Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Industrial Product/Byproduct Filter Materials
2.2. Nitrate/Phosphate/Atrazine Test Solution
2.3. Batch Test Screening Procedures
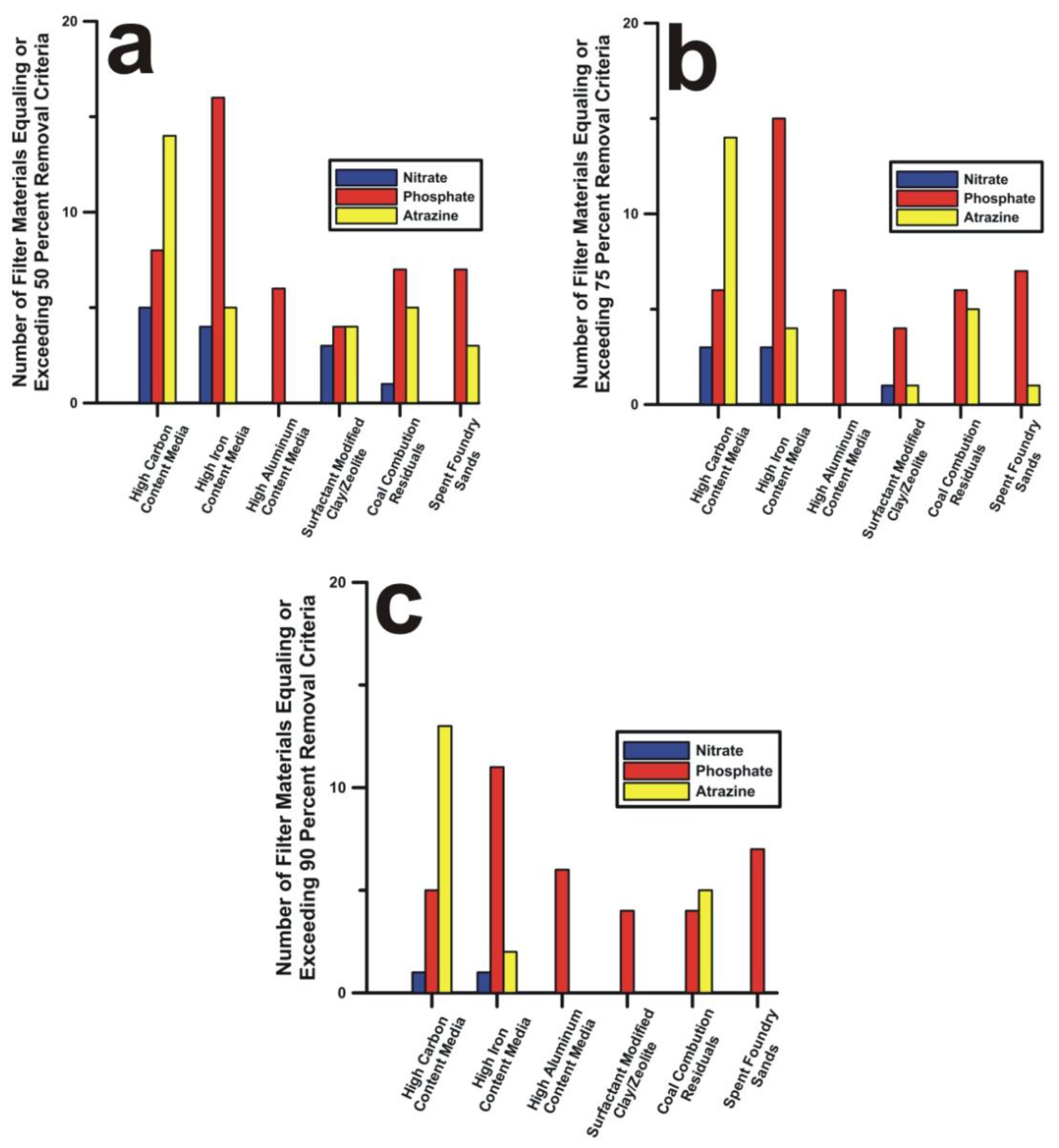
3. Results and Discussion
4. Summary and Conclusions
Conflicts of Interest
Appendix
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| COAL1 | 5.0 | 38.0 | 6.36 | 0.025 | 0.00180 | 0.00303 | 0.00304 |
| COAL2 | 5.0 | 38.0 | 2.91 | 0.359 | *** | 0.00272 | 0.00278 |
| COAL3 | 5.0 | 38.0 | 7.98 | ~0 | 0.00190 | 0.00304 | 0.00304 |
| COAL4 | 5.0 | 39.5 | 7.79 | 0.013 | 0.00191 | 0.00316 | 0.00316 |
| COAL5 | 5.0 | 38.0 | 6.51 | ~0 | 0.00186 | 0.00299 | 0.00291 |
| COAL6 | 5.0 | 38.0 | 6.64 | ~0 | 0.00099 | 0.00276 | 0.00287 |
| COKE1 | 5.0 | 40.0 | 7.25 | 0.015 | 0.00180 | 0.00315 | 0.00320 |
| COKE2 | 5.0 | 39.0 | 7.16 | 0.016 | 0.00004 | 0.00226 | 0.00271 |
| CHAR | 5.0 | 38.0 | 8.42 | ~0 | *** | 0.00302 | 0.00304 |
| AC1 | 5.0 | 38.0 | 9.01 | 0.236 | 0.00134 | 0.00303 | 0.00304 |
| AC2 | 5.0 | 40.0 | 10.13 | 0.206 | *** | 0.00320 | 0.00320 |
| AC3 | 5.0 | 38.0 | 1.97 | 0.320 | *** | 0.00304 | 0.00304 |
| AC4 | 5.0 | 40.0 | 7.81 | 0.321 | *** | 0.00320 | 0.00320 |
| AC5 | 2.5 | 39.0 | 11.91 | 0.071 | 0.00371 | 0.00623 | 0.00312 |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| ZVI1 | 4.9 (5.0) | 40.0 | 9.21 | 0.040 | 0.00195 | 0.00286 | 0.00297 |
| ZVI2 | 4.8 (5.0) | 40.0 | 9.58 | 0.025 | 0.00207 | 0.00317 | 0.00319 |
| ZVI3 | 4.3 (5.0) | 40.0 | 9.54 | ~0 | 0.00228 | 0.00020 | 0.00046 |
| ZVI4 | 3.9 (5.0) | 40.0 | 10.32 | 0.021 | 0.00255 | ~0 | 0.00051 |
| SMI1 | 4.3 (5.0) | 40.0 | 9.68 | 0.294 | 0.00230 | 0.00061 | 0.00112 |
| SMI2 | 5.0 | 40.0 | 9.12 | 0.301 | 0.00192 | 0.00086 | 0.00118 |
| SMI3 | 5.0 | 40.0 | 3.09 | 0.394 | 0.00188 | 0.00198 | 0.00264 |
| PIC | 5.0 | 40.0 | 10.70 1 | 0.145 | *** 2 | 0.00320 | - 3 |
| IS1 | 5.0 | 40.0 | 4.43 | 0.023 | 0.00147 | 0.00202 | 0.00288 |
| IS2 | 5.0 | 40.0 | 4.82 | 0.345 | 0.00198 | 0.00081 | 0.00090 |
| IS3 | 5.0 | 40.0 | 5.87 | 0.022 | 0.00179 | 0.00064 | 0.00074 |
| IO1 | 5.0 | 40.0 | 7.14 | 0.004 | 0.00179 | 0.00036 | 0.00015 |
| IO2 | 5.0 | 40.0 | 6.58 | ~0 | 0.00186 | 0.00050 | 0.00043 |
| IO3 | 5.0 | 40.0 | 5.49 | ~0 | 0.00200 | 0.00005 | 0.00094 |
| IO4 | 5.0 | 40.0 | 6.44 | ~0 | 0.00200 | ~0 | 0.00048 |
| IO5 | 0.7 (5.0) | 40.0 | 6.29 | 0.137 | 0.01091 | 0.00306 | ~0 |
| IO6 | 1.0 | 40.0 | 6.23 | 0.068 | 0.00893 | 0.00136 | 0.00218 |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| AO1 | 5.0 | 40.0 | 6.41 | ~0 | 0.00200 | 0.00021 | ~0 |
| AO2 | 5.0 | 40.0 | 6.92 | 0.016 | 0.00189 | 0.00041 | ~0 |
| AO3 | 5.0 | 40.0 | 6.10 | 0.006 | 0.00187 | 0.00060 | ~0 |
| AO4 | 5.0 | 40.0 | 8.25 | 0.007 | 0.00193 | 0.00031 | ~0 |
| AO5 | 5.0 | 40.0 | 8.08 | 0.005 | 0.00189 | 0.00033 | ~0 |
| AO6 | 1.0 | 40.0 | 6.06 | 0.034 | 0.00929 | 0.00184 | ~0 |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| SMC1 | 3.03 (5.0) | 38.0 | 7.79 | 0.442 | *** | 0.00197 | 0.00375 |
| SMC2 | 2.29 (5.0) | 40.0 | 6.25 | 0.738 | 0.00416 | 0.00507 | 0.00592 |
| SMC3 | 2.14 (5.0) | 40.0 | 8.91 | 0.031 | 0.00456 | 0.00278 | 0.00303 |
| SMZ1 | 4.00 (5.0) | 40.0 | 8.02 | 0.243 | *** | 0.00154 | 0.00276 |
| SMZ2 | 3.80 (5.0) | 40.0 | 9.33 | 0.347 | 0.00242 | 0.00252 | 0.00349 |
| SMZ3 | 4.02 (5.0) | 40.0 | 8.62 | ~0 | 0.0023 | 0.00109 | 0.00145 |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| CCR1 | 5.0 | 40.0 | 11.85 | ~0 | 0.00088 | 0.00320 | 0.00320 |
| CCR2 | 5.0 | 40.0 | 6.63 | ~0 | 0.00166 | 0.00057 | 0.00095 |
| CCR3 | 5.0 | 40.0 | 12.07 | ~0 | 0.00192 | 0.00066 | 0.00103 |
| CCR4 | 5.0 | 40.0 | 8.80 | ~0 | 0.00192 | 0.00046 | 0.00052 |
| CCR5 | 4.22 (5.0) | 40.0 | 8.99 | ~0 | 0.00209 | 0.00379 | 0.00379 |
| CCR6 | 5.0 | 40.0 | 6.32 | ~0 | 0.00139 | 0.00311 | 0.00318 |
| CCR7 | 5.0 | 40.0 | 7.99 | ~0 | 0.00197 | 0.00320 | 0.00320 |
| CCR8 | 5.0 | 40.0 | 13.05 | 0.298 | 0.00189 | 0.00320 | 0.00320 |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH 1 | Nitrate-N Average Removal (mg/g) | Phosphate-P Average Removal (mg/g) | Atrazine Immunoassay Average Removal (mg/g) | Atrazine GC-MS Average Removal (mg/g) |
|---|---|---|---|---|---|---|---|
| FS1 | 5.0 | 40.0 | 8.26 | 0.006 | 0.00190 | 0.00059 | - 2 |
| FS2 | 5.0 | 40.0 | 8.17 | 0.007 | 0.00198 | 0.00144 | - |
| FS3 | 5.0 | 40.0 | 7.82 | ~0 | 0.00189 | 0.00244 | - |
| FS4 | 5.0 | 40.0 | 7.88 | 0.011 | 0.00189 | 0.00189 | - |
| FS5 | 5.0 | 40.0 | 7.79 | 0.012 | 0.00195 | 0.00156 | - |
| FS6 | 5.0 | 40.0 | 7.80 | 0.002 | 0.00192 | 0.00162 | - |
| FS7 | 5.0 | 40.0 | 7.69 | 0.009 | 0.00190 | 0.00114 | - |
References
- Goolsby, D.A.; Battaglin, W.A. Nitrogen in the Mississippi Basin—Estimating Sources and Predicting Flux to the Gulf of Mexico; U.S. Geological Survey Fact Sheet135-00; USGS: Reston, VA, USA, 2000; pp. 1–6.
- Myers, D.N.; Thomas, M.A.; Frey, J.W.; Rheaume, S.J.; Button, D.T. Water quality in the Lake Erie—Lake Saint Clair drainages: Michigan, Ohio, Indiana, New York, and Pennsylvania, 1996–1998; U.S. Geological Survey Circular 1203; USGS: Denver, Colorado, USA, 2000; pp. 1–35.
- Sylvan, J.B.; Dortch, Q.; Nelson, D.M.; Maier Brown, A.F.; Morrison, W.; Ammerman, J.W. Phosphorous limits phytoplankton growth on the Louisiana Shelf during the period of hypoxia formation. Environ. Sci. Technol. 2006, 40, 7548–7553. [Google Scholar] [CrossRef] [PubMed]
- Scavia, D.; Donnelly, K.A. Reassessing hypoxia forecasts for the Gulf of Mexico. Environ. Sci. Technol. 2007, 41, 8111–8177. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.B.; Smith, R.A.; Schwarz, G.E.; Boyer, E.W.; Nolan, J.V.; Brakebill, J.W. Differences in phosphorous and nitrogen delivery to the Gulf of Mexico from the Mississippi River Basin. Environ. Sci. Technol. 2008, 42, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Zucker, L.A.; Brown, L.C. Agricultural Drainage: Water Quality Impacts and Subsurface Drainage Studies in the Midwest; OSU Extension Bulletin 871; Ohio State University: Columbus, OH, USA, 1998; pp. 1–40. [Google Scholar]
- Kalita, P.K.; Algoazany, A.S.; Mitchell, J.K.; Cooke, R.A.; Hirschi, M.C. Subsurface water quality from a flat tile-drained watershed in Illinois U.S.A. Agric. Ecosyst. Environ. 2006, 115, 183–193. [Google Scholar] [CrossRef]
- Kalita, P.K.; Cooke, R.A.; Anderson, S.M.; Hirschi, M.C.; Mitchell, J.K. Subsurface drainage and water quality: The Illinois experience. Trans. ASABE 2007, 50, 1651–1656. [Google Scholar] [CrossRef]
- Kladivko, E.J.; Van Scoyoc, G.E.; Monke, E.J.; Oates, K.M.; Pask, W. Pesticide and nutrient movement into subsurface tile drains on a silt loam soil in Indiana. J. Environ. Qual. 1991, 20, 264–270. [Google Scholar] [CrossRef]
- Beauchemin, S.; Simard, R.R.; Cluis, D. Forms and concentration of phosphorus in drainage water of twenty-seven tile-drained soils. J. Environ. Qual. 1998, 27, 721–728. [Google Scholar] [CrossRef]
- Sims, J.T.; Simard, R.R.; Joern, B.C. Phosphorus loss in agricultural drainage: Historical perspective and current research. J. Environ. Qual. 1998, 27, 277–293. [Google Scholar] [CrossRef]
- Laubel, A.; Jacobsen, O.H.; Kronvang, B.; Grant, R.; Andersen, H.E. Subsurface drainage loss of particles and phosphorus from field plot experiments and a tile-drained catchment. J. Environ. Qual. 1999, 28, 576–584. [Google Scholar] [CrossRef]
- Lu, J. Characteristics of phosphorus components in drainage water. Bull. Environ. Contam. Toxicol. 2004, 72, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kinley, R.D.; Gordon, R.J.; Stratton, G.W.; Patterson, G.T.; Hoyle, J. Phosphorus losses through agricultural tile drainage in Nova Scotia, Canada. J. Environ. Qual. 2007, 36, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Dousset, S.; Babut, M.; Andreux, F.; Schiavon, M. Alachlor and bentazone losses from subsurface drainage of two soils. J. Environ. Qual. 2004, 33, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.D.; MacTavish, D.C.; Findlay, W.I. Atrazine and metolachor loss in surface and subsurface runoff from three tillage treatments in corn. J. Environ. Qual. 1995, 24, 246–256. [Google Scholar] [CrossRef]
- Kladivko, E.J.; Grochulska, J.; Turco, R.F.; Van Scoyoc, G.E.; Eigel, J.D. Pesticide and nitrate transport into subsurface tile drains of different spacings. J. Environ. Qual. 1999, 28, 997–1004. [Google Scholar] [CrossRef]
- Yuan, Y.; Mitchell, J.K.; Walker, S.E.; Hirschi, M.C.; Cooke, R.A.C. Atrazine losses from corn fields in the Little Vermilion River Watershed in east central Illinois. Appl. Eng. Agric. 2000, 16, 51–56. [Google Scholar] [CrossRef]
- Gaynor, J.D.; Tan, C.S.; Drury, C.F.; Van Wesenbeeck, I.J.; Welacky, T.W. Atrazine in surface and subsurface runoff as affected by cultural practices. Water Qual. J. Can. 1995, 30, 513–531. [Google Scholar]
- Wong, M.T.F.; Huges, R.; Rowell, D.L. Retarded leaching of nitrate in acid soils from the tropics: Measurement of effective anion exchange capacity. J. Soil Sci. 1990, 41, 655–663. [Google Scholar] [CrossRef]
- Allred, B.J.; Bigham, J.M.; Brown, G.O. The impact of clay mineralogy on nitrate mobility under unsaturated flow conditions. Vadose Zone J. 2007, 6, 221–232. [Google Scholar] [CrossRef]
- Choe, S.; Chang, Y.; Hwang, K.; Khim, J. Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 2000, 41, 1307–1311. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Biswas, S.; Bose, P. Zero-valent iron-assissted autotrophic denitrification. J. Environ. Eng. 2005, 131, 1212–1220. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory evaluation of zero valent iron and sulfur-modified iron for agricultural drainage water treatment. Groundw. Monit. Remedat. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory evaluation of porous iron composite for agricultural drainage water filter treatment. Trans. ASABE 2012, 55, 1683–1697. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) Solubility Chart. In CRC Handbook of Chemistry and Physics, 75th ed.; CRC Press: Boca Raton, FL, USA, 1994; Section 8; pp. 60–62. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1981; pp. 282–285. [Google Scholar]
- Bohn, H.L.; McNeal, B.L.; O’Connor, G.A. Soil Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1985; pp. 190–194. [Google Scholar]
- Li, L.; Stanforth, R. Distinguishing adsorption and surface precipitation of phosphate on goethite (α-FeOOH). J. Colloid Int. Sci. 2000, 230, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.D.; Lombardo, P.S. Treatment of wastewater phosphate by reductive dissolution of iron: Use of ferric oxyhydroxide media. J. Environ. Qual. 2011, 40, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S. Chemical modeling of anion competition on goethite using the constant capacitance model. Soil Sci. Soc. Am. J. 1985, 49, 851–856. [Google Scholar] [CrossRef]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press, Inc.: New York, NY, USA, 1994; pp. 135–139, 372–378. [Google Scholar]
- Barrow, N.J.; Bowden, J.W.; Posner, A.M.; Quirk, J.P. Describing the effects of electrolyte on adsorption of phosphate by a variable charge surface. Aust. J. Soil Res. 1980, 18, 395–404. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D.L. Phosphate reaction dynamics in soils and soil components: A multiscale approach. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2007; pp. 135–179. [Google Scholar]
- Wang, Y.; Jiang, J.; Xu, R.; Tiwari, D. Phosphate adsorption at variable charge soil/water interfaces as influenced by ionic strength. Aust. J. Soil Res. 2009, 47, 529–536. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989; pp. 39–42. [Google Scholar]
- Dombek, T.; Dolan, E.; Schultz, J.; Klaruo, D. Rapid reductive dechlorination of atrazine by zero-valent iron under acid conditions. Environ. Pollut. 2001, 111, 21–27. [Google Scholar] [CrossRef]
- Kim, G.; Jeong, W.; Choe, S. Impact of pH buffer capacity of sediment on dechlorination of atrazine using zero valent iron. J. Environ. Sci. Health Part B 2007, 42, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Satapanajaru, T.; Anurakpongsatorn, P.; Pengthamkeerati, P.; Boparai, H. Remediation of atrazine-contaminated soil and water by nano zerovalent iron. Water Air Soil Pollut. 2008, 192, 349–359. [Google Scholar] [CrossRef]
- Bowman, R.S.; Haggerty, G.M.; Huddleston, R.G.; Neel, D.; Flynn, M.M. Chapter 5: Sorption of nonpolar organic compounds, inorganic cations, and inorganic oxyanions by surfactant-modified zeolites. In Surfactant-Enhanced Subsurface Remediation: Emerging Technologies; ACS Symposium Series 594; Sabatini, D.A., Knox, R.C., Harwell, J.H., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 54–64. [Google Scholar]
- Ahn, C.; Mitsch, W.J.; Wolfe, W.E. Effects of recycled FGD liner materail on water quality and macrophytes of constructed wetlands: A Mesocosm experiment. Water Res. 2001, 35, 633–642. [Google Scholar] [CrossRef]
- Lee, T.; Benson, C.H. Sorption and degradation of alachlor and metochlor in ground water using green sands. J. Environ. Qual. 2004, 33, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.-L.; Saha, B.; van Leeuwen, J.H. Evaluation of iron oxide and aluminum oxide as potential arsenic(V) adsorbents. Chem. Eng. Process. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res. 2011, 45, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Hornbuckle, J.W.; Christen, E.W.; Faulkner, R.D. Evaluating a multi-level subsurface drainage for improved drainage water quality. Agric. Water Manag. 2007, 89, 208–216. [Google Scholar] [CrossRef]
- Wichelns, D.; Cone, D.; Stuhr, G. Evaluating the impact of irrigation and drainage policies on agricultural sustainability. Irrig. Drain. Syst. 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Smedema, L.K.; Vlotman, W.F.; Rycroft, D.W. Modern Land Drainage: Planning, Design and Management of Agricultural Drainage Systems; A.A. Balkema Publishers: Leiden, The Netherlands, 2004; pp. 231–255. [Google Scholar]
- Henschke, C.; Hermann, T. Testing for Soil and Water Salinity; Fact Sheet 66/00; Government of South Australia—Primary Industries and Resources SA: Adelaide, Australia, 2007; pp. 1–4.
- Satapanajaru, T.; Comfort, S.D.; Shea, P.J. Enhancing metolachlor destruction rates with aluminum and iron salts during zerovalent iron treatment. J. Environ. Qual. 2003, 32, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Huang, C.Y.; Liu, J.Y. Study of different methods for enhancing the nitrate removal efficiency of a zero-valent metal process. Water Sci. Technol. 2006, 53, 81–87. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Rhee, S.; Park, J. Development of a new zero-valent iron zeolite material to reduce nitrate without ammonium release. J. Environ. Eng. 2007, 133, 6–12. [Google Scholar] [CrossRef]
- Ahn, S.C.; Oh, S.; Cha, D.K. Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J. Hazard. Mater. 2008, 156, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; pp. 1–173. [Google Scholar]
- Herzog, D.R. Immunoassays for Environmental Contaminants: (Pesticides) in Food and Water; Technical Bulletin T00037; Strategic Diagnostics Inc.: Newark, NJ, USA, 1997; pp. 1–26.
- Munch, J.W. Method 525.2: Determination of organic compounds in drinking water by liquid-solid extraction and capillary column gas chromatography/mass spectrometry. In Methods for the Determination of Organic Compounds in Drinking Water; Publication—EPA/600/R-95/131; U.S. Environmental Protection Agency: Washington, DC, USA, 1995; pp. 525.2.1–525.2.60. [Google Scholar]
- Rivera-Utrilla, J.; Sanchez-Polo, M. The role of dispersive and electrostaticinteractions in the aqueous phase adsorption of naphthalenesulphonic acids on ozone-treated activated carbons. Carbon 2002, 40, 2685–2691. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Hu, J.; Liu, M.Y.; Zhang, W.; Lai, K.C.K.; Lo, I.M.C. Activated carbon produced from waste wood pallets: Adsorption of three classes of dyes. Water Air Soil Pollut. 2007, 184, 141–155. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory batch test evaluation of effectiveness and efficiency for five filter materials potentially used to remove nutrients and pesticides from subsurface drainage waters. Trans. ASABE 2010, 53, 39–54. [Google Scholar] [CrossRef]
- Allred, B.J.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Racharaks, R. Preliminary laboratory evaluation of iron-bearing reactive media for pesticide water treatment. Appl. Eng. Agric. 2014, 30, 859–867. [Google Scholar]
- Allred, B.J.; Martinez, L.R.; Gamble, D.L. Phosphate removal from agricultural drainage water using an iron oxyhydroxide filter material. Water Air Soil Pollut. 2017, 228, 240. Available online: https://doi.org/10.1007/s11270-017-3410-9 (accessed on 8 June 2017).
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| COAL1 | 5.0 | 38.0 | 6.36 | 6.7 (0.3) | 94.9 (1.7) | 99.7 (0.5) | 100.0 (~0.0) |
| COAL2 | 5.0 | 38.0 | 2.91 | 94.6 (0.8) | *** | 89.4 (2.4) | 91.5 (0.9) |
| COAL3 | 5.0 | 38.0 | 7.98 | −1.0 (2.5) | 100.0 (~0.0) | 100.0 (~0.0) | 100.0 (~0.0) |
| COAL4 | 5.0 | 39.5 | 7.79 | 3.3 (0.7) | 96.5 (3.1) | 100.0 (~0.0) | 100.0 (~0.0) |
| COAL5 | 5.0 | 38.0 | 6.51 | −1.8 (0.4) | 98.1 (2.9) | 98.4 (0.9) | 95.7 (0.6) |
| COAL6 | 5.0 | 38.0 | 6.64 | −1.2 (1.6) | 52.6 (6.0) | 90.8 (0.9) | 94.5 (0.6) |
| COKE1 | 5.0 | 40.0 | 7.25 | 3.7 (0.3) | 89.8 (4.7) | 98.3 (0.6) | 100.0 (~0.0) |
| COKE2 | 5.0 | 39.0 | 7.16 | 4.0 (0.1) | 2.3 (1.5) | 72.5 (2.8) | 87.0 (1.8) |
| CHAR | 5.0 | 38.0 | 8.42 | −4.3 (0.8) | *** | 99.3 (0.7) | 100.0 (~0.0) |
| AC1 | 5.0 | 38.0 | 9.01 | 62.1 (1.1) | 70.6 (3.0) | 99.7 (0.3) | 100.0 (~0.0) |
| AC2 | 5.0 | 40.0 | 10.13 | 51.6 (9.2) | *** | 100.0 (~0.0) | 100.0 (~0.0) |
| AC3 | 5.0 | 38.0 | 1.97 | 84.2 (0.5) | *** | 100.0 (~0.0) | 100.0 (~0.0) |
| AC4 | 5.0 | 40.0 | 7.81 | 80.4 (1.9) | *** | 100.0 (~0.0) | 100.0 (~0.0) |
| AC5 | 2.5 | 39.0 | 11.91 | 9.1 (0.3) | 95.2 (1.9) | 99.8 (0.5) | 100.0 (~0.0) |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| ZVI1 | 4.9 (5.0) | 40.0 | 9.21 | 9.8 (1.1) | 95.7 (4.3) | 87.6 (2.6) | 91.1 (0.6) |
| ZVI2 | 4.8 (5.0) | 40.0 | 9.58 | 6.0 (0.5) | 99.2 (0.7) | 95.2 (1.7) | 95.8 (0.1) |
| ZVI3 | 4.3 (5.0) | 40.0 | 9.54 | −0.2 (0.3) | 98.0 (3.1) | 5.3 (6.8) | 12.3 (3.8) |
| ZVI4 | 3.9 (5.0) | 40.0 | 10.32 | 4.0 (1.8) | 99.6 (1.9) | −8.0 (5.7) | 12.5 (5.7) |
| SMI1 | 4.3 (5.0) | 40.0 | 9.68 | 63.2 (2.5) | 98.9 (0.4) | 16.4 (3.3) | 30.2 (10.7) |
| SMI2 | 5.0 | 40.0 | 9.12 | 75.3 (3.0) | 96.0 (0.4) | 26.8 (3.9) | 37.0 (5.7) |
| SMI3 | 5.0 | 40.0 | 3.09 | 98.6 (0.3) | 94.0 (0.7) | 61.8 (5.8) | 82.5 (2.1) |
| PIC | 5.0 | 40.0 | 10.70 1 | 36.3 (1.9) | *** 2 | 100.0 (~0.0) | - 3 |
| IS1 | 5.0 | 40.0 | 4.43 | 5.8 (0.5) | 73.4 (22.2) | 63.1 (4.6) | 90.3 (3.4) |
| IS2 | 5.0 | 40.0 | 4.82 | 86.3 (0.1) | 99.0 (0.6) | 25.2 (4.1) | 28.0 (6.0) |
| IS3 | 5.0 | 40.0 | 5.87 | 5.6 (1.9) | 89.4 (0.4) | 19.9 (4.7) | 23.0 (11.1) |
| IO1 | 5.0 | 40.0 | 7.14 | 1.1 (5.6) | 89.7 (8.8) | 11.3 (3.5) | 4.7 (4.8) |
| IO2 | 5.0 | 40.0 | 6.58 | −4.6 (0.8) | 93.2 (1.5) | 15.6 (3.7) | 13.5 (6.5) |
| IO3 | 5.0 | 40.0 | 5.49 | −2.4 (0.2) | 100.0 (~0.0) | 1.7 (12.4) | 29.4 (3.7) |
| IO4 | 5.0 | 40.0 | 6.44 | −3.2 (0.4) | 100.0 (~0.0) | −2.4 (9.2) | 15.0 (5.9) |
| IO5 | 0.7 (5.0) | 40.0 | 6.29 | 4.8 (0.2) | 76.4 (0.6) | 13.4 (12.0) | −5.8 (8.7) |
| IO6 | 1.0 | 40.0 | 6.23 | 3.4 (0.4) | 89.3 (0.8) | 8.5 (3.3) | 13.6 (12.5) |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| AO1 | 5.0 | 40.0 | 6.41 | −2.6 (0.3) | 100.0 (~0.0) | 6.7 (8.9) | 22.8 (12.0) |
| AO2 | 5.0 | 40.0 | 6.92 | 4.1 (0.3) | 94.6 (0.5) | 12.7 (3.1) | −47.8 (6.6) |
| AO3 | 5.0 | 40.0 | 6.10 | 1.6 (0.1) | 93.3 (2.1) | 18.9 (3.3) | −9.7 (5.9) |
| AO4 | 5.0 | 40.0 | 8.25 | 1.8 (0.1) | 96.5 (0.1) | 9.7 (4.9) | −32.0 (17.9) |
| AO5 | 5.0 | 40.0 | 8.08 | 1.3 (0.3) | 94.6 (0.4) | 10.4 (5.6) | −22.7 (16.3) |
| AO6 | 1.0 | 40.0 | 6.06 | 1.7 (0.1) | 92.9 (0.5) | 11.5 (7.4) | −24.9 (6.4) |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| SMC1 | 3.03 (5.0) | 38.0 | 7.79 | 66.9 (0.9) | *** | 39.2 (11.4) | 74.7 (3.5) |
| SMC2 | 2.29 (5.0) | 40.0 | 6.25 | 84.5 (0.1) | 95.3 (0.7) | 72.6 (1.4) | 84.8 (1.5) |
| SMC3 | 2.14 (5.0) | 40.0 | 8.91 | 3.3 (0.3) | 97.6 (0.2) | 37.2 (2.3) | 40.5 (14.4) |
| SMZ1 | 4.00 (5.0) | 40.0 | 8.02 | 48.5 (4.8) | *** | 38.4 (8.1) | 68.9 (6.1) |
| SMZ2 | 3.80 (5.0) | 40.0 | 9.33 | 66.0 (0.4) | 92.3 (1.4) | 59.9 (2.6) | 82.8 (1.2) |
| SMZ3 | 4.02 (5.0) | 40.0 | 8.62 | −1.9 (0.1) | 93.2 (0.3) | 27.5 (6.3) | 36.4 (15.8) |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | Avg. pH | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| CCR1 | 5.0 | 40.0 | 11.85 | −3.6 (0.4) | 44.1 (9.1) | 100.0 (~0.0) | 100.0 (~0.0) |
| CCR2 | 5.0 | 40.0 | 6.63 | −5.2 (1.2) | 83.2 (3.6) | 17.8 (6.6) | 29.6 (15.0) |
| CCR3 | 5.0 | 40.0 | 12.07 | 13.8 (3.1) | 96.1 (0.1) | 20.6 (3.9) | 32.3 (6.2) |
| CCR4 | 5.0 | 40.0 | 8.80 | −3.3 (1.5) | 96.0 (0.8) | 14.4 (10.4) | 16.3 (7.6) |
| CCR5 | 4.22 (5.0) | 40.0 | 8.99 | −2.5 (4.4) | 88.4 (2.7) | 100.0 (0.0) | 100.0 (~0.0) |
| CCR6 | 5.0 | 40.0 | 6.32 | −2.5 (0.5) | 69.3 (1.9) | 97.2 (0.9) | 99.3 (8.3) |
| CCR7 | 5.0 | 40.0 | 7.99 | −1.4 (0.6) | 98.5 (0.4) | 100.0 (~0.0) | 100.0 (~0.0) |
| CCR8 | 5.0 | 40.0 | 13.05 | 74.5 (1.6) | 94.6 (2.3) | 100.0 (~0.0) | 100.0 (~0.0) |
| Industrial Product/Byproduct | Dry (Wet) Filter Material Amount (g) | Test Solution Amount (g) | pH 1 | Nitrate-N Average of Percent Removal and (Std. Dev.) | Phosphate-P Average of Percent Removal and (Std. Dev.) | Atrazine Immunoassay Average of Percent Removal and (Std. Dev.) | Atrazine GC-MS Average of Percent Removal and (Std. Dev.) |
|---|---|---|---|---|---|---|---|
| FS1 | 5.0 | 40.0 | 8.26 | 1.6 (1.0) | 95.1 (2.4) | 18.5 (8.7) | - 2 |
| FS2 | 5.0 | 40.0 | 8.17 | 1.7 (1.9) | 98.8 (0.3) | 45.2 (4.2) | - |
| FS3 | 5.0 | 40.0 | 7.82 | −1.0 (0.2) | 94.3 (2.5) | 76.5 (0.9) | - |
| FS4 | 5.0 | 40.0 | 7.88 | 2.9 (0.7) | 94.4 (0.5) | 59.1 (6.8) | - |
| FS5 | 5.0 | 40.0 | 7.79 | 2.9 (0.6) | 97.3 (0.8) | 49.0 (9.2) | - |
| FS6 | 5.0 | 40.0 | 7.80 | 0.6 (0.6) | 96.2 (2.8) | 50.5 (3.7) | - |
| FS7 | 5.0 | 40.0 | 7.69 | 2.2 (0.7) | 94.9 (1.9) | 35.7 (5.3) | - |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allred, B.J. Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment. Water 2017, 9, 791. https://doi.org/10.3390/w9100791
Allred BJ. Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment. Water. 2017; 9(10):791. https://doi.org/10.3390/w9100791
Chicago/Turabian StyleAllred, Barry J. 2017. "Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment" Water 9, no. 10: 791. https://doi.org/10.3390/w9100791
APA StyleAllred, B. J. (2017). Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment. Water, 9(10), 791. https://doi.org/10.3390/w9100791




