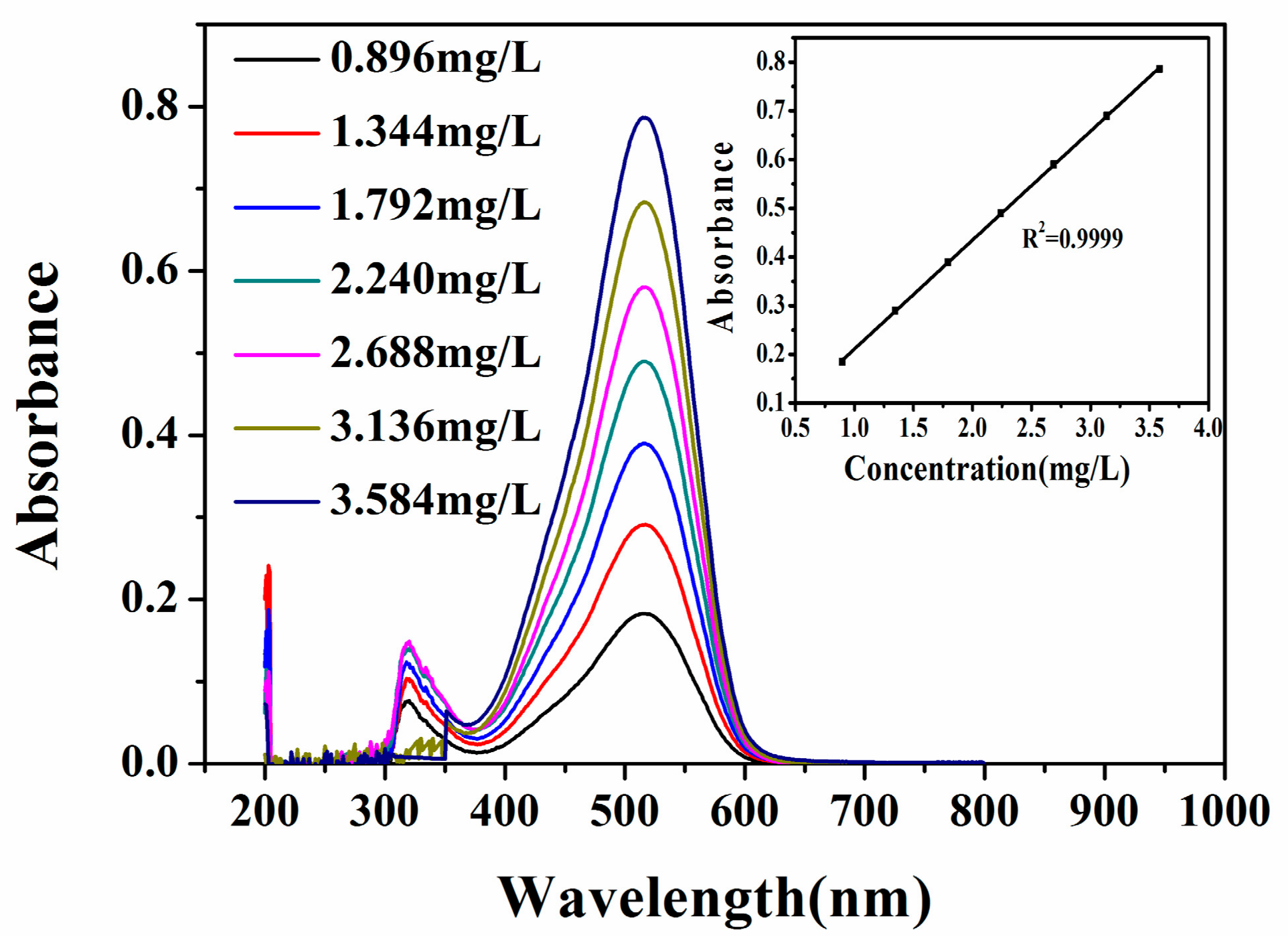
3.2. Analytical Determination of 1-NAP
3.3. 1-NAP Absorption Experiment
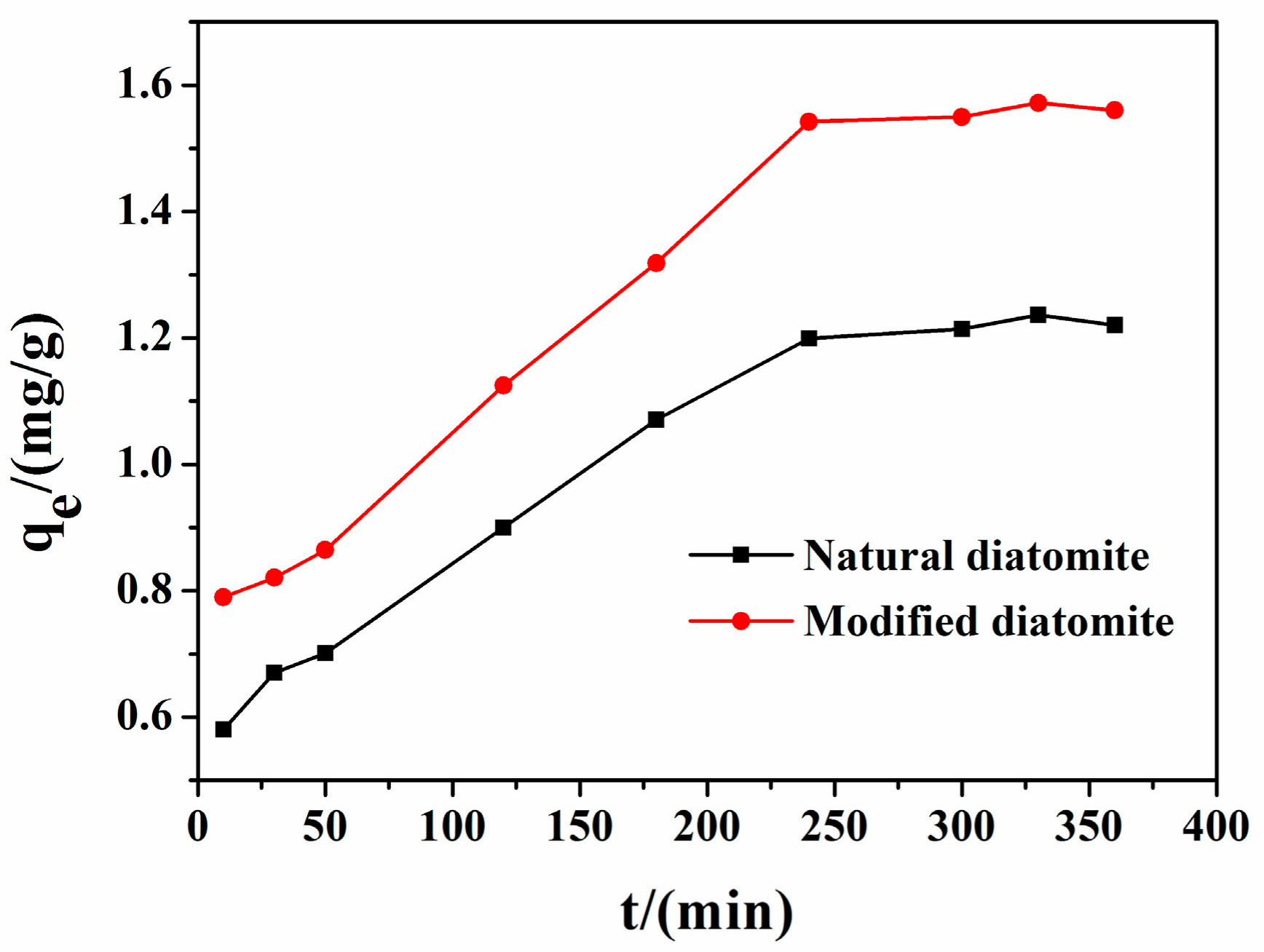
3.3.1. Effect of Contact Time
3.3.2. Effect of pH
3.3.3. Effect of Diatomite Dose
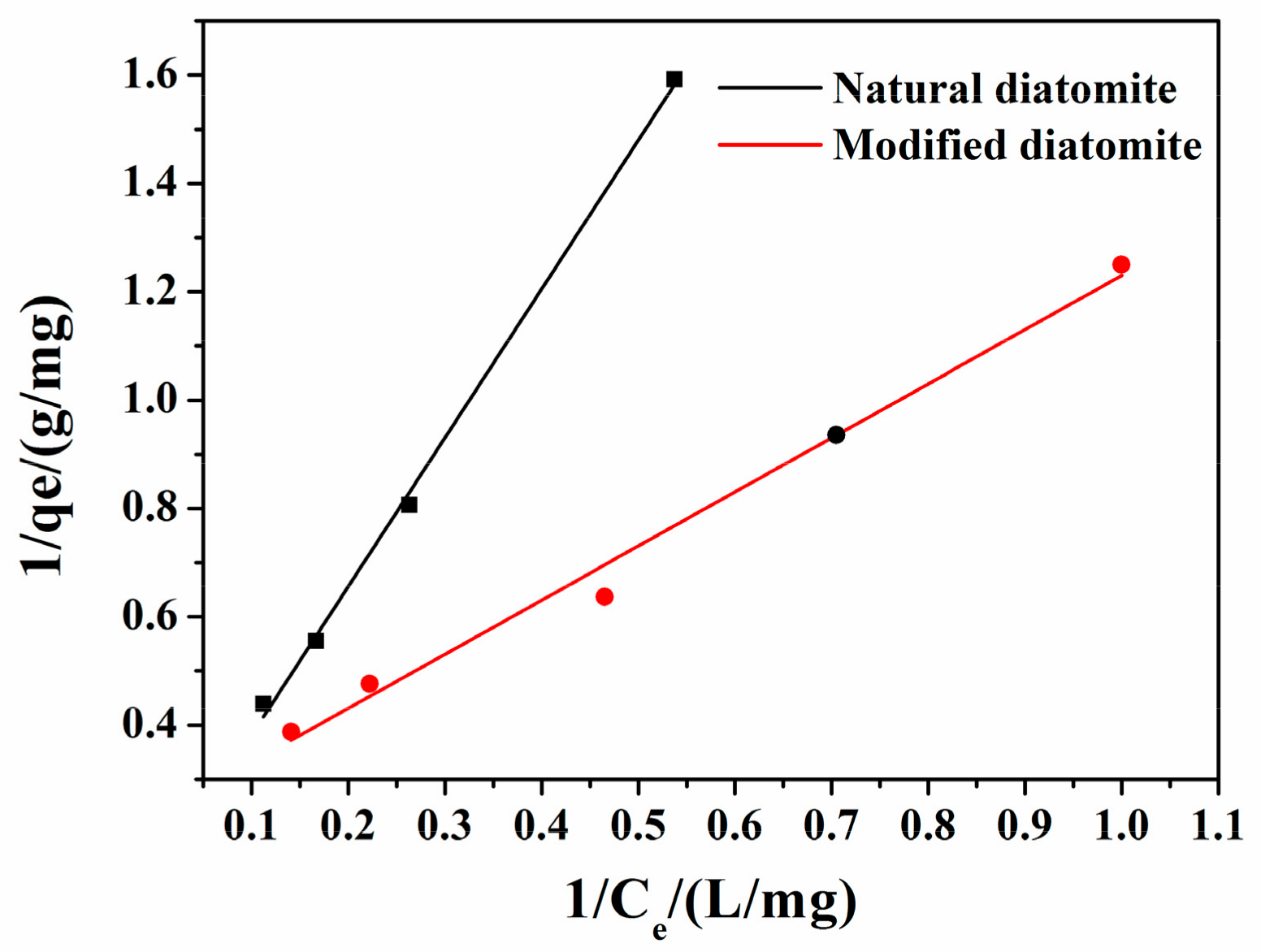
3.3.4. Adsorption Isotherms
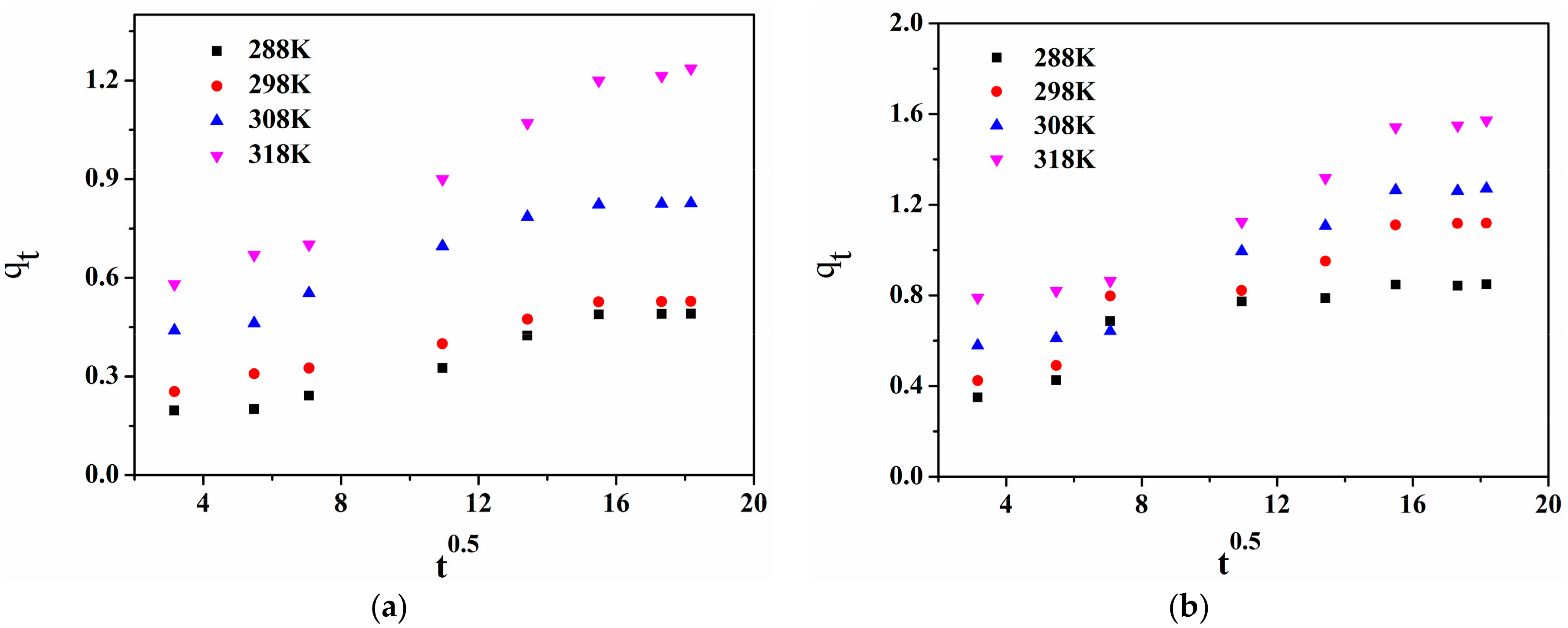
3.3.5. Kinetics of Adsorption
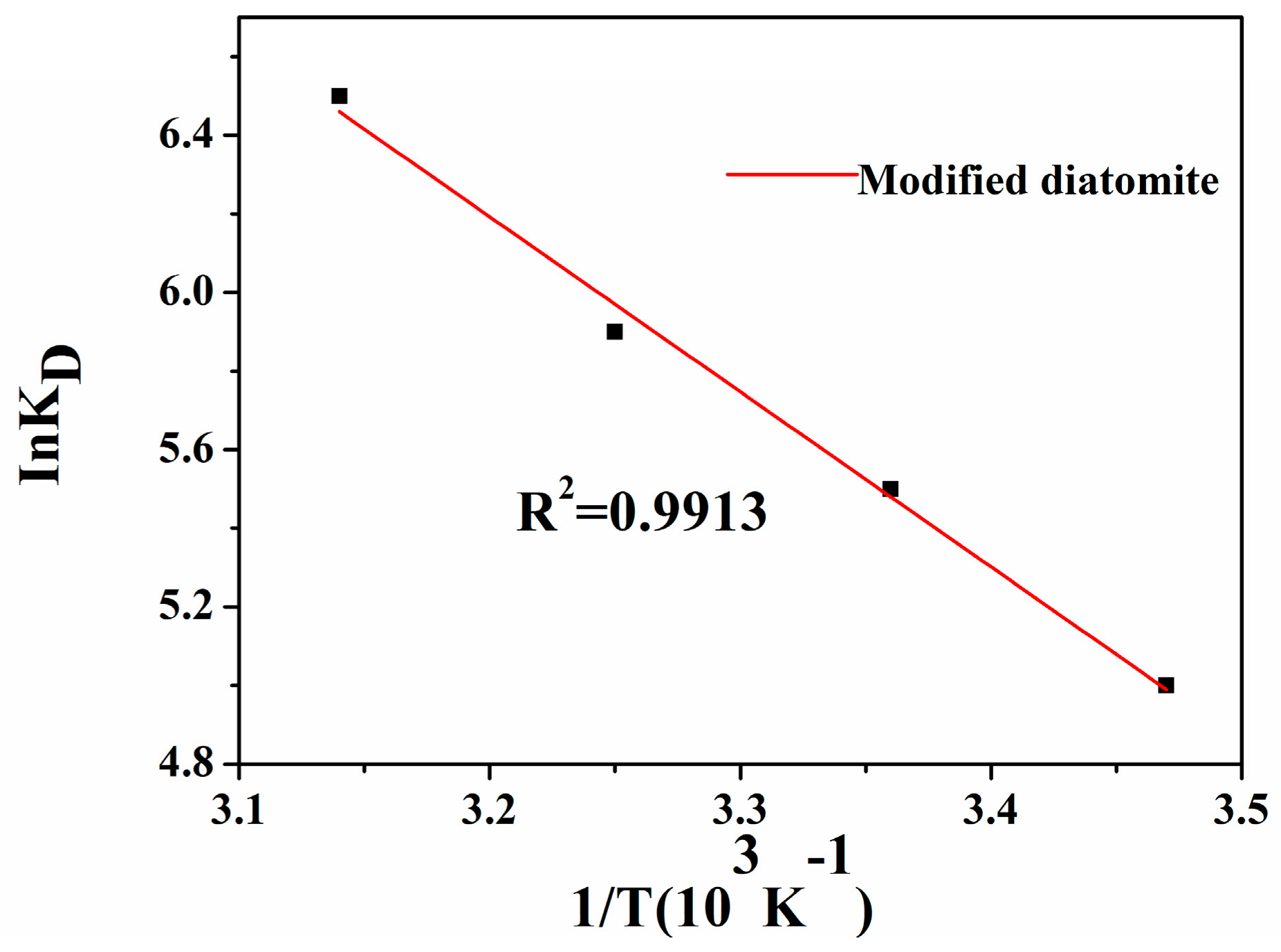
3.3.6. Thermodynamic Parameters
4. Conclusions
- (1)
- The adsorption of 1-NAP on raw and thermal modified diatomite was endothermic and spontaneous processes, controlled by both physical and chemical mechanisms
- (2)
- It was found that the adsorption of 1-NAP on raw and thermally modified diatomite could best be explained by the pseudo second-order model.
- (3)
- For raw and thermal modified diatomite, the experimental data have been applied on Langmuir, Freundlich and D-R isotherm models. The Langmuir adsorption isotherm model fitted better in the temperature gradients studied.
- (4)
- The sorption of 1-NAP is strongly affected by pH, temperature and diatomite dose. Compared with natural diatomite, it can be observed that the modified diatomite significantly improve the adsorption capacity (about 2 times at 298 K).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, G.X.; Li, J.X.; Wang, X.K. Kinetic and thermodynamic study of 1-naphthol adsorption from aqueous solution to sulfonated graphene nanosheets. Chem. Eng. J. 2011, 173, 185–190. [Google Scholar] [CrossRef]
- Zhang, W.M.; Hong, C.H.; Pan, B.C.; Zhang, Q.J.; Jiang, P.J.; Jia, K. Sorption enhancement of 1-naphthol onto a hydrophilic hyper-cross-linked polymer resin. J. Hazard. Mater. 2009, 163, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Burgos, W.D.; Pisutpaisal, N.; Tuntoolavest, M.; Chorover, J.; Unz, R.F. Biodegradation of 1-naphthol in the presence of humic acid. Environ. Eng. Sci. 2000, 17, 343–351. [Google Scholar] [CrossRef]
- Zhu, H.C.; Shen, Z.M.; Tang, Q.L.; Ji, W.C.; Jia, L.J. Degradation mechanism study of organic pollutants in ozonation process by QSAR analysis. Chem. Eng. J. 2014, 255, 431–436. [Google Scholar] [CrossRef]
- Gcina, M.; Xavier, Y.K.; Mbianda, A.K. Photocatalytic degradation of the diazo dye naphthol blue black in water using MWCNT/Gd,N,S-TiO2 nanocomposites under simulated solar light. J. Environ. Sci. 2015, 33, 219–228. [Google Scholar]
- Sheng, G.D.; Shao, D.D.; Ren, X.M.; Wang, X.Q.; Li, J.X.; Chen, Y.X.; Wang, X.K. Kineticsand thermodynamics of adsorption ofionizable aromatic compounds fromaqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2010, 178, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.X.; Chen, Q.H.; Pan, J.M.; Zhang, Y.L.; Huang, Y.; Qi, X.Y. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Application of activated carbon derived from ‘waste’ bamboo culms for the adsorption of azo disperse dye: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2012, 102, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Tian, F.; Wu, Z.L.; Yan, Y.J.; Cravotto, G.; Wu, Z.S. Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects. Chem. Eng. Process. 2015, 91, 67–77. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.J.; Wang, X.; Song, J.K.; Wang, H.; Zhang, J.; Zhao, J.F. Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO loaded diatomite. Coll. Surf. A Physicochem. Eng. Asp. 2016, 506, 220–227. [Google Scholar] [CrossRef]
- Li, W.G.; Gong, X.J.; Li, X.; Zhang, D.Y.; Gong, H.N. Removal of Cr (VI) from low-temperature micro-polluted surface water by tannic acid immobilized powdered activated carbon. Bioresour. Technol. 2012, 113, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Hu, Z.J.; Wang, Y.; Gao, H.W. Magnetic SN-functionalized diatomite for effective removals of phenols. Int. J. Miner. Process. 2017, 162, 1–5. [Google Scholar] [CrossRef]
- Li, J.; Guan, P.; Zhang, Y.; Xiang, B.; Tang, X.H.; She, H.D. A diatomite coated mesh with switchable wettability for on-demand oil/water separation and methylene blue adsorption. Sep. Purif. Technol. 2017, 174, 275–281. [Google Scholar] [CrossRef]
- Aivalioti, M.; Vamvasakis, I.; Gidarakos, E. BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazard. Mater. 2010, 178, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Sadeghian, B.; Pebdani, A.A.; Sahraei, R.; Daneshfar, A.; Duran, C. Kinetics, thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chem. Eng. J. 2012, 187, 133–141. [Google Scholar] [CrossRef]
- Kaçan, E.; Kütahyalı, C. Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J. Anal. Appl. Pyrol. 2012, 97, 149–157. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Sogut, E.G.; Alacabey, I. Adsorption of Zinc (II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dural, M.U.; Cavas, L.; Papageorgiou, S.K.; Katsaros, F.K. Methylene blue adsorption on activated carbon prepared from Posidoniaoceanica (L.) dead leaves: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 168, 77–85. [Google Scholar] [CrossRef]
- Singh, V.K.; Tiwari, P.N. Removal and recovery of chromium (VI) from industrial waste water. J. Chem. Technol. Biot. 1997, 69, 376–382. [Google Scholar] [CrossRef]
- Yang, X.Z.; Shi, Z.; Liu, L.S. Adsorption of Sb from aqueous solution by GFGO in batch and fixed-bed systems. Chem. Eng. J. 2015, 260, 444–453. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Adsorption characteristics of industrial solid waste derived activated carbon prepared by microwave heating for methylene blue. Fuel Process. Technol. 2012, 99, 103–109. [Google Scholar] [CrossRef]
- Wu, F.C.; Ruling, T.; Rueyshin, J. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Li, H. Dye adsorption on unburned carbon: Kinetics and equilibrium. J. Hazard. Mater. 2005, 126, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Farhan, A.M. Equilibrium, Kinetic and Thermodynamics of Biosorption of Lead (II) Copper (II) and Cadmium (II) Ions from Aqueous Solutions onto Olive Leaves Powder. Am. J. Chem. 2012, 2, 238–244. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.C.; Hou, L.; Zhu, D.Q.; Ji, R.; Chen, W. Enhance Transport of Phenanthrene and 1-Naphthol by Colloidal Graphene Oxide Nanoparticles in Saturated Soil. Environ. Sci. Technol. 2014, 48, 10136–10144. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.R.; Kut, O.M.; Lenzner, J.; Maire, H. Kinetics of the diazo coupling between 1-naphthol and diazotized sulfanilic acid. Ind. Eng. Chem. Res. 1990, 29, 1761–1765. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Li, B.; Yang, L.; Luo, L.; Yang, X. Adsorption of Cr(VI) and Phosphate on Mg–Al Hydrotalcite Supported Kaolin Clay Prepared by Ultrasound-Assisted Coprecipitation Method Using Batch and Fixed-Bed Systems. Ind. Eng. Chem. Res. 2014, 53, 7746–7757. [Google Scholar] [CrossRef]







| Adsorbent | T/K | qe, Experimental (mg/g) | Pseudo-First-Order Constants | Pseudo-Second-Order Constants | Intra-Particle Diffusion Constants | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe, Calculated (mg/g) | R2 | k2 (g/mg/min) | qe, Calculated (mg/g) | R2 | kid | R2 | |||
| Natural diatomite | 288 | 0.49 | 2.11 × 10−2 | 0.75 | 0.857 | 5.90 × 10−2 | 0.57 | 0.954 | 2.30 × 10−2 | 0.960 |
| 298 | 0.53 | 2.10 × 10−2 | 0.61 | 0.858 | 3.32 × 10−2 | 0.57 | 0.984 | 2.91 × 10−2 | 0.975 | |
| 308 | 0.83 | 2.00 × 10−2 | 0.75 | 0.944 | 4.70 × 10−2 | 0.89 | 0.994 | 3.20 × 10−2 | 0.947 | |
| 318 | 1.24 | 1.21 × 10−2 | 0.95 | 0.940 | 2.15 × 10−2 | 1.34 | 0.978 | 4.72 × 10−2 | 0.981 | |
| Modified diatomite | 288 | 0.85 | 2.01 × 10−2 | 0.58 | 0.807 | 4.97 × 10−2 | 0.91 | 0.996 | 3.20 × 10−2 | 0.807 |
| 298 | 1.12 | 1.79 × 10−2 | 1.39 | 0.849 | 2.25 × 10−2 | 1.22 | 0.978 | 4.73 × 10−2 | 0.917 | |
| 308 | 1.27 | 1.70 × 10−2 | 1.28 | 0.845 | 1.77 × 10−2 | 1.31 | 0.977 | 5.42 × 10−2 | 0.953 | |
| 318 | 1.57 | 1.32 × 10−2 | 1.30 | 0.885 | 1.42 × 10−2 | 1.69 | 0.971 | 6.00 × 10−2 | 0.963 | |
| Adsorbent | ΔH (kJ/mol) | ΔS (J/k/mol) | ΔG (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | |||
| Raw diatomite | 25.70 | 124 | −10.06 | −11.15 | −12.55 | −13.75 |
| Modified diatomite | 37.04 | 170 | −11.97 | −13.63 | −15.11 | −17.19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, Y.; Wang, L.; Cao, L.; Li, K.; Hursthouse, A. Preparation of a Thermally Modified Diatomite and a Removal Mechanism for 1-Naphthol from Solution. Water 2017, 9, 651. https://doi.org/10.3390/w9090651
Yang X, Zhang Y, Wang L, Cao L, Li K, Hursthouse A. Preparation of a Thermally Modified Diatomite and a Removal Mechanism for 1-Naphthol from Solution. Water. 2017; 9(9):651. https://doi.org/10.3390/w9090651
Chicago/Turabian StyleYang, Xiuzhen, Yuezhou Zhang, Liping Wang, Lili Cao, Kelin Li, and Andrew Hursthouse. 2017. "Preparation of a Thermally Modified Diatomite and a Removal Mechanism for 1-Naphthol from Solution" Water 9, no. 9: 651. https://doi.org/10.3390/w9090651
APA StyleYang, X., Zhang, Y., Wang, L., Cao, L., Li, K., & Hursthouse, A. (2017). Preparation of a Thermally Modified Diatomite and a Removal Mechanism for 1-Naphthol from Solution. Water, 9(9), 651. https://doi.org/10.3390/w9090651






