Mixing Precipitation of CaCO3 in Natural Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Sample No. | pH | t °C | Cl− mg/L | SO42− mg/L | CO32− mg/L | HCO3− mg/L | Na+ mg/L | K+ mg/L | Ca2+ mg/L | Mg2+ mg/L | SIc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.65 | 35.0 | 4.20 | 431.00 | 0 | 392.00 | 6.90 | 26.20 | 232.00 | 47.30 | 0.927 |
| 2 | 6.50 | 35.5 | 532.00 | 441.00 | 0 | 1870.00 | 980.00 | 98.00 | 33.20 | 6.58 | 0.313 |
| 3 | 6.80 | 58.0 | 37.60 | 39.90 | 0 | 1963.60 | 717.50 | 62.60 | 14.00 | 1.80 | 0.779 |
| 4 | 7.00 | 51.5 | 328.00 | 192.00 | 0 | 2230.00 | 970.00 | 73.00 | 50.60 | 22.90 | 3.723 |
| 5 | 7.00 | 27.0 | 2.90 | 327.00 | 0 | 359.00 | 6.10 | 19.70 | 204.00 | 36.20 | 1.599 |
| 6 | 7.25 | 25.0 | 3.40 | 279.00 | 0 | 306.00 | 4.40 | 13.70 | 164.00 | 27.90 | 2.073 |
| 7 | 6.71 | 35.0 | 4.20 | 431.00 | 0 | 393. 00 | 6.80 | 26.30 | 232.00 | 47.20 | 1.106 |
| 8 | 7.26 | 25.0 | 3.40 | 284.00 | 0 | 306.00 | 4.40 | 14.40 | 167.00 | 28.40 | 2.155 |
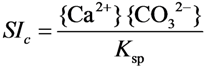
2.2. Methods
| Major components | Derived species | |||
|---|---|---|---|---|
| No. | Components | No. | Species | Chemical reactions |
| 1 | Cl- | 1 | NaCl | 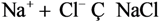 |
| 2 | SO42- | 2 | KCl |  |
| 3 | CO32- | 3 | H2SO4 | 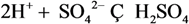 |
| 4 | Na+ | 4 | HSO4− | 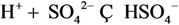 |
| 5 | K+ | 5 | NaSO4− |  |
| 6 | Ca2+ | 6 | KSO4− | 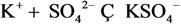 |
| 7 | Mg2+ | 7 | CaSO4 | 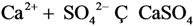 |
| 8 | H+ | 8 | MgSO4 |  |
| 9 | OH- | 9 | H2CO3 | 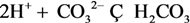 |
| 10 | HCO3− |  | ||
| 11 | CaCO3 |  | ||
| 12 | MgCO3 |  | ||
| 13 | CaHCO3+ | 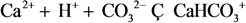 | ||
| 14 | MgHCO3+ | 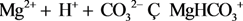 | ||
| 15 | CaOH+ | 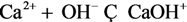 | ||
| 16 | MgOH+ |  | ||
| 17 | H2O |  | ||
3. Results and Discussion
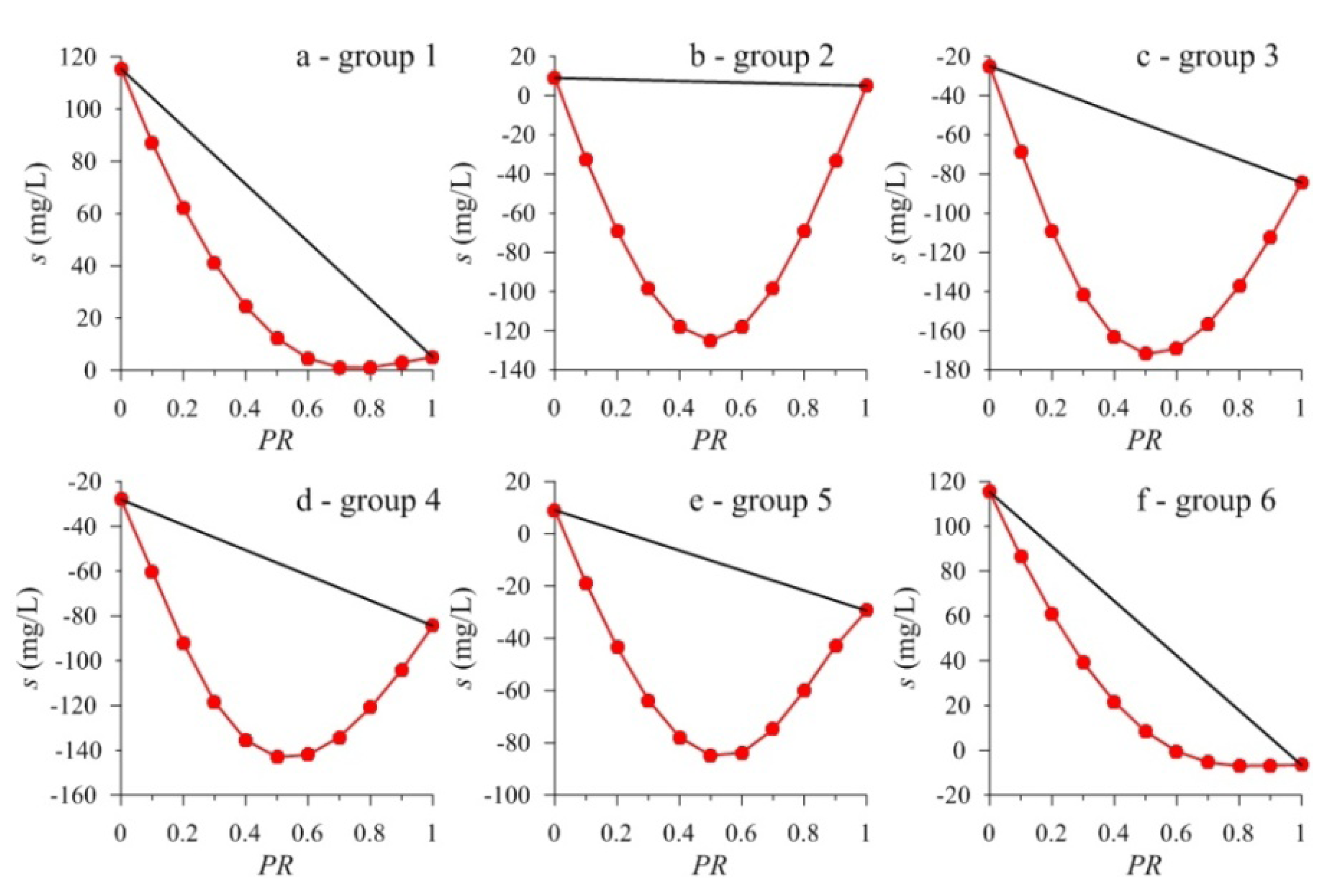
3.1. Results
3.2. Discussion
3.2.1. Mixing Precipitation between Two Unsaturated Water Samples
| Group | Item | Mixing ratios | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | ||
| 1 | pH0 | 6.50 | 6.50 | 6.51 | 6.51 | 6.52 | 6.53 | 6.54 | 6.55 | 6.57 | 6.60 | 6.65 |
| SIc | 0.31 | 0.48 | 0.62 | 0.74 | 0.84 | 0.91 | 0.97 | 0.99 | 0.99 | 0.97 | 0.93 | |
| s1 | 115.36 | 86.99 | 62.06 | 41.06 | 24.39 | 12.26 | 4.61 | 1.14 | 1.04 | 3.00 | 5.02 | |
| s2 | 115.36 | 104.33 | 93.29 | 82.26 | 71.22 | 60.19 | 49.16 | 38.12 | 27.09 | 16.05 | 5.02 | |
| Δs | 0.00 | −17.34 | −31.23 | −41.20 | −46.83 | −47.93 | −44.55 | −36.98 | −26.05 | −13.05 | 0.00 | |
| pHe | 6.59 | 6.58 | 6.57 | 6.56 | 6.55 | 6.54 | 6.54 | 6.55 | 6.57 | 6.61 | 6.67 | |
| 2 | pH0 | 6.80 | 6.79 | 6.79 | 6.78 | 6.78 | 6.77 | 6.76 | 6.75 | 6.73 | 6.70 | 6.65 |
| SIc | 0.78 | 1.72 | 2.35 | 2.74 | 2.90 | 2.87 | 2.69 | 2.38 | 1.96 | 1.47 | 0.93 | |
| s 1 | 9.01 | −32.72 | −69.33 | −98.65 | −118.01 | −124.96 | −118.28 | −98.72 | −69.07 | −33.04 | 5.02 | |
| s 2 | 9.01 | 8.61 | 8.21 | 7.81 | 7.41 | 7.02 | 6.62 | 6.22 | 5.82 | 5.42 | 5.02 | |
| Δ s | 0.00 | −41.33 | −77.54 | −106.46 | −125.42 | −131.98 | −124.90 | −104.94 | −74.89 | −38.46 | 0.00 | |
| pHe | 6.81 | 6.76 | 6.72 | 6.67 | 6.63 | 6.59 | 6.57 | 6.56 | 6.57 | 6.60 | 6.67 | |
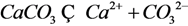

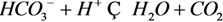
3.2.2. Mixing Precipitation between Two Oversaturated Water Samples
| Group | Item | Mixing ratios | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | ||
| 3 | pH0 | 7.00 | 7.02 | 7.02 | 7.02 | 7.02 | 7.02 | 7.01 | 7.01 | 7.01 | 7.00 | 7.00 |
| SIc | 1.60 | 2.47 | 3.22 | 3.83 | 4.32 | 4.65 | 4.83 | 4.85 | 4.68 | 4.31 | 3.72 | |
| s1 | −24.97 | −68.42 | −109.24 | −141.95 | −163.23 | −171.96 | −169.14 | −156.73 | −137.22 | −112.62 | −84.41 | |
| s2 | −24.97 | −30.91 | −36.86 | −42.80 | −48.75 | −54.69 | −60.63 | −66.58 | −72.52 | −78.47 | −84.41 | |
| Δ s | 0.00 | −37.51 | −72.38 | −99.15 | −114.48 | −117.27 | −108.51 | −90.15 | −64.70 | −34.15 | 0.00 | |
| pHe | 6.86 | 6.77 | 6.73 | 6.71 | 6.72 | 6.75 | 6.78 | 6.81 | 6.85 | 6.88 | 6.92 | |
| 4 | pHo | 7.25 | 7.16 | 7.12 | 7.09 | 7.07 | 7.05 | 7.04 | 7.02 | 7.02 | 7.01 | 7.00 |
| SIc | 2.07 | 2.61 | 3.12 | 3.56 | 3.91 | 4.16 | 4.32 | 4.36 | 4.29 | 4.08 | 3.72 | |
| s1 | −28.08 | −60.13 | −92.28 | −118.38 | −135.48 | −143.03 | −142.04 | −134.11 | −120.97 | −104.07 | −84.41 | |
| s2 | −28.08 | −33.71 | −39.35 | −44.98 | −50.61 | −56.25 | −61.88 | −67.51 | −73.14 | −78.78 | −84.41 | |
| Δ s | 0.00 | −26.42 | −52.93 | −73.40 | −84.87 | −86.79 | −80.16 | −66.60 | −47.83 | −25.29 | 0.00 | |
| pHe | 7.01 | 6.89 | 6.83 | 6.80 | 6.80 | 6.81 | 6.83 | 6.85 | 6.87 | 6.90 | 6.92 | |
3.2.3. Mixing Precipitation between an Unsaturated Water Sample and an Oversaturated Water Sample
| Group | Item | Mixing ratios | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | ||
| 5 | pH0 | 6.80 | 6.81 | 6.81 | 6.83 | 6.84 | 6.86 | 6.89 | 6.92 | 6.97 | 7.06 | 7.26 |
| SIc | 0.78 | 1.42 | 1.87 | 2.15 | 2.31 | 2.36 | 2.32 | 2.22 | 2.09 | 1.97 | 2.16 | |
| s1 | 9.01 | −18.93 | −43.54 | −63.65 | −77.87 | −84.83 | −83.75 | −74.85 | −60.03 | −42.89 | −29.43 | |
| s2 | 9.01 | 5.17 | 1.32 | −2.52 | −6.37 | −10.21 | −14.05 | −17.90 | −21.74 | −25.59 | −29.43 | |
| Δs | 0.00 | −24.10 | −44.86 | −61.13 | −71.50 | −74.62 | −69.70 | −56.95 | −38.29 | −17.30 | 0.00 | |
| pHe | 6.81 | 6.79 | 6.77 | 6.75 | 6.73 | 6.73 | 6.73 | 6.75 | 6.79 | 6.87 | 7.01 | |
| 6 | pHo | 6.50 | 6.51 | 6.51 | 6.52 | 6.53 | 6.54 | 6.55 | 6.57 | 6.59 | 6.64 | 6.71 |
| SIc | 0.31 | 0.48 | 0.62 | 0.75 | 0.86 | 0.94 | 1.00 | 1.05 | 1.07 | 1.08 | 1.11 | |
| s1 | 115.36 | 86.47 | 60.94 | 39.16 | 21.60 | 8.36 | −0.51 | −5.35 | −7.01 | −6.69 | −6.57 | |
| s2 | 115.36 | 103.17 | 90.97 | 78.78 | 66.59 | 54.40 | 42.20 | 30.01 | 17.82 | 5.62 | −6.57 | |
| Δs | 0.00 | −16.70 | −30.03 | −39.62 | −44.99 | −46.04 | −42.71 | −35.36 | −24.83 | −12.31 | 0.00 | |
| pHe | 6.59 | 6.58 | 6.57 | 6.56 | 6.55 | 6.55 | 6.55 | 6.56 | 6.58 | 6.62 | 6.68 | |
3.2.4. General Characteristics of End Member Samples for Mixing Precipitation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crandall, C.A.; Katz, B.G.; Hirten, J.J. Hydrochemical evidence for mixing of river water and groundwater during high-flow conditions, lower Suwannee River basin, Florida, USA. Hydrogeol. J. 1999, 7, 454–467. [Google Scholar] [CrossRef]
- Doria, M.F.; Pidgeon, N.; Hunter, P.R. Perceptions of drinking water quality and risk and its effect on behaviour: A cross-national study. Sci. Total Environ. 2009, 407, 5455–5464. [Google Scholar] [CrossRef]
- Inoue, R.; Yoshida, J.; Hiroe, Y.; Komatsu, K.; Kawasaki, K.; Yasuda, I. Modification of North Pacific intermediate water around mixed water region. J. Oceanogr. 2003, 59, 211–224. [Google Scholar] [CrossRef]
- Mao, B.Y.; Xu, M.; Zhong, B.A.; Huang, R.Y. Study on the karst development law and mechnism HESAN Coal Field Guangxi. Res. Soil Water Conserv. 2007, 14, 137–140. (in Chinese). [Google Scholar]
- Paulsen, S.; List, E.J. A study of transport and mixing in natural waters using ICP−MS: Water-particle interactions. Water Air Soil Poll. 1997, 99, 149–156. [Google Scholar]
- Gong, Z.Z. Mixing corrosion tests of natrual water in Guilin karst region. Carsologica Sinica 1987, 6, 245–254. (in Chinese). [Google Scholar]
- Huang, F.; Tang, W.; Wang, J.M.; Cao, J.H.; Yin, J.J. The influence of allogenic water on karst carbon sink: A case study in the Maocum Subterranean River in Guilin, China. In Carsologica Sinica; 2011; Volume 30, pp. 417–421. (in Chinese) [Google Scholar]
- Rygaard, M.; Arvin, E.; Binning, P.J. The valuation of water quality: Effects of mixing different drinking water qualities. Water Res. 2009, 43, 1207–1218. [Google Scholar] [CrossRef]
- Shen, H.T.; Zhang, C.L. Mixing of salt water and fresh water in the Changjiang River estuary and its effects on suspended sediment. Chin. Geogr. Sci. 1992, 2, 373–381. [Google Scholar] [CrossRef]
- Bögli, A. Karst Hydrology and Physical Speleology; Springer Verlag: Berlin, Germany, 1980; pp. 1–284. [Google Scholar]
- Chen, H.H.; Zou, S.Z.; Zhu, Y.F.; Chen, CX. An experimental study of mixture corrosion effects of carbonate rocks in the transitional zone of littoral karst areas. Acta Geol. Sinica 2001, 75, 298–302. [Google Scholar]
- Ramos-Leal, J.A.; Martínez-Ruiz, V.J.; Rangel-Mendez, J.R.; Alfaro de la Torre, M.C. Hydrogeological and mixing process of waters in aquifers in arid regions: A case study in San Luis Potosi Valley, Mex. Environ. Geol. 2007, 53, 325–337. [Google Scholar] [CrossRef]
- Zou, S.Z.; Zhu, Y.F.; Chen, H.H.; Wang, C.K. Chemistry process of seawater in littoral karst area of Daweijia, Dalian city. Mar. Geol. Quat. Geol. 2004, 24, 61–68. (in Chinese). [Google Scholar]
- Qian, H.; Li, P.Y. Mixing corrosion of CaCO3 in natural waters. E J. Chem. 2011, 8, 1124–1131. [Google Scholar] [CrossRef]
- Kaufmann, G. Numerical models for mixing corrosion in natural and artificial karst environments. Water Resour. Res. 2003, 39, 1157–1168. [Google Scholar] [CrossRef]
- Dreybrodt, W. Kinetics of the dissolution of calcite and its applications to karstification. Chem. Geol. 1980, 31, 245–269. [Google Scholar] [CrossRef]
- Dreybrodt, W. Mixing corrosion in CaCO3/1bCO2/1bH2O systems and its role in the karstification of limestone areas. Chem. Geol. 1981, 32, 221–236. [Google Scholar] [CrossRef]
- Qian, H.; Li, P.Y. Proportion dependent mixing effects of CaCO3 in natural waters. Asian J. Chem. 2012, 24, 2257–2261. [Google Scholar]
- Tong, W.; Zhang, M.; Zhang, Z.; Liao, Z.; You, M.; Zhu, M.; Guo, G.; Liu, S. Geothermal Resources in Tibet; (in Chinese). Science Press: Beijing, China, 1981; pp. 24–57. [Google Scholar]
- Crerar, D.A. A method for computing multicomponent chemical equilibria based on equilibrium constants. Geochim. Cosmochim. Acta 1975, 39, 1375–1384. [Google Scholar] [CrossRef]
- Qian, H. A calculation method of the possible distribution of the dissolved species and the Eh values in aqueous solution. J. Xi'an Coll. Geol. 1987, 9, 69–80. (in Chinese). [Google Scholar]
- Arnórsson, S.; Sigurdsson, S.; Svavarsson, H. The chemistry of geothermal waters in Iceland. I. calculation of aqueous speciation from 0° to 370°. Geochim. Cosmochim. Acta 1982, 46, 1513–1532. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, X.D.; Li, P.Y. Calculation of CaCO3 solubility (precipitability) in natural waters. Asian J. Chem. 2012, 24, 668–672. [Google Scholar]
- Plummer, L.N.; Parkhurst, D.L.; Kosiur, D.R. MIX2: A Computer Program for Modeling Chemical Reactions in Natural Waters; U.S. Department of the Interior, Geological Survey: Reston, VA, USA, 1975; pp. 1–70. [Google Scholar]
- Qian, H.; Song, X.; Zhang, X.D.; Yang, C.; Li, P.Y. Calculation of PH values for mixed waters. E J. Chem. 2011, 8, 657–664. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Qian, H.; Li, P. Mixing Precipitation of CaCO3 in Natural Waters. Water 2013, 5, 1712-1722. https://doi.org/10.3390/w5041712
Chen J, Qian H, Li P. Mixing Precipitation of CaCO3 in Natural Waters. Water. 2013; 5(4):1712-1722. https://doi.org/10.3390/w5041712
Chicago/Turabian StyleChen, Jie, Hui Qian, and Peiyue Li. 2013. "Mixing Precipitation of CaCO3 in Natural Waters" Water 5, no. 4: 1712-1722. https://doi.org/10.3390/w5041712
APA StyleChen, J., Qian, H., & Li, P. (2013). Mixing Precipitation of CaCO3 in Natural Waters. Water, 5(4), 1712-1722. https://doi.org/10.3390/w5041712






