Abstract
There are many factors to consider for the design of appropriate water treatment systems including: cost, the concentration and type of biological and/or chemical contamination, concentration limits at which contaminant(s) are required to be removed, required flow rate, level of local expertise for on-going maintenance, and social acceptance. An ideal technology should be effective at producing clean, potable water; however it must also be low-cost, low-energy (ideally energy-free) and require low-maintenance. The use of packed beds containing metallic iron (Fe0 filters) has the potential to become a cheap widespread technology for both safe drinking water provision and wastewater treatment. Fe0 filters have been intensively investigated over the past two decades, however, sound design criteria are still lacking. This article presents an overview of the design of Fe0 filters for decentralized water treatment particularly in the developing world. A design for safe drinking water to a community of 100 people is also discussed as starting module. It is suggested that Fe0 filters have the potential for significant worldwide applicability, but particularly in the developing world. The appropriate design of Fe0 filters, however, is site-specific and dependent upon the availability of local expertise/materials.
1. Introduction
1.1. Background
In recent years the use of decentralized water treatment systems has increased worldwide, but particularly in the developing world [1,2,3,4,5,6,7,8,9,10]. Due to their relatively small spatial scale and low carbon footprint, decentralized water filtration systems have a low environmental impact on water resources because they do not discharge effluent into waterways [4]. Furthermore, each decentralized water treatment system can be customized to suit local water quality objectives, climatic and topographic conditions as well as aesthetic requirements. Conventional water treatment often calls for complex multistage processes (namely coagulation, disinfection, flocculation, sand filtration, screen-filtration, ozonization, sedimentation) and requires a wide array of chemicals (namely chlorine, flocculents, hydrogen peroxide, lime, ozone). The processes therefore also typically require specialist expertise for installation and maintenance [4,10,11,12,13]. As a result conventional wastewater treatment facilities are often expensive to install and maintain and have a high carbon footprint. This has motivated the development of alternative “one-step” technologies which include membrane filters (e.g., reverse osmosis, ultra-filtration), functionalized adsorbents, and ion exchange resins.
Membrane filtration technologies typically exhibit a number of advantageous attributes including [3,7]: (i) the ability to produce very high quality water (low aqueous contaminant concentrations); (ii) simple modular design and the ability to be automated; (iii) no requirement for chemicals; and (iv) the ability to effectively remove bacteria, viruses and other microorganisms. Conventional membrane filtration can be divided into three stages: pre-filtration (media filtration), ultra-filtration and reverse osmosis. However, three key disadvantages associated with membrane filtration systems are the high installation cost, high energy requirement, and the necessity for maintenance (removal of membrane fouling material). As a consequence, over the past few decades much research has been conducted into the development of more cost effective and simple water treatment systems for the developing world [3,4,7,12,13,14,15,16,17,18]. These efforts include the development of renewable energy powered membrane (RE-membrane) technologies [19,20]. Resulting water treatment systems are flexibly scalable from devices using individual modules for household water supply (e.g., Lifestraws and Homespring) to large scale water supplies for mega-cities [20]. Process selection can be adjusted to any water quality and desired contaminant removal. The Portable Aqua Unit for Lifesaving (PAUL), also known as Water Backpack [13] is presented as an example in the following section.
1.2. Membrane Technology Can Be a Bridging Solution
PAUL is a portable membrane water filter (pore size: 20–100 nm) developed at the University of Kassel (Germany) for humanitarian aid [13]. It is designed for the specific purpose of decentralized water treatment in emergency and disaster situations. PAUL functions without chemicals or energy input and during its “lifetime” there is no need for technical maintenance. A PAUL device can produce safe water for up to 700 persons for several months. Furthermore PAUL has been demonstrated as highly effective for the removal of 99.999% of bacteria (Escherichia coli and coliform) and 99.9% of viruses (coliphages). The performance of PAUL and other similar gravity driven membrane filtration systems for the treatment of chemical contaminant species (e.g., hydrocarbons, metals, metalloids, radionuclides), however, has been demonstrated as less effective. PAUL and similar membrane filtration systems are therefore inappropriate for the procurement of potable water in remote communities where such aqueous contaminant species (including arsenic, fluoride, and nitrate) are above minimal threshold levels. Accordingly, there remains a need for the development of alternative and/or complimentary technologies for water treatment. Gravity filtration using Fe0 has been discussed in the literature as a potential candidate technology due to the ability of Fe0 to remove microorganisms, degrade organic contaminants and also immobilize metal and metalloid species [14,15,18,19,20,21,22,23,24,25,26,27].
1.3. The Suitability of Fe0 Filters for Safe Water Provision in the Developing World
The idea of using metal corrosion for the in-situ generation of metal hydroxides for aqueous contaminant removal is the basis of electro-coagulation, essentially using Al0 and Fe0 as electrodes. Noubactep and Schöner [28] discussed the similarities between decontamination by electro-coagulation and by using granular metallic materials. According to Bojic et al. [29], the great efficacy of voluminous insoluble Al(OH)3 for aqueous removal of many chemical and microbiological pollutants implies efficient water decontamination by a microalloyed aluminium based composite. Bojic et al. [29] positively tested this idea to eliminate Escherichia coli from a model surface water and later for various chemical contaminants including Cr, Cu, halogenated trihalomethanes, and Zn [30,31,32].
In an independent approach, Fe0 was used as a reducing agent or generator of iron hydroxides for water treatment [33,34,35]. By 2002, Fe0 had already been tested for many relevant groups of chemical contamination. With the publication of the work of You et al. [36] entitled “Removal and inactivation of waterborne viruses using zerovalent iron”, Fe0 was demonstrated to be a universal material for water treatment. Since then, a great deal of work has established the potential of Fe0 for water disinfection [37,38,39,40,41].
The suitability of Fe0 filters for safe water provision in the developing world arises from their ability to treat chemical and biological contamination. Pathogens, arsenic and fluoride are arguably the three main pollutants of worldwide relevance [42,43,44,45]. The ability of Fe0 filters to remove salt ions (e.g., fluoride) and trace contaminants (e.g., arsenic) makes this technology more suitable than gravity-based membrane technology for deployment in remote areas.
1.4. Fe0 Filters for Self-Reliance in Water Supply
This article presents a comprehensive overview of the science and rationale for the use of Fe0 for decentralized water treatment. In particular a working methodology is presented which is intended to function as a basis for future work; this comprises a hypothetic scenario wherein Fe0 is used for the provision of potable water to a community with 100 inhabitants (1 m3 water/day). This population size has been selected because it is considered to be one “production module” which can then be scaled upwards for communities of different population. For example, a community with 400 inhabitants would require four production modules. Downwards scaling for communities with less than 100 inhabitants is not discussed but the expertise from the present effort would also enable downscaling.
2. Water Supply Systems
2.1. Centralized Water Supply
Centralized water supply represents the current conventional water supply approach [4,6]. This approach is based on providing water through water supply schemes including components such as: water source development (namely boreholes, rainwater, or rivers), water distribution systems (namely piping systems), water storage systems (namely overhead tanks). A ready source of power supply is needed to run the schemes and a distribution network [4,6,18,46,47]. Individual homes are expected to be connected to the distribution network (Figure 1). Centralized water supply systems are typically available in cities both in the developed and developing world, and small communities in the developed world. In urban areas of the developing world, water is often quantitatively available. However, ineffective water supply chains frequently result in low quality [11,46].
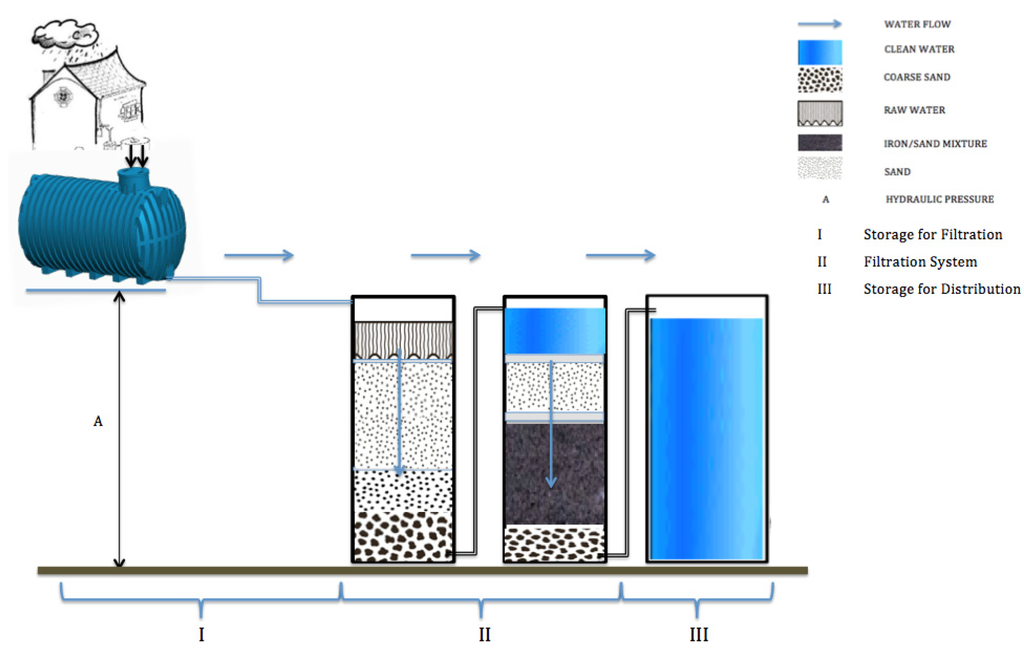
Figure 1.
Concept of water treatment train based on filtration on granular materials and including at least one sand filter for media filtration (roughing filter). Further units may comprise: (i) biosand filter; (ii) activated carbon filter, Fe0/sand filter. Treated water is stored for distribution.
2.2. Disadvantages of the Centralized Water Supply
Centralized water supply systems may be prohibitively expensive to install, operate, and maintain for low-income and/or remote communities. This is due to a number of reasons including: (i) intermittent power supply (e.g., lack of fuel); (ii) lack of infrastructure; and (iii) lack of technical knowledge to maintain infrastructure [1,6,11,21]. Accordingly, there is an urgent need to develop affordable yet also low maintenance technologies for the water supply of low-income and remote communities.
2.3. Decentralized Water Supply
Some rural locations are equipped with obsolete water supply systems with an overhead storage tank. In the developing world, however, such small scale centralized water supply systems are fraught with financial and managerial problems [6,11,46]. Boreholes are sometimes available for domestic water supply systems; however, the quality of these and all other water sources (rain, river, source) is not typically monitored [11,48]. Alternative decentralized technologies for the developing world should be small-scale, energy efficient, environmentally sound, and use locally available resources [7]. They should also be capable of being controlled and maintained by the local community.
2.4. Appropriateness of Decentralized Water Treatment Systems
Decentralized water treatment systems have three main unique advantages: (i) the ability to tailor the technology for specific contaminant species; (ii) low cost; and (iii) ability for deployment in remote locations. Ideal systems should be able to produce clean drinking water without power input, or with energy input via renewable sources such as solar power [19,20]. Ideally, the system should also be constructed using local materials and use local technical labor. These requirements exclude the use of chemicals (including chlorine), particularly in low skill communities. The requirement of using endogenous or easily transferable technical skills exclude membrane technology (Section 1.2) as long as used membranes are not locally manufactured [49].
3. Decentralized Water Treatment with Metallic Iron
3.1. An Overview of the Fe0/H2O System for Contaminant Mitigation
The potential utility of Fe0 filters for decentralized safe drinking water provision has been intensively investigated over the past 15 years [14,15,16,18,24,26,27,50,51,52,53,54]. Fe0 is considered an appropriate material for water treatment because it is a relatively strong reducing agent (E0 = −0.44 V) and was applied initially to transform recalcitrant halogenated organic compounds into less toxic and/or biodegradable species [55,56,57,58,59]. More recently, Fe0 was demonstrated as highly effective for the treatment of several other classes of substances, including aromatic nitro compounds, bacteria, heavy metals, herbicides, nitrates, pesticides, radionuclides and viruses [18,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. Fe0 materials have also been used in Fenton oxidation reactions [71,72,80,81,82]. It appears from the overview of treatable contaminants that Fe0 might be regarded as a technology with the potential to manage all classes of contaminants (inorganic ions, organic poisons, and harmful germs). This impression is supported by articles which reported on quantitative removal of species (e.g., 1,2-dichloroethane, dichloromethane, methylene blue, triazoles) which were proven to be not reducible by Fe0 [83,84,85].
The performance of Fe0 materials for water treatment has been adapted in recent years via multiple different methodologies, for a wide range of applications, including: (i) decreasing the particle size to nano-scale in order to enhance the reactivity of the material as a function of mass [86]; (ii) embedding noble bimetallic particles into the Fe0 structure in order to improve the galvanic properties of the material [87]; (iii) embedding Fe0 into appropriate porous support materials [14,48]; and (iv) embedding Fe0 with complimentary adsorbent materials [88,89,90]. In addition, other metallic elements (namely Al0, Cu0, Sn0, Ti0 and Zn0) have also been tested as alternatives to Fe0 [91,92,93,94,95]. However, Fe0 has typically been demonstrated as superior due to its cost-effectiveness, bio-compatibility and long-term reactivity under natural conditions [96,97]. The presentation herein is limited to granular mm- and μm-scale particles (d > 215 μm) [25,98]. Furthermore, the suitability of micron scale Fe0 particles arises from the evidence that resulting system (Fe0 filters) must be efficient in the long term, with sub-micron scale particles becoming exhausted over relatively short timescales [25,71,72,99,100].
3.2. The Nature of the Fe0/H2O System
It has been demonstrated/recalled that under environmental conditions, the Fe0/H2O interface does not exist [101,102,103,104,105,106]. Rather, there is a minimum of two interfaces: Fe0/Fe-oxides and Fe-(hydr)oxide/H2O, with the material comprising a “core-shell” structure [80,81,107,108,109,110,111,112]. Moreover, the (hydr)oxide layer comprises the location for H/H2 and Fe2+ formation which is driven by Fe0 corrosion. Given that the outermost (hydr)oxide layer is not typically electronic conductive, extensive chemical reduction of aqueous contaminant species upon this interface does not typically occur [113]. In contrast, chemical reduction of aqueous contaminants due to chemical interactions with H/H2 or Fe2+ has been demonstrated as more likely [114,115,116,117,118]. As a consequence Fe0 corrosion is an electrochemical reaction mediated by water (H2O or H+) and resulting in H2 evolution. However, contaminant reduction, when it occurs, is not the primary coupled cathodic reaction [82,112,118,119].
This clarification coupled to the consideration of the formation of voluminous Fe0 corrosion products is the theoretical starting point for the design of next generation Fe0 filters [16,17,25,98,100,120,121,122,123]. No consideration of these key issues has led to controversial reports which render the assessment of progress in designing Fe0 filters difficult. For example, in a recent article entitled “South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers”, Trois and Cibati [54] demonstrated the suitability of admixing 25 or 50% (v/v) Fe0 with natural sand to treat as contamination. The title of their article is justified by the volumetric abundance of sand but the experimental design did not test any pure sand system (100% sand).
4. Rationale for Fe0 Filter Design
At present there are conflicting views with regard to the design of Fe0 filters [8,9,54,58,88,89,90,124,125,126]. In particular, the usefulness of mixing Fe0 and inert (anthracite, pumice, sand) or reactive but non-expansive (Fe3O4, MnO2, TiO2) materials is still controversially discussed [125,127,128]. Furthermore, an empirical approach has been used to screen selected operational factors like grain sizes and grain size distributions [126], grain packing [129] or the mixture of Fe3O4 and external FeII solutions [88,89,90]. This empirical approach is certainly costly but not necessarily effective [121,122,123]. An alternative approach is to develop the science of the system, which will serve as compass to evaluate experimental results [25,130,131].
A key factor which prevents cross correlation between Fe0 water filtration studies is the absence of a standard reference material for Fe0 [132]. Accordingly, even results obtained under similar conditions are not really comparable. A methodology to compare the intrinsic reactivity of Fe0 materials was introduced [133], and was recently revisited [132,134], however it is yet to receive universal acceptance. On the other hand, a universal design rationale for the design of Fe0 filters was presented by Noubactep and Caré [130,131] and progressively revisited [25,98,121,122,123].
The following text provides the example of a pioneering work by Westerhoff and James [124] and enumerates the lesson that could have been learnt from it. The discussion is limited to relevant design aspects.
4.1. A Non-Exploited Pioneering Work
Westerhoff and James [124] investigated nitrate removal in Fe0 packed beds both at lab and field scales. Laboratory columns (V = 600 mL, D = 5 cm, L = 30 cm) were used. The columns were packed with two different Fe0 samples (Fe0 is generally termed as zero-valent iron (ZVI)): 1636 g of ZVI1 (6.32 g/cm3) and 2271 g of ZVI2 (7.89 g/cm3). The field columns were packed with ZVI1. The dimensions of the field columns were: V = 4000 mL, D = 7.5 cm, L = 91 cm. While the lab columns contained 100% Fe0, a series of field experiments implied a column with a bottom half packed with 50% sand and 50% Fe0 to sustain the permeability. The other run had 100% Fe0 throughout the column (Figure 2).
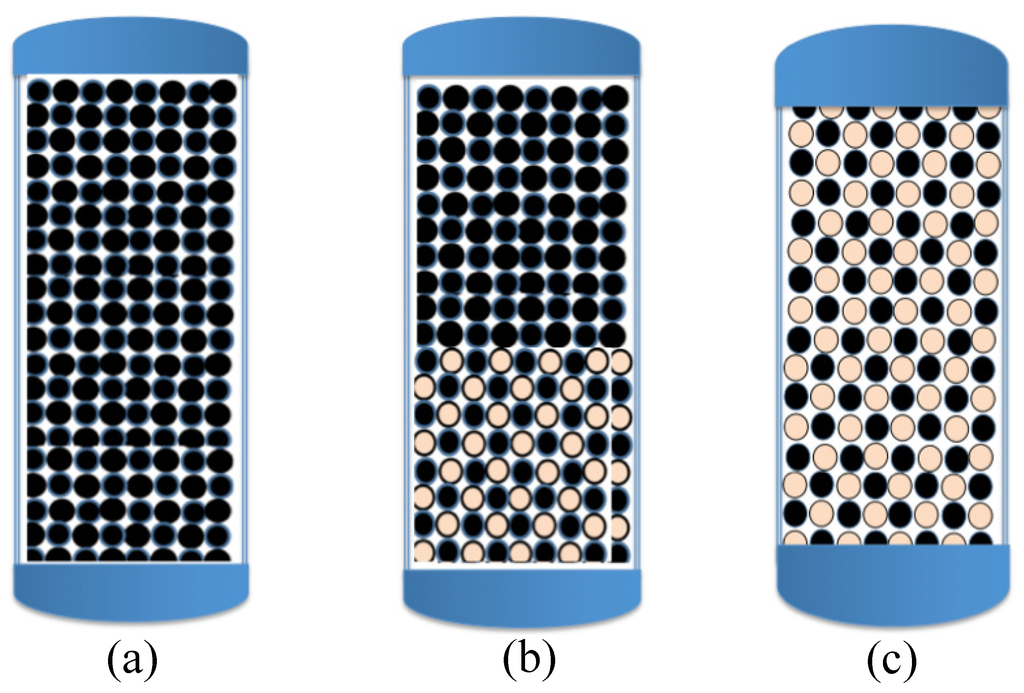
Figure 2.
Schematic diagrams of the common design of individual Fe0-based filters: (a) pure Fe0 (100%); (b) a pre-treatment hybrid zone (e.g., Fe0/sand) followed by 100% Fe0; and (c) a fully hybrid system. It has now been established that only fully hybrid systems are sustainable because of the volumetric expansive nature of iron corrosion at pH > 4.5.
Field experiments lasted for approximately 12 months. During this period, operational problems occurred frequently in the pure Fe0 system. The most significant operational problem was a decrease in hydraulic conductivity (permeability loss) over time. Permeability loss was also documented in the hybrid Fe0/sand system, but to a lower extent. The experiments were stopped when the residual value of the hydraulic conductivity was less than 10% of the initial value (for the hybrid Fe0/sand system). Other operational problems included: (i) air entrainment; (ii) electrical power outage at the site; and (iii) cracks in reactor.
The most important results of Westerhoff and James [124] could be summarized as follows: (i) permeability loss was more pronounced under field conditions (due to a continuous supply of dissolved O2); (ii) there was a deficiency in the nitrogen mass balance (co-precipitation or enmeshment); (iii) a large difference in intrinsic reactivity was documented between the two tested Fe0 materials; and (iv) from the bottom (influent side) to the top the material in the column exhibited differential compaction behavior. In particular, for the hybrid system (bottom Fe0/sand, top pure Fe0) the three following layers were observed: (i) the bottom 10 cm consisted of highly cemented Fe0 and sand; (ii) the intermediate layer (still within the Fe0/sand zone) was black in color and was visually similar to the original Fe0 material, but the Fe0 particles were irregular in shape; and (iii) the upper pure Fe0 layer maintained the irregular shape of the iron but changed to a black-gray color. Despite increased cementation in the influent zone, the same trend was observed in the pure Fe0 column.
4.2. Lessons from the Pioneering Work
The observations from the hybrid system of Westerhoff and James [124] correspond to the recent results of Miyajima [135] and Phukan [136]. These authors used a 1:1 Fe0:sand (vol/vol) for methylene blue discoloration in column studies for four and three months respectively and observed that at the influent of the column, about 3 cm was brown colored (Figure 3) and hardly compacted, while the remaining Fe0/sand layer was less or not compacted and black colored. Recent works have recalled that the availability of dissolved O2 is the major factor causing particle cementation and permeability loss [25,100,122,123]. However, even without this “recent” knowledge, a pragmatic approach would have consisted of comparing the density of Fe0 (7.8 g/cm3) and Fe oxides (e.g., β-FeOOH; 3.6 g/cm3) [137]. Because the volume of a filter is constant, iron corrosion is necessarily coupled with a decrease of the pore volume because in-situ generated oxides are less dense or more voluminous than parent Fe0. If Fe0 (ZVI) is to be transformed to Akageneite (β-FeO(OH,Cl)), a volume of about 2VZVI is necessary for the reaction to be quantitative.
4.3. Disregarding Lessons from the Pioneering Work
Despite the clear message from Westerhoff and James [124] that pure Fe0 systems are not sustainable, research with pure systems has continued. For example, Ruhl et al. [125] recently tested four binary mixtures (Fe0/anthracite, Fe0/gravel, Fe0/pumice and Fe0/sand) for their efficiency in removing trichloroethylene (TCE) in column experiments for up to 200 days. No accompanying Fe0 system (reference system) was tested, the work of Westerhoff and James [124] was not considered for the discussion. The authors concluded that “the mixed reactive filters” are not applicable for treatment of the “tested groundwater with its indigenous microorganisms”. This conclusion is erroneous for at least three reasons: (i) it is unlikely that used Fe0 is depleted within 200 d; (ii) a pure Fe0 system is not tested (no operative reference); and (iii) TCE removal by binary systems containing only 22% Fe0 (w/w) was the system used to demonstrate the feasibility of Fe0 for groundwater remediation [58].
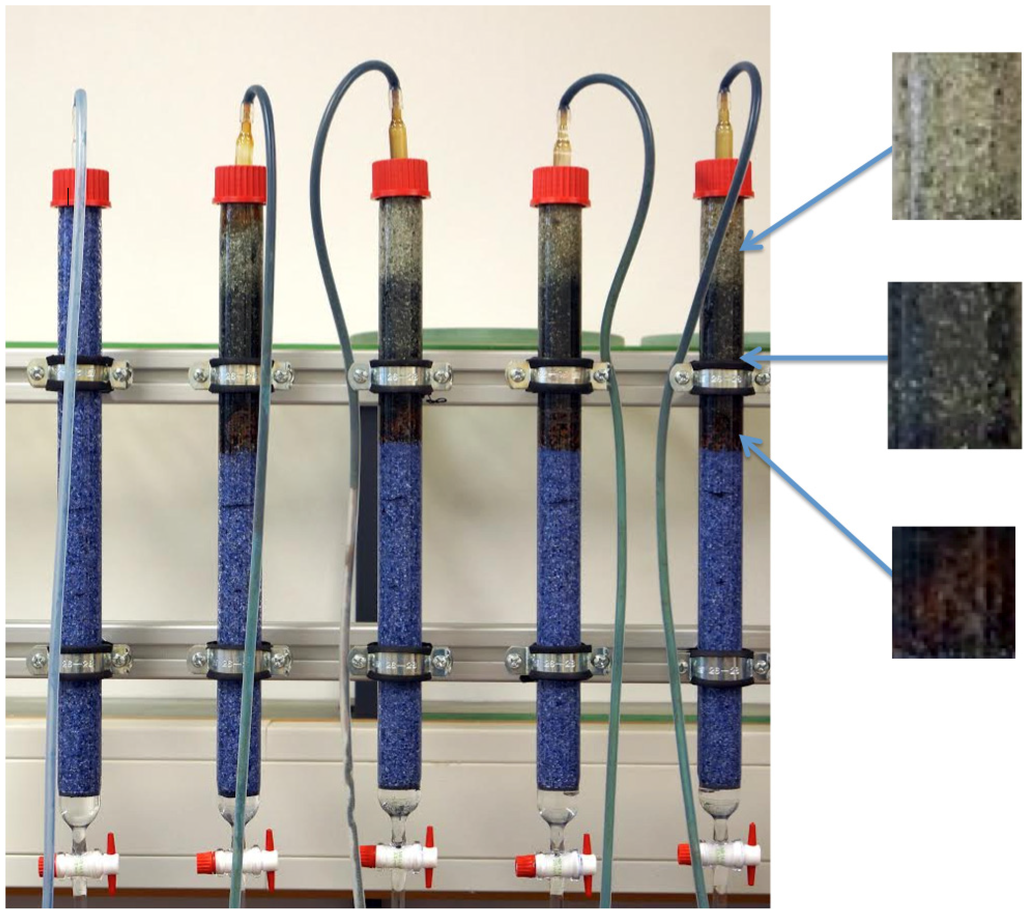
Figure 3.
Photograph of a column design depicting the typical sequence of coloration described by Westerhoff and James [124]. It can be seen that the entrance zone of the Fe0/sand zone is colored brown while the upper section is black. The sand layer after the Fe0/sand zone more or less maintains its “white” color or is brownish by FeIII iron oxide from the Fe0/sand zone. Sand in the reference system (first column) and the sand layers preceding the Fe0/sand zone is blue colored by methylene blue (see text).
A second example showing how the results of Westerhoff and James [124] were not properly considered is presented by Huang et al. [88,89,90]. The authors reported on a new efficient hybridized Fe0/Fe3O4/FeII system for environmental remediation and water treatment. However, it is not clear from the related works, what makes the system sustainable and why magnetite is the suitable additive. In a system in which FeII is generated (iron corrosion) in-situ and is suitable at the long-term, the introduction of external FeII could be regarded as counterintuitive (LeChatelier Principle). In other words, Huang and colleagues have not yet rationalized the functionality of their system.
4.4. Evaluation
The two examples in Section 4.3 and the parameters in Table 1 and Table 2 testify that various μm- and mm-scale Fe0 materials have been tested as filter materials for water treatment. Most of the experiments were performed at laboratory scale. Field experiments were reported as well. Some 200 Fe0 permeable reactive barriers (Fe0 PRBs) have been installed worldwide [138,139,140]. Results from laboratory experiments (controlled conditions) have typically shown promising treatment efficiencies. Results from the field trials, including commercial Fe0 PRBs, have been more various. In particular, failures of Fe0 PRBs [83,141] have not been satisfactorily rationalized [122,123]. It is evident that the composition of contaminated waters and effluents vary to a large extent. This is also one of the reasons why comparison of published results is challenging.
Table 1 and Table 2 clearly relate the diversity among Fe0 material tested or used for environmental remediation and water treatment. Although Fe0 has been used in some 200 PBRs, little progress has been made toward characterizing the variability in reactivity among Fe0 samples from different sources [132,133,134,142,143].

Table 1.
Selected operational conditions of column studies presented as examples in Section 4 with regard to their diversities. X is the contaminant of concern and “n.s.” stands for non specified. Generally, diversities of operational conditions including the amount of Fe0 materials and their proportion in hybrid systems render fixation of reported discrepancies between studies difficult.
| Fe0/Solid Ratio | Column Dimensions | Flow Rate | Duration | X | Reference | |
|---|---|---|---|---|---|---|
| D | L | |||||
| (vol:vol) | (cm) | (cm) | (mL/min) | (Days) | ||
| Fe0 (100%) | 5.0 | 30 | variable | weeks | NO3− | [124] |
| Fe0/sand (3:1) | 7.5 | 91 | n.s. | 365 | NO3− | [124] |
| Fe0 (100%) | 7.5 | 91 | n.s. | 365 | NO3− | [124] |
| Fe0/sand (3:1) | 16 | 107 | 4.2 to 201 | 0.21 | As | [54] |
| Fe0/sand (1:1) | 16 | 107 | 4.2 to 202 | 0.21 | As | [54] |
| Fe0 (100%) | 16 | 107 | 4.2 to 203 | 0.21 | As | [54] |
| Fe0/anthracite (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/gravel (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/pumice (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0/sand (n.s.) | 2.5 | 21.5 | 0.075 | 200 | TCE | [125] |
| Fe0 (100%) | 5.1 | 15.0 | n.s. | 14 | PO43− | [8] |
| Fe0 (100%) | 5.1 | 15.0 | 0.8 to 1.0 | 14 | As, Cr, Se | [9] |
| Fe0 (100%) | 5.1 | 15.0 | 0.8 to 1.0 | 14 | Cd, Cu, Pb | [9] |

Table 2.
Selected characteristic of Fe0-based filter materials used in studies presented as examples in § 4.
| Material | Availability | Origin | Mass | d | Reference |
|---|---|---|---|---|---|
| (g) | (mm) | ||||
| Iron filings | scrap iron | Masterbuilders Inc. | 1636 | 0.05–0.6 | [124] |
| Iron chips | commercial | Baker Iron | 2271 | 0.5–5.0 | [124] |
| Iron fillings | commercial | Connelly-GPM Inc. | n.s. | 0.08–2.4 | [54] |
| Iron fillings | commercial | Gotthart Maier | n.s. | 1.0–2.0 | [54] |
| Granulated cast iron | commercial | Gotthart Maier | 100 | 0.3–2.0 | [125] |
| Zero-valent iron (ZVI) | commercial | Connelly-GPM | n.s. | 0.1–2.0 | [8,9] |
| Porous iron composite (PIC) | commercial | NA Höganäs Inc. | n.s. | 0.1–2.0 | [8,9] |
| Sulfur modified iron (SMI) | commercial | SMI_PS, Inc. | n.s. | 0.1–2.0 | [8,9] |
The Fe0 literature review has also revealed that it is very hard to normalize the data from different independent studies [144,145]. In fact, despite an observed linear relationship between the first-order rate constant (kobs) and the specific surface area of Fe0, the introduction of the kSA-concept (surface area normalized rate constant) did not enable comparison of experiments obtained by different Fe0 types. There are several reasons for this including: (i) the intrinsic reactivity of tested Fe0; (ii) the various physical properties (shape, size, specific surface area) of tested Fe0; (iii) the proportion of Fe0 in the system; and (iv) the solution pH and the redox potential. Furthermore, a standard protocol is missing which might enable the comparison of Fe0 materials based on their treatment efficiency for a given volume of water, with a given level of contamination, and flow through the Fe0-based filter at a certain flow velocity. Only under such defined conditions can the complexity of processes occurring in Fe0/H2O systems be properly addressed.
Summarizing the Fe0 literature, it can be observed that despite 20 years of intensive research and numerous field scale treatment facilities, the explanations for contaminant removal from water by Fe0 are still in their infancy [25]. Drawbacks have not been reported to a large extent, attention seems to have been focused on success stories [118,122,123]. The use of Fe0 as filter materials for water treatment is necessarily connected to two major “drawbacks” [41]: (i) “reactivity loss” or non-linearity of Fe0 corrosion; and (ii) permeability loss resulting from the loss of interconnectivity of the initial pore space (in-situ generation of “cementing” agents). In essence, both “reactivity loss” and permeability loss are inherent to Fe0 filtration for water treatment. This implies that both apparent “disadvantages” have occurred (to different extents) at success sites. Therefore, the way forward is further systematic research. Such a collaborative research path has recently been initiated in our laboratories. Achieved results are summarized in the following section.
4.5. Rationally Designing Fe0 Filters
A critical literature review (Section 4.1, Section 4.2, Section 4.3 and Section 4.4) has revealed unsatisfactory aspects related to the design of Fe0 filters. It has also shown that, based on Westerhoff and James [124], the Fe0 system design could have been given more attention during the past 10 years. This approach would have been beneficial for the procurement of new systems as well as the modification and operation of existing systems (e.g., three-pitcher household filter). It is of vital importance that these systems are designed according to the intrinsic properties of Fe0 [101,104,108,109], and known principles for designing conventional granular filters [146,147,148]. Moreover, the intrinsic properties of contaminants should be considered because classifications like “organic contaminants (e.g., dyes, pesticides, and pharmaceuticals/drugs)” or “industrial organic wastes (e.g., phenols and aromatic amines)” say nothing about the chemical reactivity or the affinity of the species of concern for the Fe0/H2O system.
The realization that aqueous contaminant removal in the presence of Fe0 (Fe0/H2O system) is not a property of the reduction by Fe0 but a characteristic of corroding Fe0 [115,149,150,151] was decisive for the rational design of Fe0 filters. In other words, contaminant removal is not mediated by reductive degradation or reductive precipitation but by the interactions between dissolved contaminants and primary (FeII, H/H2), secondary (FeII/FeIII-hydroxides/oxides) and tertiary (FeIII-hydroxides/oxides) products of Fe0 oxidative dissolution. Here, Fe0 is oxidized by water (H+ or H2O). This observation corresponds to reports of several researchers [33,35,152,153,154,155] with the subtle but decisive difference that reduction (if applicable) is subordinated to adsorption, co-precipitation, and size-exclusion. Clearly, under experimental conditions, there is reduction in the presence of Fe0 (in Fe0/H2O systems) but not by Fe0 (no direct reduction).
The finding that direct reduction (reduction by Fe0) is of secondary importance for the process of contaminant removal in Fe0/H2O systems implies that observed reduction was mediated by primary corrosion products (FeII and H/H2) and that contaminants are removed by adsorption, co-precipitation, and size-exclusion. This finding was followed by a theoretical work discussing the suitability of using hybrid systems instead of pure Fe0 layers [120,130,131]. Results challenged the still popular view, that mixing Fe0 with non-reactive materials (e.g., pumice, sand) is a chance to save Fe0 costs while satisfying width requirements [125,127,128]. Rather, it can be demonstrated that mixing Fe0 and a non-expansive aggregate is a prerequisite for sustainable Fe0 filters, the most sustainable system containing 25% Fe0 (v/v) [98,100,121]. These theoretical results have been experimentally validated [135,156,157,158,159].
The ion-selective nature (charge exclusion) of Fe0/H2O systems was also demonstrated using methylene blue (MB) as operational indicator [135,156,158,159]. The suitability of MB for this purpose arises from the fact that MB (cationic) has a very low affinity for iron hydroxides/oxides (pHpzc > 6.0) covering the surface of Fe0 in Fe0/H2O systems. Under these conditions, the most reactive system is the one quantitatively producing iron oxides, or the one in which “early” MB breakthrough is observed [132]. From Figure 3, a summary of the behavior of the Fe0/sand/MB system can be read as: (i) negatively charged sand is an excellent adsorbent for MB; (ii) in the Fe0/sand zone, MB discoloration is first “disturbed” by FeII and FeIII ions (concurrence for negatively charged sand surface), and later by ion oxides, coated in-situ on sand [135,158]; and (iii) the preferential flow created in the Fe0/sand zone makes the sand layer upwards not fully available for MB discoloration (no blue coloration).
This hypothesis from MB discoloration (charge exclusion) was further confirmed/validated using two anionic dyes: Orange II and Reactive Red 120 [136,160]. Using MB as operative indicator has solved the long lasting problem of reducing the experimental duration while achieving reliable results [113,161,162,163,164,165,166]. When using up to 100 g Fe0 packed in fixed beds, it has been clearly demonstrated that reliable results could be obtained only after two months. This should be regarded as the absolute minimal duration of a column study. Table 1 recalls that larger Fe0 amounts have been typically used for sometimes shorter experimental duration.
The demonstration that (i) the Fe0/H2O system is ion-selective and (ii) the evidence that only hybrid systems are sustainable are the cornerstones for the design of better laboratory and field Fe0 filters. This issue is addressed in the following section. The ion-selective nature of the Fe0/H2O system suggests that available literature on the interaction of iron oxides/hydroxides with contaminants should be considered in designing Fe0 filters. In other words, classifications like “heavy metals”, “industrial organic wastes”, “organic contaminants” or “personal care products” are not really useful. As an example, a recent review on dyes adsorptive removal [167,168] reveals that the science of dye interaction with metal oxides (including iron oxides) dates back to the years 1951–1970. Since then, it has been established that there are three classes of dye: anionic, cationic and non-ionic, behaving differently with various metal oxides and hydroxides [169,170,171,172,173,174,175,176]. The main open question for the Fe0 research is how to correlate available knowledge on contaminant adsorption on iron oxides and the fact that these adsorbents are progressively generated in-situ in Fe0/H2O systems. A further question would be when the generation of iron oxides stops or when available Fe0 will be completely exhausted in the filters. Answering this question would enable the prediction of service life of Fe0 filters.
5. Designing Fe0 Filters for On-Site Water Treatment
The Fe0 amount necessary to treat a given volume of water depends on five main parameters: (i) the nature of the contaminant (solubility, affinity to iron corrosion products); (ii) the contamination level; (iii) the Fe0 intrinsic reactivity; (iv) the water flow velocity and the resulting interaction time as well as (v) the water chemistry (including pH value and hardness). The first intrinsic issue associated with Fe0 is that its physical structure and chemistry constantly changes during aqueous corrosion. This inherent difficulty makes stoichiometric determinations between Fe0 and aqueous contaminant species extremely challenging, i.e., it is difficult to define operational parameters such as adsorption capacity which is a trivial process for non-reactive adsorbents [13,177,178,179].
5.1. Modular Fe0 Filter Design
The present paper intends to provide rationale for the design of a pilot plan in which a Fe0 filter operates as an independent treatment unit, similar to the pioneering work by Westerhoff and James [124]. The scientific basis for this design is summarized in Noubactep et al. [121] and Rahman et al. [98]. The basic treatment system should consist of a modular series of treatment processes and includes in the following sequence (i) roughing filters; (ii) slow sand filters (SSF) and (iii) Fe0/sand filters. Fe0/sand filters could also be followed by SSF or iron removing filters for maximum effluent water quality. As stated above, the goal is to build a module for the treatment of drinking water for 100 people. The World Health Organization estimates that the average daily water requirement for drinking and cooking per person is 7.5 L, which equates to 750 L for 100 persons. The system presented herein is therefore designed to treat a minimum of 1000 L (1 m3) water per day.
It should be explicitly stated that slow sand filter (SSF) are currently more or less successfully used for drinking water provision at household and small community levels [54,120]. However, sand itself does very little in cleaning water contaminated with organic and inorganic species (chemical contamination) and the removal of biological contamination is not resolved to this day. In essence, sand filters in study [180] work only when the groundwater to be cleaned is FeII-rich. This FeII is oxidized to FeIII and derived FeIII-(hydr)oxides in-situ coat sand grains making it suitable for the removal of contaminants such as As [50]. The effort presented herein could be regarded as an attempt to improve SSF.
5.2. Appropriateness of Fe0 Filters
An implicit shortcoming for Fe0/sand filters is that Fe0 corrosion by natural water cannot be accelerated “on request”. Positively tested approaches to accelerate Fe0 corrosion include (see Section 4.1) (i) the reduction of the particle size down to nano-scale [86]; (ii) using porous materials [8,9,14]; (iii) using electro-dissolution and internal electrolysis [27,181]; (iv) using oxidizing agents like H2O2 or O3 [24,52]; (v) using multi-metallic systems, including sulfur modified iron [8,9,27,69]; (vi) using minerals with the potential to act as FeII scavengers (MnO2) [159,182]; and (vii) controlling the O2 level [124]. Of these approaches, only those implying no chemicals and no technical skills are suitable for small communities (in the developing world). These are: using porous materials, using multi-metallic systems, using minerals like MnO2, controlling the O2 level, and combinations thereof.
In preliminary studies, the initial water flow rate should correspond to that of a slow sand filter. This initial flow rate will be stepwise increased to take the maximum advantage of microbial processes in the SSF and chemical and physical processes in the Fe0/sand filters.
5.3. Improving Available Designs
A compilation of literature data [24,121,124,183], suggests that an effective pilot system is likely to comprise: (i) a series of polyethylene tanks (≥1200 L) installed for instance beside a municipal water treatment plant (raw water storage); (ii) a series of roughing filters; (iii) a series of slow sand filter (SSF); (iv) a series of Fe0/sand filters, eventually (v) a series of filters for the removal of Fe escaping from the Fe0/sand filters; and (vi) a second series of polyethylene tanks to collect and store drinking water. The number of filters in each series depends on the quality of the raw water and the quality of potable water to be delivered to the community. In a pilot study, after each treatment step, the quality of water should be monitored.
As a starting point, a modified field column of Westerhoff and James [124] can be applied: V = 4400 mL, d = 7.5 cm, L = 100 cm. Each Fe0/sand column comprises a 70 cm reactive layer sandwiched between 2 layers of gravel or fine sand (each 15 cm thick). Roughing filters and slow sand filters are filled in the same manner but the reactive layer (70 cm) is replaced by gravel and fine sand respectively (Figure 4).
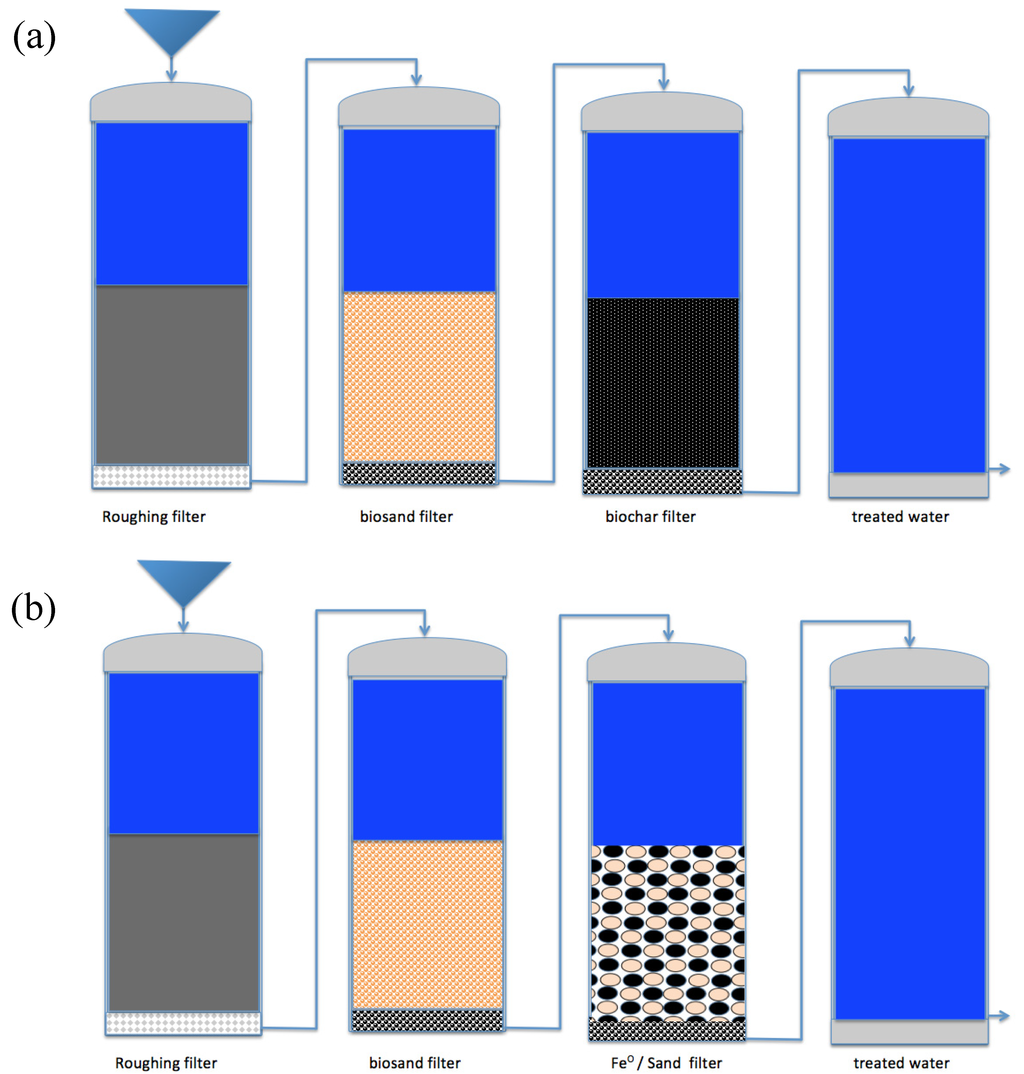
Figure 4.
Concept for the configuration of multi-barrier water treatment systems: (a) the sustainable biochar-based system; and (b) a comparable Fe0-based system. Adapted from Kearns, 2012 [184]. A comparison of the cleaning efficiency of any material to Fe0 should be performed by replacing the Fe0/sand unit by the corresponding unit(s) containing the material of concern. This approach will enable the extension of the multi-barrier concept for efficient water treatment.
5.4. Ways for Efficient Fe0 On-Site Water Treatment Plants
Section 4.4 demonstrated that a minimum of six variables (Fe0 intrinsic reactivity, Fe0 shape and size, Fe0 content (amount and proportion), solution pH, and the redox potential) have been shown to have an important impact on the decontamination efficiency of Fe0/sand filters. This makes comparisons and correlation of available data difficult. However, a profound analysis of the fundamental reactions involved, together with some recently obtained design criteria, bring out a number of important considerations which may simplify design efforts. They can be summarized as (i) use only volumetric Fe0 ratios ≤ 50%; (ii) characterize the intrinsic reactivity of used Fe0; (iii) use as little Fe0 as necessary for reliable observations; and (iv) avoid too short experimental durations.
Testing Fe0 materials at pilot scale (a given model water) can be summarized in the following: (i) test several well-characterized Fe0 materials; (ii) test several Fe0/sand ratios (Fe0 < 50%), for a given Fe0 material and a Fe0/sand ratio; (iii) test the number of each unit for satisfactory water treatment; (iv) insert wood charcoal units before Fe0/sand units; (v) partly or totally replace sand by porous materials including: anthracite, gravel, MnO2 and pumice.
At each site and for each design water samples should be analyzed on a weekly basis. Besides the concentrations of relevant contaminants (including pathogen indicators), the Fe level, the turbidity and the pH values should also be monitored. The hydraulic conductibility should also be recorded. This ambitious program implies the equipment of water laboratories wherever pilot scales are planned: ideally in any country water contamination is potentially a problem. Clearly the whole developing world should be equipped with good water laboratories. At the end of the field operation, the Fe0/sand column should be dismantled and thoroughly characterized. In this effort the seminal work of Westerhoff and James [124] can be used as guide.
5.5. Comparing Fe0 Filters to Other Technologies
The constancy that the nature of Fe0 filters has not been properly considered in filter design efforts implies the necessity to revisit the approach to (i) compare Fe0 and other affordable filter materials for water treatment [185,186]; and (ii) implement Fe0 in a combination of treatment methods for more efficient systems [187,188]. The presentation above has already shown that O2 scavengers including SSF should precede Fe0/sand filters. The situation of multiple contaminant mixtures which may not be efficiently treated by a single technology [189] is an opportunity to compare Fe0 filtration to other available affordable filtration systems [190] and shape multi-barrier systems involving Fe0 filters. Contaminants possess different properties with respect to adsorptive behavior, degradation potential, molecular size, solubility, and surface charge [191]. Fe0 filters are ionic selective in nature because at neutral pH values, the surface of iron oxides is positively charged [160,191]. This means that negatively charged contaminants are readily removed in Fe0 filters. Because multiple contaminant mixtures may contain positively charged species as well, it is important to comparatively test Fe0 and other materials under the same operational conditions. Relevant filter materials include activated carbons, bark, bio-carbons (biochar), wood charcoal, calcareous shale, chitin, chitosan, commercial ion exchangers, dairy manure compost, dolomite, fly ash, lignite, limestone, olivine, peat, rice husks, steel slag materials, vegetal compost, yeast, and zeolites [190,192].
Comparing Fe0 filters to other filtration systems is continued by replacing Fe0/sand units in a treatment train as described in Section 5.3 by a unit filled with the material to be tested (70 cm per unit). Here the materials are not compared based on their mass or their chemical reactivity, but on the efficiency of a 70 cm packed filter. Results of such an experiment give a strong basis for the design of multi-barrier systems involving Fe0/sand units.
5.6. Economic Considerations
The economy of Fe0/sand filters has already been discussed. According to Gottinger et al. [24,52], Fe0/sand filtration for removal of As and U is economically feasible for small-communities. The total treatment cost is less than $ 0.01/L water in their modular treatment train, including filter installation, media, operation and maintenance, and disposal. The system presented herein should be less expensive because no ozonization and no aeration are needed.
The mass of iron needed for each Fe0/sand unit can be estimated using the data of Westerhoff and James [124]. Here, 2271 g of the densest Fe0 material fill 600 mL with a porosity of 40%. Assuming the same porosity, the volume corresponding to L = 70 cm reactive zone can be calculated using Equation (1):
V = L·S = π*L*D2/4
where S = π*D2/4 is the cross section of the column with a diameter D = 7.5 cm. The calculations yield a volume of 3094 cm3 or 3094 mL. The corresponding Fe0 mass is given by the rule of proportion like Equation (2):
m = 3094 × 2271/600
The calculations yield a mass of 11,710 g or 11.71 kg of Fe0 for a 100% Fe0 unit. For a 1:1 Fe0 to sand mixture, just 5.86 or about 6 kg of Fe0 is need. If it is further supposed that 4 Fe0/sand units will be needed and the system will operate for one year, then a 100-inhabitant-community may need just 24 kg Fe0 for one year.
In addition, having good water and the know-how to produce more at low cost or low money expense, small communities have the possibility to commercialize excess water. That is some 250 L per day for the model community of this study can be bottled and offered to consumers. In other words, Fe0/sand filters are not only affordable for communities in need; they are also a potential source of income. Business models for commercialization of treated water in the developing world have already been presented [7].
5.7. Implementation of Fe0-Based Water Treatment Plants
There are important aspects of the implementation of Fe0 filters that should be pointed out before sending a construction crew to the field. The maintenance of the Fe0 filters is not addressed herein as it will be considered during pilot testing under real life conditions. In recent years, there have been numerous pilot plant testing for water filters, partly involving Fe0 [24,26,27,52,124,184,193,194,195]. Construction materials (ceramic, concrete, metallic, plastic) are numerous and include: (i) plastic tanks (e.g., polyetheylene); (ii) modified shipping container; (iii) ferro-cement tanks and (iv) polyvinylchlorid (PVC) tubing. Fe0 materials can be commercially obtained. In general, manufacturing of Fe0 filters is comparable to other existing filters, making it a priori a feasible technology. This section enumerates the materials used by Kearns [195] and adaptable for Fe0 filters.
5.7.1. Siting
Water is ideally moved by gravity. The water system is sited at lower elevation than the source water and at higher elevation than the location(s) where treated water will be used. This circumstance enables completely passive operation of the treatment system and very simple control using only a float valve. When water is withdrawn from the storage tank (Figure 4a) the water level in the system drops, opening the float valve. When the system is full, the float valve closes.
5.7.2. Containment
The Fe0-based water treatment system presented herein is an open architecture which could be constructed, modified, adapted, and improved on a site-specific basis. Filters containers can be locally built, for example from stackable prefabricated concrete rings commonly used for tank construction. Commercially available plastic tanks can be also modified and used. Appropriately skilled masons can construct custom ferro-cement tanks. In this case the dimensions should enable facile filling of filter media and routine maintenance including the removal/replacement of all materials.
Some cover material (lid) should be used to exclude sunlight and inhibit the growth of photosynthetic microorganisms (algae, cyanobacteria) in the system. Tank tops should be wrapped in fine mesh screening to prevent entrance of insects, bird droppings, leaves, and bits of debris, etc. into the system.
5.7.3. Plumbing
PVC pipe is ubiquitous and cheap in most locations. The most suitable diameter for most connections to and from the water system and between the tanks should be used. Plumbing in the bottom of filter tanks should be protected from physical damage and blockage by under drains made from rock and coarse gravel at least 20 cm in depth.
5.7.4. Filter Materials
Standard gravel (1.0–4.0 cm) should be used for the roughing filter. Standard fine sand (0.15–0.35 cm) should be used for the sand filter [194]. The sand to be used in the reactive zone (Fe0/sand) should be coarser (e.g., 0.5–2.0 cm) and of comparable particle size. Fe0 should be abundantly available and selected for its appropriate reactivity.
5.7.5. Implementation Plan
Whichever materials are tested at pilot scale, there is always the possibility to find cheaper options. A pilot plan should be able to be implemented in a single phase. Firstly the tanks, piping materials and other equipment listed need to be sourced, bought and transported to the pilot site. The transport can be made by any common trailer truck. Once at the site, it will be relatively simple to build, operate, and monitor the Fe0-based plant for at least six months.
6. Concluding Remarks
The need for a scientific-based approach to design and evaluate the efficiency of Fe0 filters is corroborated in this study. The urgency of such a consensual approach is evident as it has been established herein that the required knowledge (science of aqueous iron corrosion) is available, but has not been considered in the right way. All is needed is a systematic holistic approach enabling the characterization of aqueous iron corrosion as influenced by the water chemistry, including the pH value, the presence of contaminants and co-solutes. The present study intends to create/initiate a synergy among researchers who are working for more efficient Fe0 filtration systems for decentralized water treatment. The adoption or at least the general consideration of fundamental aspects presented herein would accelerate the understanding of the operating mode of Fe0 filters and thus, outline the strengths and limitations of this still innovative, but potentially highly efficient technology.
This study specifically restricted its attention to aspects relevant for filter design and the evaluation of their performance in long-term experiments, in particular at pilot scale. The specific objectives of the paper were to make recommendations based on the current state of the science concerning (i) aqueous iron corrosion and (ii) contaminant removal by iron oxides/hydroxides in the environment. The achievement can be summarized by a number of questions, including the following: (i) Which Fe0 materials are suitable for water treatment (intrinsic reactivity, porosity, size)? (ii) What level of dissolved O2 is needed for sustainable systems? (iii) How can one warrant the required level of dissolved O2? (iv) What is the general filter design (depth of filter, layering arrangement with particles of different sizes, compaction of the media during construction)? (v) Is it feasible to classify chemicals according to their affinities to the Fe0/H2O system? (vi) What types of data are needed for the evaluation of chemicals (molecular size, molecular weight, solubility)? (vii) How does one proceed when data for critical inputs are missing (molecular size)? (viii) Are transformation half-lives of any realistic significance in assessing the relative removal efficiency of species in Fe0/H2O system? (ix) Which other treatment units, beside sand filters (e.g., activated carbons) are necessary to optimize the efficiency of the system (on a site specific basis)? (x) What is the optimal frequency of filtration events (intermittent filters).
If future experiments are performed with these ten questions in mind, it will soon be possible to establish the science of “Fe0 for environmental remediation” based on the science of aqueous iron corrosion and knowledge on the interactions of iron oxides and hydroxides with aqueous species.
Acknowledgments
Thanks are due to Sabine Caré (Université ParisEst/France); Emmanuel Chimi (Université Douala/Cameroon); Mohammad A. Rahman (Leibniz Universität Hannover/Germany) and Paul Woafo (Université Yaoundé 1/Cameroon) and for fruitful collaboration on investigating the evolution of the porosity of iron filtration systems. Gerhard Max Hundertmark is acknowledged for technical support. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Author Contributions
The text of this article was written by Chicgoua Noubactep and Richard Crane with contributions from Raoul Tepong-Tsindé, Achille Nassi and Hans Ruppert. Raoul Tepong-Tsindé conducted background research on the design of a pilot plan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Gujer, W.; Pronk, W. Intermittent operation of ultra-low pressure ultrafiltration for decentralized drinking water treatment. Water Res. 2012, 46, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, S. Improving the sustainability of water treatment systems: Opportunities for innovation. Solutions 2010, 1, 42–49. [Google Scholar]
- Tellen, V.; Nkeng, G.; Dentel, S. Improved filtration technology for pathogen reduction in rural water supplies. Water 2010, 2, 285–306. [Google Scholar] [CrossRef]
- Grady, C.; Younos, T. Bottled water technology and its global ramifications: An overview. Int. Water Technol. J. 2012, 2, 185–194. [Google Scholar]
- Sima, L.C.; Elimelech, M. More than a drop in the bucket: Decentralized membrane-based drinking water refill stations in Southeast Asia. Environ. Sci. Technol. 2013, 47, 7580–7588. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Zhang, B.; Li, F.; Toure, B.; Omosa, I.B.; Chiramba, T.; Abdel-Monem, M.; Pradhan, M. Water and wastewater treatment in Africa—Current practices and challenges. Clean Soil Air Water 2014, 42, 1029–1035. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Obi, C.L.; Thompson, P. Survey of disinfection efficiency of small drinking water treatment plants: Challenges facing small water treatment plants in South Africa. Water SA 2009, 35, 485–494. [Google Scholar]
- Frechen, F.-B.; Exler, H.; Romaker, J.; Schier, W. Long-term behaviour of a gravity-driven dead end membrane filtration unit for potable water supply in cases of disasters. Water Sci. Technol. Water Supply 2011, 11, 39–44. [Google Scholar] [CrossRef]
- Ali, I. Water treatment by adsorption columns: Evaluation at ground level. Sep. Purif. Rev. 2014, 43, 175–205. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water worldwide. Chem. Eng. J. 2010, 165, 740–749. [Google Scholar] [CrossRef]
- Chiu, P.C. Applications of zero-valent iron (ZVI) and nanoscale ZVI to municipal and decentralized drinking water systems—A review. In Novel Solutions to Water Pollution; Ahuja, S., Hristovski, K., Eds.; ACS Symposium Series; Volume 1123, American Chemical Society: Washington, DC, USA, 2013; pp. 237–249. [Google Scholar]
- Schäfer, A.I.; Broeckmann, A.; Richards, B.S. Renewable energy powered mem-brane technology. 1. Development and characterization of a photovoltaic hybrid membrane system. Environ. Sci. Technol. 2007, 41, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.I.; Hughes, G.; Richards, B.S. Renewable energy powered membrane technology: A leapfrog approach to rural water treatment in developing countries? Renew. Sustain. Energy Rev. 2014, 40, 542–556. [Google Scholar] [CrossRef]
- Johnson, D.M.; Hokanson, D.R.; Zhang, Q.; Czupinski, K.D.; Tang, J. Feasibility of water purification technology in rural areas of developing countries. J. Environ. Manag. 2008, 88, 416–427. [Google Scholar] [CrossRef]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Gottinger, A.M.; McMartin, D.W.; Wild, D.J.; Moldovan, B. Integration of zero valent iron sand beds into biological treatment systems for uranium removal from drinking water wells in rural Canada. Can. J. Civ. Eng. 2013, 40, 945–950. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.-B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Kowalski, K. New Method for Arsenic Compounds Elimination from Naturally Contaminated Drinking Water Systems. Ph.D. Thesis, Aalborg Universitet, Esbjerg, Denmark, January 2014. [Google Scholar]
- Kowalski, K.P.; Søgaard, E.G. Implementation of zero-valent iron (ZVI) into drinking water supply—Role of the ZVI and biological processes. Chemosphere 2014, 117, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Schöner, A. Metallic iron for environmental remediation: Learning from electrocoagulation. J. Hazard. Mater. 2010, 175, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Bojic, A.; Purenovic, M.; Kocic, B.; Perovic, J.; Ursic-Jankovic, J.; Bojic, D. The inactivation of Escherichia coli by microalloyed aluminium based composite. Facta Univ. 2001, 2, 115–124. [Google Scholar]
- Bojic, A.; Purenovic, M.; Bojic, D. Removal of chromium(VI) from water by micro-alloyed aluminium based composite in flow conditions. Water SA 2004, 30, 353–359. [Google Scholar] [CrossRef]
- Bojic, A.L.; Purenovic, M.; Bojic, D.; Andjelkovic, T. Dehalogenation of trihalomethanes by a micro-alloyed aluminium composite under flow conditions. Water SA 2007, 33, 297–304. [Google Scholar]
- Bojic, A.L.; Bojic, D.; Andjelkovic, T. Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction–coagulation process in flow conditions. J. Hazard. Mater. 2009, 168, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Lackovic, J.A.; Nikolaidis, N.P.; Dobbs, G.M. Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 2000, 17, 29–39. [Google Scholar] [CrossRef]
- Scherer, M.M.; Richter, S.; Valentine, R.L.; Alvarez, P.J.J. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Rev. Environ. Sci. Technol. 2000, 30, 363–411. [Google Scholar] [CrossRef]
- Mantha, R.; Taylor, K.E.; Biswas, N.; Bewtra, J.K. A continuous system for Fe0 reduction of nitrobenzene in synthetic wastewater. Environ. Sci. Technol. 2001, 35, 3231–3236. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. On the mechanism microbe inactivation by metallic iron. J. Hazard. Mater. 2011, 198, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wie, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Cheng, C.; Liu, P.; Shi, L.; Ma, Z.; Zheng, X. Removal of bacteriophage f2 in water by nanoscale zero-valent iron and parameters optimization using response surface methodology. Chem. Eng. J. 2014, 252, 150–158. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Yang, M.; Hashimoto, T.; Hoshi, N.; Myoga, H. Fluoride removal in a fixed bed packed with granular calcite. Water Res. 1999, 33, 3395–3402. [Google Scholar] [CrossRef]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef] [PubMed]
- García, M.G.; Borgnino, L.; Bia, G.; Depetris, P.J. Mechanisms of arsenic and fluoride release from Chacopampean sediments (Argentina). Int. J. Environ. Health 2014, 7, 41–57. [Google Scholar] [CrossRef]
- Trikha, R.; Sharma, B.K. Studies on factors affecting fluoride removal from water using passive system. J. Environ. Chem. Eng. 2014, 2, 172–176. [Google Scholar] [CrossRef]
- Omenka, E. Improvement of Decentralised Wastewater Treatment in Asaba, Nigeria. Master’s Thesis, Lund University, Lund, Sweden, July 2010. [Google Scholar]
- Younos, T. Paradigm shift: Holistic approach for water management in urban environments. Front. Earth Sci. 2011, 5, 421–427. [Google Scholar]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Noubactep, C. Affordable safe drinking water for victims of natural disasters. In Natural Disasters and Sustainable Development, Proceedings of the International Seminar Held in Göttingen, Germany, 17–19 April 2013; Kätsch, C., Meliczek, H., Eds.; Cuvillier Verlag: Göttingen, Germany, 2014; pp. 57–75. [Google Scholar]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for groundwater of Bangladesh. J. Environ. Sci. Health A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- Antia, D.D.J. Modification of aquifer pore-water by static diffusion using nano-zero-valent metals. Water 2011, 3, 79–112. [Google Scholar] [CrossRef]
- Gottinger, A.M.; Wild, D.J.; McMartin, D.; Moldovan, B.; Wang, D. Development of an iron-amended biofilter for removal of arsenic from rural Canadian prairie potable water. In Water Pollution X; Marinov, A.M., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2010; pp. 333–344. [Google Scholar]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Trois, C.; Cibati, A. South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers: Column tests. Chem. Eng. J. 2015, 259, 981–989. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J. Iron-mediated reductive transformations: Investigation of reaction mechanism. Environ. Sci. Technol. 1996, 30, 716–719. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Murugan, S.; Paulpandian, P. Synergistic antibacterial evaluation of commercial antibiotics combined with nanoiron against human pathogens. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 183–190. [Google Scholar]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Comba, S.; Di Molfetta, A.; Sethi, R. A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 2011, 215, 595–607. [Google Scholar] [CrossRef]
- Crane, R.A.; Dickinson, M.; Popescu, I.C.; Scott, T.B. Magnetite and zero-valent iron nanoparticles for the remediation of uranium contaminated environmental water. Water Res. 2011, 45, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Scott, T.B.; Popescu, I.C.; Crane, R.A.; Noubactep, C. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J. Hazard. Mater. 2011, 186, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J. Laboratory evaluation of porous iron composite for agricultural drainage water filter treatment. Trans. ASABE 2012, 55, 1683–1697. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory evaluation of zero valent iron and sulfur-modified iron for agricultural drainage water treatment. Ground Water Monit. Remediat. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Crane, R.; Noubactep, C. Elemental metals for environmental remediation: Lessons from hydrometallurgy. Fresenius Environ. Bull. 2012, 21, 1192–1196. [Google Scholar]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Crane, R.A. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kawase, Y. Kinetic modeling and simulation of zero-valent iron wastewater treatment process: Simultaneous reduction of nitrate, hydrogen peroxide, and phosphate in semiconductor acidic wastewater. Ind. Eng. Chem. Res. 2013, 52, 17829–17840. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. The removal of uranium onto nanoscale zero-valent iron particles in anoxic batch systems. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N. Effect of reduced iron on the degradation of chlorinated hydrocarbons in contaminated soil and ground water: A review of publications. Eurasian Soil Sci. 2014, 47, 119–133. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Artificial permeable redox barriers for purification of soil and ground water: A review of publications. Eurasian Soil Sci. 2014, 47, 1058–1068. [Google Scholar] [CrossRef]
- Crane, R.A.; Dickinson, M.; Scott, T.B. Nanoscale zero-valent iron particles for the remediation of plutonium and uranium contaminated solutions. Chem. Eng. J. 2015, 262, 319–325. [Google Scholar] [CrossRef]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; CRC Press: Taylor & Francis Group, London, UK, 2015; p. 333. ISBN ISBN 9781482224474. [Google Scholar]
- Ghauch, A.; Abou Assi, H.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Abou Assi, H.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-Based Metallic Systems: An Excellent Choice for Sustainable Water Treatment. Ph.D. Thesis, University of Grenoble, Grenoble, France, November 2013. [Google Scholar]
- Lai, K.C.K.; Lo, I.M.C.; Birkelund, V.; Kjeldsen, P. Field monitoring of a permeable reactive barrier for removal of chlorinated organics. J. Environ. Eng. 2006, 132, 199–210. [Google Scholar] [CrossRef]
- Jia, Y.; Aagaard, P.; Breedveld, G.D. Sorption of triazoles to soil and iron minerals. Chemosphere 2007, 67, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Characterizing the discoloration of methylene blue in Fe0/H2O systems. J. Hazard. Mater. 2009, 166, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Nanoscale iron particles for environmental remediation: An overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Korte, N.E.; Zutman, J.L.; Schlosser, R.M.; Liang, L.; Gu, B.; Fernando, Q. Field application of palladized iron for the dechlorination of trichloroethene. Waste Manag. 2000, 20, 687–694. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tang, C.L.; Zeng, H. Removing molybdate from water using a hybridized zero-valent iron/magnetite/Fe(II) treatment system. Chem. Eng. J. 2012, 200, 205–263. [Google Scholar]
- Huang, Y.H.; Peddi, P.K.; Zeng, H.; Tang, C.L.; Teng, X.J. Pilot-scale demonstration of the hybrid zero-valent iron process for treating flue-gas-desulfurization wastewater: Part I. Water Sci. Technol. 2013, 67, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Peddi, P.K.; Zeng, H.; Tang, C.L.; Teng, X.J. Pilot-scale demonstration of the hybrid zero-valent iron process for treating flue-gas-desulfurization wastewater: Part II. Water Sci. Technol. 2013, 67, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.A.; Roberts, A.L. Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn(0). Environ. Sci. Technol. 1998, 32, 3017–3025. [Google Scholar] [CrossRef]
- Arnold, W.A.; Ball, W.P.; Roberts, A.L. Polychlorinated ethane reaction with zero-valent zinc: Pathways and rate control. J. Contam. Hydrol. 1999, 40, 183–200. [Google Scholar] [CrossRef]
- Suresh, S. Reductive remediation of pollutants using metals. Open Waste Manag. J. 2009, 2, 6–16. [Google Scholar] [CrossRef]
- Salter-Blanc, A.J.; Tratnyek, P.G. Effects of solution chemistry on the dechlorination of 1,2,3-trichloropropane by zero-valent zinc. Environ. Sci. Technol. 2011, 45, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Han, V.; Chen, Z.; Tong, L.N.; Yang, L.; Shen, J.M.; Wang, B.Y.; Liu, Y.; Liu, Y.; Chen, Q. Reduction of N-Nitrosodimethylamine with zero-valent zinc. Water Res. 2013, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.E.; Gray, H.B.; Haight, G.P., Jr. Chemical Principles, 3rd ed.; Benjamin/Cummings Inc.: San Francisco, CA, USA, 1979; p. 944. [Google Scholar]
- Noubactep, C. The suitability of metallic iron for environmental remediation. Environ. Progr. Sust. En. 2010, 29, 286–291. [Google Scholar] [CrossRef]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu-K., B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Gillham, R.W. Discussion of Papers/Discussion of nano-scale iron for dehalogenation. Ground Water Monit. Remediat. 2003, 23, 6–8. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabro, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Sato, N. 1989 Whitney award lecture: Toward a more fundamental understanding of corrosion processes. Corrosion 1989, 45, 354–368. [Google Scholar] [CrossRef]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Sato, N. Surface oxides affecting metallic corrosion. Corros. Rev. 2001, 19, 253–272. [Google Scholar]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Dickinson, M.; Scott, T.B.; Crane, R.A.; Riba, O.; Barnes, R.J.; Hughes, G.M. The effects of vacuum annealing on the structure and surface chemistry of iron: nickel alloy nanoparticles. J. Nanoparticle Res. 2010, 12, 2081–2092. [Google Scholar] [CrossRef]
- Scott, T.B.; Dickinson, M.; Crane, R.A.; Riba, O.; Hughes, G.M.; Allen, G.C. The effects of vacuum annealing on the structure and surface chemistry of iron nanoparticles. J. Nanoparticle Res. 2010, 12, 1765–1775. [Google Scholar] [CrossRef]
- Caule, E.J.; Cohen, M. An electron-micrograph study of oxide films on electropolished surfaces of iron. Can. J. Chem. 1953, 31, 237–241. [Google Scholar] [CrossRef]
- Cohen, M. The formation and properties of passive films on iron. Can. J. Chem. 1959, 37, 286–291. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior. J. Electrochem. Soc. 2000, 147, 4087–4092. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 2011, 196, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0-H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for water treatment: A critical review. Clean Soil Air Water 2013, 41, 702–710. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Sauter, M. Significance of oxide-film in discussing the mechanism of contaminant removal by elemental iron materials. In Photo-Electrochemistry & Photo-Biology for the Sustainablity; Kaneco, S., Viswanathan, B., Katsumata, H., Eds.; Union Press: Osaka, Japan, 2012; pp. 97–122. [Google Scholar]
- Noubactep, C. Processes of contaminant removal in “Fe0-H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. An analysis of the evolution of reactive species in Fe0/H2O systems. J. Hazard. Mater. 2009, 168, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 2010, 36, 663–670. [Google Scholar] [CrossRef]
- Noubactep, C. Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 2011, 37, 419–426. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, L. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water production. Freib. Online Geosci. 2011, 27, 38. [Google Scholar]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Noubactep, C. Flaws in the design of Fe(0)-based filtration systems? Chemosphere 2014, 117, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Designing metallic iron packed-beds for water treatment: A critical review. Clean Soil Air Water 2015. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, A.S.; Ünal, N.; Jekel, M. Evaluation of two-component Fe(0) fixed bed filters with porous materials for reductive dechlorination. Chem. Eng. J. 2012, 209, 401–406. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Martin, J. Impacts of Fe(0) grain sizes and grain size distributions in permeable reactive barriers. Chem. Eng. J. 2012, 213, 245–250. [Google Scholar] [CrossRef]
- Bi, E.; Devlin, J.F.; Huang, B. Effects of mixing granular iron with sand on the kinetics of trichloroethylene reduction. Ground Water Monit. Remediat. 2009, 29, 56–62. [Google Scholar] [CrossRef]
- Ulsamer, S. A Model to Characterize the Kinetics of Dechlorination of Tetrachloroethylene and Trichloroethylene by a Zero Valent Iron Permeable Reactive Barrier. Master’s Thesis, Worcester Polytechnic Institute, Worcester, UK, 2011; p. 73. [Google Scholar]
- Firdous, R.; Devlin, J.F. Consideration of grain packing in granular iron treatability studies. J. Contam. Hydrol. 2014, 164, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S. Dimensioning metallic iron beds for efficient contaminant removal. Chem. Eng. J. 2010, 163, 454–460. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Designing laboratory metallic iron columns for better result comparability. J. Hazard. Mater. 2011, 189, 809–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Btatkeu-K., B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 60. [Google Scholar]
- Phukan, M. Characterizing the Fe0/Sand System by the Extent of Dye Discoloration. Master’s Thesis, University of Göttingen, Göttingen, Germany, January 2015. [Google Scholar]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics: Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China; Chen, Y., Tang, X., Zhan, L., Eds.; Springer: Berlin, Germany, 2010; pp. 5–15. [Google Scholar]
- Phillips, D.H.; van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468–469, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Mushovic, P.S.; Niesen, P.L. Early breakthrough of molybdenum and uranium in a permeable reactive barrier. Environ. Sci. Technol. 2006, 40, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.L.; Gillham, R.W.; Reardon, E.J.; Fagan, R.; Focht, R.M.; Vogan, J.L. An examination of zero-valent iron sources used in permeable reactive barriers. In Proceedings of the 3rd International Containment Technology Conference, Tallahassee, Orlando, FL, USA, 10–13 June 2001; Florida State University; p. 5.
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Scherer, M.M.; Tratnyek, P.G. Kinetics of halogenated organic compound degradation by iron metal. Environ. Sci. Technol. 1996, 30, 2634–2640. [Google Scholar] [CrossRef]
- McGeough, K.L.; Kalin, R.M.; Myles, P. Carbon disulfide removal by zero valent iron. Environ. Sci. Technol. 2007, 41, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.-M.; Habibian, M.T.; O’melia, C.R. Water and waste water filtration: Concepts and applications. Environ. Sci. Technol. 1971, 5, 1105–1112. [Google Scholar] [CrossRef]
- Bedrikovetsky, P.; Siqueira, F.D.; Furtado, C.A.; Souza, A.L.S. Modified particle detachment model for colloidal transport in porous media. Transp. Porous Med. 2011, 86, 353–383. [Google Scholar] [CrossRef]
- You, Z.; Osipov, Y.; Bedrikovetsky, P.; Kuzmina, L. Asymptotic model for deep bed filtration. Chem. Eng. J. 2014, 258, 374–385. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in ground water: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Merkel, J.B. Investigating the mechanism of uranium removal by zerovalent iron materials. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium (VI) fixation by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, N.P.; Dobbs, G.M.; Lackovic, J.A. Arsenic removal by zero-valent iron: field, laboratory and modeling studies. Water Res. 2003, 37, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.; Wang, J.; O’Day, P.; Conklin, M. Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ. Sci. Technol. 2001, 35, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Burris, D.R.; Delcomyn, C.A.; Smith, M.H.; Roberts, A.L. Reductive dechlorination of tetrachloroethylene catalyzed by vitamin B12 in homogeneous and heterogeneous systems. Environ. Sci. Technol. 1996, 30, 3047–3052. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kim, J.-W.; Watkins, J.; Wilkin, R.T. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ. Sci. Technol. 2002, 36, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for water treatment. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P. The application of rapid small-scale column tests in iron-based packed bed arsenic treatment systems. In Advances in Arsenic Research, ACS Symp. Ser.; Oxford University Press: Oxford, UK, 2005; Volume 915, pp. 268–283. [Google Scholar]
- Gu, B.; Phelps, T.J.; Liang, L.; Dickey, M.J.; Roh, Y.; Kinsall, B.L.; Palumbo, A.V.; Jacobs, G.K. Biogeochemical dynamics in zero-valent iron columns: Implications for permeable reactive barriers. Environ. Sci. Technol. 1999, 33, 2170–2177. [Google Scholar] [CrossRef]
- Farrell, J.; Kason, M.; Melitas, N.; Li, T. Investigation of the long-term performance of zero-valent iron for reductive dechlorination of trichloroethylene. Environ. Sci. Technol. 2000, 34, 514–521. [Google Scholar] [CrossRef]
- Melitas, N.; Wang, J.P.; Conklin, M.; O’Day, P.; Farrell, J. Understanding soluble arsenate removal kinetics by zerovalent iron media. Environ. Sci. Technol. 2002, 36, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. Are accelerated column tests used in permeable reactive barriers design sufficiently reliable? In Proceedings of the 3rd International Conference “Hazardous and Industrial Waste Management”, Create, Greece, 12–14 September 2012.
- Moraci, N.; Bilardi, S.; Calabrò, P. Critical aspects related to Fe0 and Fe0/pumice PRB design. Environ. Geotech. 2014. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Matis, K.A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials 2013, 6, 5131–5158. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Review on dye removal from its aqueous solution into alternative cost effective and non-conventional adsorbents. J. Chem. Proc. Eng. 2014, 1, 1–11. [Google Scholar]
- Chapman, A.C.; Siebold, A. On the application of adsorption to the detection and separation of certain dyes. Analyst 1912, 37, 339–345. [Google Scholar] [CrossRef]
- Ewing, W.W.; Liu, F.W.J. Adsorption of dyes from aqueous solutions on pigments. J. Colloid Sci. 1953, 8, 204–213. [Google Scholar] [CrossRef]
- Whetstone, J. The adsorption of dyes by crystals. Discuss. Faraday Soc. 1954, 16, 132–140. [Google Scholar] [CrossRef]
- Haldeman, R.G.; Emmett, P.H. Specific adsorption of alkyl orange dyes on silica gel. J. Phys. Chem. 1955, 59, 1039–1043. [Google Scholar] [CrossRef]
- Mitchell, G.; Poole, P.; Segrove, H.D. Adsorption of methylene blue by high-silica sands. Nature 1955, 176, 1025–1026. [Google Scholar] [CrossRef]
- Prasad, R.; Dey, A.K. Adsorption of dyestuffs by hydrous thorium oxide: Heat of adsorption of the dyes by various samples of the hydroxide. Kolloid Z. Z. Polym. 1962, 183, 153–155. [Google Scholar] [CrossRef]
- Brooks, C.S. Mechanism of methylene blue dye adsorption on siliceous minerals. Kolloid Z. Z. Polym. 1964, 199, 31–36. [Google Scholar] [CrossRef]
- Padday, J.F. Adsorption of cyanine dyes at silver-halide surfaces. Trans. Faraday Soc. 1964, 60, 1325–1334. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Luft, C.P.; Hand, D.W. Prediction of multicomponent adsorption equilibria in background mixtures of unknown composition. Water Res. 1985, 19, 1537–1548. [Google Scholar] [CrossRef]
- Worch, E. Fixed-bed adsorption in drinking water treatment: A critical review on models and parameter estimation. J. Water Supply Res. Technol. AQUA 2008, 57, 171–183. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, J.; Pan, B. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univers. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Lan, V.M.; Trang, P.T.K.; Viet, P.H.; Berg, M.; Voegelin, A.; Planer-Friedrich, B.; Zahoransky, J.; Müller, S.-K.; Byrne, J.M.; et al. Arsenic removal from drinking water by a household sand filter in Vietnam—Effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci. Total Environ. 2015, 502, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura Mbenguela, B. Water treatment with Fe0/H2O systems: Learning from internal electrolysis. Fresenius Environ. Bull. 2014, 23, 2663–2669. [Google Scholar]
- Burghardt, D.; Kassahun, A. Development of a reactive zone technology for simultaneous in situ immobilisation of radium and uranium. Environ. Geol. 2005, 49, 314–320. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–740. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J. Sustainable decentralized water treatment for rural and developing communities using locally generated biochar adsorbents. Water Cond. Purif. Int. 2012, 54, 7–12. [Google Scholar]
- Tillman, D.E. Combination of Zero-Valent Iron and Granular Activated for the Treatment of Groundwater Contaminated with Chlorinated Solvents. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 1996. [Google Scholar]
- Indelicato, B.M. Comparision of Zero-Valent Iron and Activated Carbon for Treating Chlorinated Contaminants in Groundwater. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 1998. [Google Scholar]
- Bayer, P.; Finkel, M. Modelling of sequential groundwater treatment with zero valent iron and granular activated carbon. J. Contam. Hydrol. 2005, 78, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Van Nooten, T.; Diels, L.; Bastiaens, L. Design of a multifunctional permeable reactive barrier for the treatment of landfill leachate contamination: Laboratory column evaluation. Environ. Sci. Technol. 2008, 42, 8890–8895. [Google Scholar] [CrossRef] [PubMed]
- Doula, M.K. Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Res. 2009, 43, 3659–3672. [Google Scholar] [CrossRef] [PubMed]
- Westholm, L.J.; Repo, E.; Sillanpää, M. Filter materials for metal removal from mine drainage—A review. Environ. Sci. Pollut. Res. 2014, 21, 9109–9128. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge: V. Update. J. Colloid Interf. Sci. 2011, 353, 1–15. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Gottinger, A.M. Chemical-Free Arsenic Removal from Potable Water with a ZVI-Amended Biofilter. Master’s Thesis, University of Regina, Saskatchewan, Canada, January 2010; p. 90. [Google Scholar]
- Clark, P.A.; Pinedo, C.A.; Fadus, M.; Capuzzi, S. Slow-sand water filter: Design, implementation, accessibility and sustainability in developing countries. Med. Sci. Monit. 2012, 18, RA105–RA117. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J. Sustainable Decentralized Water Treatment for Rural and Developing Communities Using Gasifier Biochar Version 1.0, March 2012. Available online: http://www.aqsolutions.org/ (accessed on 30 January 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).