Abstract
The investigation of electric arc furnace (EAF) steel slag as a viable add-on technology to existing stormwater systems for the removal of dissolved phosphorus (P) was extended to explore the effects of varying environmental and treatment system conditions. Parameters such as stormwater composition, P concentration, metal concentration, pH, temperature, slag mass and slag particle size were varied. Observations relating to the method of P removal via EAF slag were also carefully considered to explain removal mechanisms involved. Results demonstrated that, although physisorption contributed to P reduction, it was not the key P removal mechanism. Instead, precipitation was observed to be a key removal pathway as evidenced by the correlation between the loss of iron (Fe) from slag and the amount of P removed from solution. The reduced removal of P by slag in a copper-dominant stormwater solution was attributed to the formation of a stable complex formed by the interaction of copper with the slag via the ion-exchange surface model. The stability of this complex inhibits the loss of Fe from the EAF slag and, consequently, P removal by means of precipitation. In terms of the effect of changing environmental and treatment system conditions on the P removal process, stormwater composition, P concentration, metal concentration, pH, temperature, slag mass and slag particle size were found to significantly influence the effectiveness of EAF slag in removing P from a given stormwater system. It was also established that a number of combinations of these factors influence P uptake differently.
1. Introduction
Stormwater is non-point source (NPS) pollution that occurs when runoff from rainfall or snowmelt gathers pollutants accumulated on lands or impermeable urban surfaces and conveys these to receiving water bodies. The pollutants include pathogens, organic matter, nutrients, sediments and toxins. The United States Environmental Protection Agency (USEPA) recognized NPS as its country’s largest source of water quality problems [1]. Urban runoff was found to be one of the leading causes of water quality degradation in surface waters; it accounted for loss of quality in 13% of rivers and streams surveyed, 18% of lakes, ponds and reservoirs and 32% of estuaries [2]. Furthermore, provinces in Canada have long recognized the severe contamination and fish kills caused by urban stormwater runoff. For example, in British Columbia, the provincial Ministry of Environment estimated that over half of the approximately 1,750 stormwater outfalls in the Greater Vancouver Regional District discharged directly into fish-bearing waters, causing extensive destruction of aquatic life [3].
Of the constituents found in stormwater, phosphorus (P) is an essential nutrient for the growth of aquatic organisms. Sources of P include detergents, fertilizers, compromised septic systems, animal wastes, plant debris, sediments, gasoline and tires. However, when present in high concentrations, P can cause eutrophication in freshwater systems resulting in a significant loss of water quality, biodiversity, and recreational or economic value [4,5,6].
Stormwater as a source of P has received lesser attention than have treatment processes for point source P pollution from wastewater from industrial and residential discharges. However, the control of P in discharged urban stormwater is becoming more commonly recognized as highly important for the sustained health and vitality of receiving waters [7]. Today, many countries around the world have stormwater quality regulations and guidelines in place to curb the potential degradation of water resources by the different stormwater constituents, including phosphorus [8]. With these guidelines and regulations, a suite of stormwater treatment technologies such as grassed swales [9], constructed wetlands [10] and detention ponds [11,12] has also emerged. However, some of these systems have proven ineffective for the removal of dissolved nutrients and heavy metals [12,13,14]. Furthermore, the efficiency and efficacy of growing systems for stormwater treatment in extreme climates, particularly where deep freezing conditions exist for extended periods, are generally insufficient for environmentally adequate P reduction [15,16]. Therefore, many dissolved inorganic nutrients and heavy metals continue to be discharged to surface waters, resulting in potential reductions in quality and overall ecosystem health. To address these challenges, an effective and sustainable add-on technology that can function efficiently under a wide variety of climatological conditions is required. This add-on component can be introduced prior to the release of treated stormwater to the receiving water body.
Stormwater programs are usually budget-constrained. Thus, apart from being effective, a successful stormwater treatment initiative must be affordable and make use of inexpensive materials. Low-cost industrial by-products such as fly ash [17,18], dried alum sludge [19], cement kiln dust [20] and different varieties of slag [21,22,23] that have been used for P removal from wastewaters would also be attractive for stormwater treatment. Of these materials, electric arc furnace steel slag (EAF slag) has consistently proven to be a superior material for P sequestration [24,25,26,27]. EAF slag exhibits the most desirable physico-chemical properties, highest P retention capacity (~2.2 g P kg−1), higher P removal efficiency than other slag types, and a potential to regenerate for reuse in P retention [28,29]. For these reasons, and for the fact that it is widely available and easily sourced in Saskatchewan and other North American locations such as Iowa and Alabama, it is an important focus of this study. The current research investigates EAF slag as a viable add-on technology for the retention of P in stormwater treatment systems. As well, interactions between P and some of the most commonly-occurring metals in urban stormwater—cadmium (Cd), copper (Cu), lead (Pb) and Zinc (Zn) [30,31]—are considered.
The specific objectives of this research are to: (1) To characterize EAF slag so as to propose a theory on the pathway for phosphorus removal from stormwater, and (2) To study the effect of environmental and treatment system conditions on phosphorus removal using EAF slag in bench scale experiments.
2. Materials and Methods
2.1. Characterization of Slag
The source of EAF slag, the methods of adsorbent preparation for testing, and the characterization methods used in these experiments were previously described in detail [32]. Briefly, EAF slag was obtained from the EVRAZ steel recycling facility in Regina, Saskatchewan, Canada. The slag was pulverized and sieved with an Endecolts Octagon 200 Test Sieve Shaker (London, England). A particle size (dp) between 2.36 and 3.35 mm, equivalent to commercially available granular activated carbon, was used in experiments. The resulting EAF slag particles were washed with distilled water to remove residual fines, dried in a furnace at 105 °C for 24 h and stored in a desiccator until use.
In this study, additional surface area determination, Powder X-ray Diffraction (XRD) and Energy Dispersive X-ray (EDS) characterization analyses were conducted on spent slag samples obtained from the different stormwater solutions. These analyses were included to gain insight into the mechanisms involved in the competitive reduction of P in the systems evaluated.
Fresh and spent slags were the terms used to describe the slag as obtained before and after sorption, respectively.
2.2. Synthetic Stormwater Solutions
The Nationwide Urban Runoff Program [30] was one of the most comprehensive studies ever conducted on the quality characteristics of urban runoff and its degradation impacts on receiving waters. The study confirmed the presence of a wide range of constituents in urban runoff which varied significantly from one geographic location or land use category to another in terms of characteristics and pollution levels. It recommended the soluble P, Cu, Pb and Zn concentrations in Table 1 for general urban runoff planning requirements. Except where otherwise indicated, a series of synthetic stormwater samples was therefore produced comprising combinations of P and one or more of the identified metal ions in a metal to P concentration ratio (M:P) of 0.2. This M:P value is within the range of relative values of metal to P concentration ratios observed in actual stormwater [30,31]. This study assumes that only dissolved P and heavy metals remain in the stormwater at discharge after treatment with conventional systems such as grassed swales, constructed wetlands and detention ponds.
Actual stormwater is complex mixture of organic, inorganic and toxic substances such as suspended solids, debris, pathogens, nutrients, pesticides, heavy metals and hydrocarbons. Therefore, it should be noted that the synthetic stormwater samples used in this study may not fully mimic the behavior of actual stormwater.

Table 1.
Water quality characteristics of urban runoff a.
| Constituent | Event to event b variability in EMC’s | Site median EMC c | |
|---|---|---|---|
| For median urban site | For 90th percentileurban site | ||
| Soluble Phosphorus, mg/L | 0.5–1.0 | 0.12 | 0.21 |
| Total Copper (Cu), µg/L | 0.5–1.0 | 34 | 93 |
| Total Lead (Pb), µg/L | 0.5–1.0 | 144 | 350 |
| Total Zinc (Zn), µg/L | 0.5–1.0 | 160 | 500 |
a. Reproduced from “Results of the Nationwide Urban Runoff Program. Volume 1—Final Report” [30]; b. Event: Refers to a rainfall runoff (or storm) event; c. EMC: “Event Mean Concentration” is a flow weighted mean concentration of a water quality parameter.
As the possible combinations of absolute P and metal concentrations in stormwater are infinite, the focus in the development of the synthetic stormwater solutions was on blending relative values of the stormwater constituents as opposed to their absolute concentration values. All relative P to metal concentration ratios were calculated from a phosphorus concentration basis of 5 mg/L. This P concentration value was selected based on observations from prior experiments showing that it was an adequate concentration from which to satisfactorily observe both gradual and immediate phosphorus changes in the stormwater system. The use of much lower concentrations resulted in greater difficulty detecting and studying the effect of system changes, and led to more inconsistencies, errors and uncertainties. Ultimately, the aim was to observe the P removal patterns occurring with changes in M:P concentrations. The synthetic stormwater solutions used in these experiments and their methods of preparation have been previously described in detail [32]. A summary of the synthesized stormwater solutions is presented in Table 2.
The terms “stormwater systems” and “stormwater matrices” are used interchangeably to mean the different stormwater solutions.

Table 2.
Composition of the six synthetic stormwater solutions (M:P = 0.2).
| No. | Description | Composition |
|---|---|---|
| 1 | Metal-free stormwater (P-only) | Cd, Cu, Pb, Zn = 0 |
| 2 | Cd-dominant stormwater (P + Cd) | Cd:P = 0.2, Cu, Pb, Zn = 0 |
| 3 | Cu-dominant stormwater (P + Cu) | Cu:P = 0.2, Cd, Pb, Zn = 0 |
| 4 | Pb-dominant stormwater (P + Pb) | Pb:P = 0.2, Cd, Cu, Zn = 0 |
| 5 | Zn-dominant stormwater (P + Zn) | Zn:P = 0.2, Cd, Cu, Pb = 0 |
| 6 | Multi-metal stormwater (P + Mix) | Cd:P, Cu:P, Pb:P, Zn:P = 0.2 |
2.3. Kinetics of Phosphate Removal
Volumes of 250 mL of the synthetic stormwater samples were contacted with 1 g EAF slag placed in Erlenmeyer flasks. The adsorption studies were conducted in a platform shaker (Lab-line Instruments Inc., IL, USA) at 80 rpm for 120 h, under which equilibrium was reached and no slag disintegration was observed. All experiments were run at room temperature (298 ± 5 K) and an influent pH of 7. The pH was adjusted with 0.1 M HCl or 0.1 M NaOH to achieve neutrality. Samples were collected every 24 h and filtered through a 0.45 µm Nalgene membrane prior to P analysis as dissolved reactive phosphorus, i.e., orthophosphate (PO4-P). The analysis was carried out using a modified colorimetric Stannous Chloride method [33] and a Helios Alpha scanning spectrophotometer (Thermo Electron Corporation, England) set at 650 nm. All blank, control and test samples were taken and analyzed in triplicate. P removal as presented in this study was calculated as the net decrease in dissolved P in solution. Once slag was present in a stormwater mixture, it was assumed that all of the removed dissolved P was taken up by the slag. Results were expressed in terms of % P removal or in terms of adsorption density (mg P/g slag).
Excepting certain cases, all the experiments conducted in this study, and described in the sections below, were carried out according to the procedure and conditions described in the preceding paragraph. The comparative effects of varying P concentration (mg/L) as a ratio of phosphorus to metal concentration (P:M = 0–10), metal concentration as a ratio of metal to phosphorus concentration (M:P = 0–1.4), slag mass (0–6 g), slag particle size (0.50–5.56 mm), pH (3.0–9.0), and temperature (298–333 K) were studied for the removal of P in the different stormwater systems. The experimental ranges for the environmental and system conditions were selected to clearly observe changes to system behavior, as judged via P-removal efficiency.
2.4. Effect of Phosphate Concentration
Initial P concentration in the stormwater samples was varied as phosphorus to metal ratios (P:M) to determine impacts on the kinetics of competitive P removal in the presence of Cd, Cu, Pb and Zn in urban stormwater. P:M = 0, 2.5, 5.0, 7.5 and 10.0 were used in these experiments.
2.5. Effect of Metal Concentration
In a second series of experiments, initial heavy metal concentrations in the stormwater were adjusted as metal to phosphorus ratios (M:P) over the range of 0 to 1.4 (i.e., at M:P = 0, 0.2, 0.6, 1.0 and 1.4). These experiments were designed to identify and quantify the effects of varying heavy metal concentrations on P removal.
2.6. Effect of Slag Mass
In this series of experiments, only metal-free (P-only) stormwater was evaluated. A range of slag masses, from 0 to between 4 and 6 g, was contacted with the P-supplemented solution (lower concentration) to elucidate the influence of EAF slag density on overall P uptake. To further investigate the relationship and corresponding P removal pattern between slag mass and P-concentration, the experiment was repeated at three times the concentration of P-only (higher concentration). Experiments were run for 72 h instead of 120 h, as the increased slag mass was expected to more rapidly remove P from the aqueous phase.
2.7. Effect of Slag Particle Size
EAF slag particle size in the ranges of 0.50–0.85 mm, 0.85–1.00 mm, 1.00–1.40 mm, 1.40–1.70 mm, 2.36–3.35 mm and 4.75–5.56 mm were tested to calculate the influence of surface area on P physisorption. Unlike other experiments, these were conducted at 293 ± 5 K, which is cooler than the remaining experiments. The different particle sizes were contacted with P-only solution to provide information about the effect of slag diameter and surface area on P uptake in the stormwater systems.
2.8. Effect of pH
Influent pH was adjusted to 3.0, 5.0, 7.0, and 9.0, and the influence of initial pH on P removal in a variety of stormwater solutions was subsequently monitored.
2.9. Effect of Temperature
To determine the influence of temperature on the competitive sorption process, all batch experiments using all stormwater systems in Table 1 were repeated at temperatures of 313 and 333 K. This wide temperature range was selected to clearly observe the variability in P adsorption density as a result of temperature.
2.10. Statistical Analysis
The relative standard deviation (%) was calculated for the measured P adsorption densities (mg/g). One-way analysis of variance (ANOVA) was applied to analyze the differences in P removal data obtained from the slag particle size experiments. Also, Scheffé’s test was conducted in post-hoc comparisons to examine the significance of differences existing between P removal results for pairs of slag particle size tests.
Data from all other experiments were processed using a two-way ANOVA with consideration for replication and interaction effects between a given stormwater composition and a particular environmental parameter, or in the case of experiments on varying slag masses, between a given slag quantity and a given P concentration.
Statistical significance was set at the probability level, α = 0.05, for all analyzed data. Microsoft Excel 2010 was used for all statistical work.
3. Results and Discussion
Previous work [32] led to the following observations: (1) The nature of competing metal ions in stormwater has a significant effect on the extent of P elimination; (2) The presence of Cu in stormwater resulted in much lower P removal by EAF slag; and (3) There was loss of Fe from the EAF slag.
The effect of this Fe loss and the impact of Cu on P sorption are further discussed in this section.
3.1. Characterization of Slag
Detailed characterization of the slag before and after interaction with P-only stormwater was previously completed and presented [32]. Those prior characterization tests resulted in the following major findings: (1) The EAF slag used in these experiments were principally composed of iron (Fe) and calcium (Ca), as previously noticed in other studies on EAF slag [34,35,36], and were present as Wüstite (FeO), Larnite (β-Ca2SiO4), Brownmillerite (Ca2(Al,Fe)O5), Srebrodolskite (Ca2Fe3+2O5) and Lime (CaO); (2) Fe content was the highest in the fresh slag, according to the following metal composition (%, g/g): Fe—23.93, Ca—20.71, Si—5.83, Mn—4.6, Al—4.52, Cr—1.66, and Ni—0.023; (3) Physisorption was a contributor to P removal by EAF slag; (4) Fe depletion from slag was evident.
In this study, characterization was extended to spent slag specimens from the treatment of other synthetic stormwater systems in Table 2. BET (Brunauer-Emmett-Teller) surface area determination was carried out to ascertain if physisorption occurred in all cases, especially in the case of the Cu-dominant system, P + Cu, where significantly lower P removal had been observed. The BET surface area (m2/g) of the fresh EAF slag sample was 1.12. Table 3 shows the results for the BET surface area of the spent slag samples from treatment of the different stormwater matrices.

Table 3.
BET (Brunauer-Emmett-Teller) Surface Area of spent slag samples.
| No. | Source of spent slag sample | BET Surface Area (m2/g) |
|---|---|---|
| 1 | P-only | 0.48 |
| 2 | P + Cd | 0.50 |
| 3 | P + Cu | 0.45 |
| 4 | P + Pb | 0.79 |
| 5 | P + Zn | 0.47 |
| 6 | P + Mix | 0.58 |
A decrease in the BET surface area was observed for all the used slag specimens when compared with the fresh slag. This indicates the likelihood of physisorption as a P removal mechanism in all the stormwater systems, irrespective of their composition. Also, the difference in the surface area decrease of the slag sample retrieved from P-only and those applied to the other metal-containing stormwater solutions may be negligible. When the surface area of spent slag from P-only was compared to that from P + Cu, there was essentially no difference. It can therefore be deduced that though physical adsorption contributes to P reduction, it may not be the key P removal mechanism. Some other mechanism appears to be responsible for the increased P adsorption density noticed in P-only versus the lower P uptake observed in P + Cu.
XRD results of spent slag samples from the treatment of the P-only, P + Cu and P + Mix stormwater matrices (Table 2) are presented in Figure 1, Figure 2, Figure 3. In Figure 1(a), the XRD spectrum of spent slag from P-only [32] has been reproduced. This has been compared with the XRD patterns of spent slag from both P + Cu (Figure 1b), in which P-removal was statistically different as against P-reduction in P-only and P + Mix (Figure 1c), in which P-removal was statistically insignificant as was also the case for P + Cd, P + Pb and P + Zn.
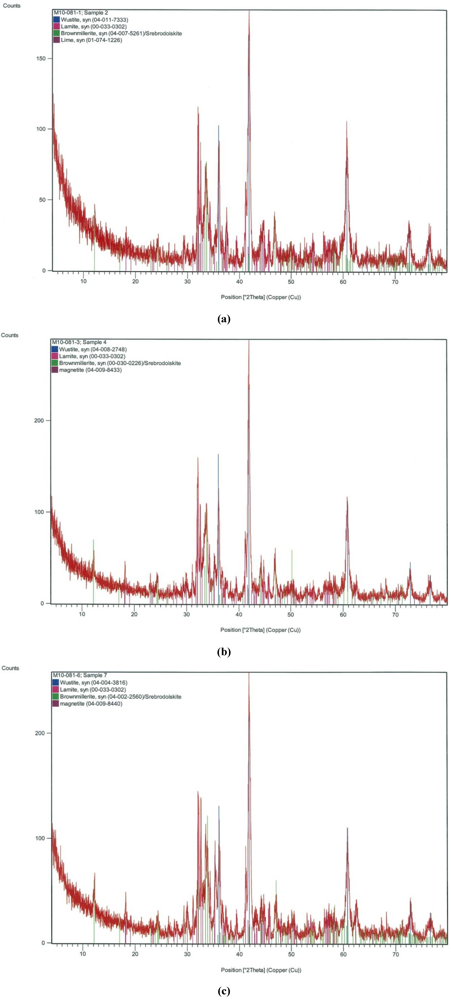
Figure 1.
X-ray diffraction pattern of spent slag from (a) P-only (P removal: 95%); (b) P + Cu (Premoval: 68%); (c) P + Mix (Premoval: 95%).
Figure 1.
X-ray diffraction pattern of spent slag from (a) P-only (P removal: 95%); (b) P + Cu (Premoval: 68%); (c) P + Mix (Premoval: 95%).
XRD results of the spent slag samples from P + Cu and P + Mix also showed that the major volume fraction was amorphous. Just as in the case of spent slag from P-only, Wüstite (FeO), Larnite (β-Ca2SiO4), Brownmillerite (Ca2(Al,Fe)O5), Srebrodolskite (Ca2Fe3+2O5), Lime (CaO) were identified as common constituents in the representative spent slag samples from those stormwater types. Magnetite (Fe3O4) was also noticed in some cases. For example, while lime (CaO) was identified in the spent slag from P-only, it was not noticeable in the slag samples used on P + Cu and P+ Mix. Instead, the latter slag samples contained magnetite (Fe3O4), which on the contrary, was not identified in the slag used to treat P-only. This observed variation in slag composition indicates that the EAF slag is heterogeneous. However, the disparity in the slag make-up did not appear to be significant in fully explaining the resulting P removal observed in the different stormwater solutions. Overall, Fe and Ca were consistently proven to be key components of the EAF slag under study, as previously observed [37].
The semi-quantitative EDS results for the spent slag samples from P-only [32], P + Cu and P + Mix are shown in Figure 2.
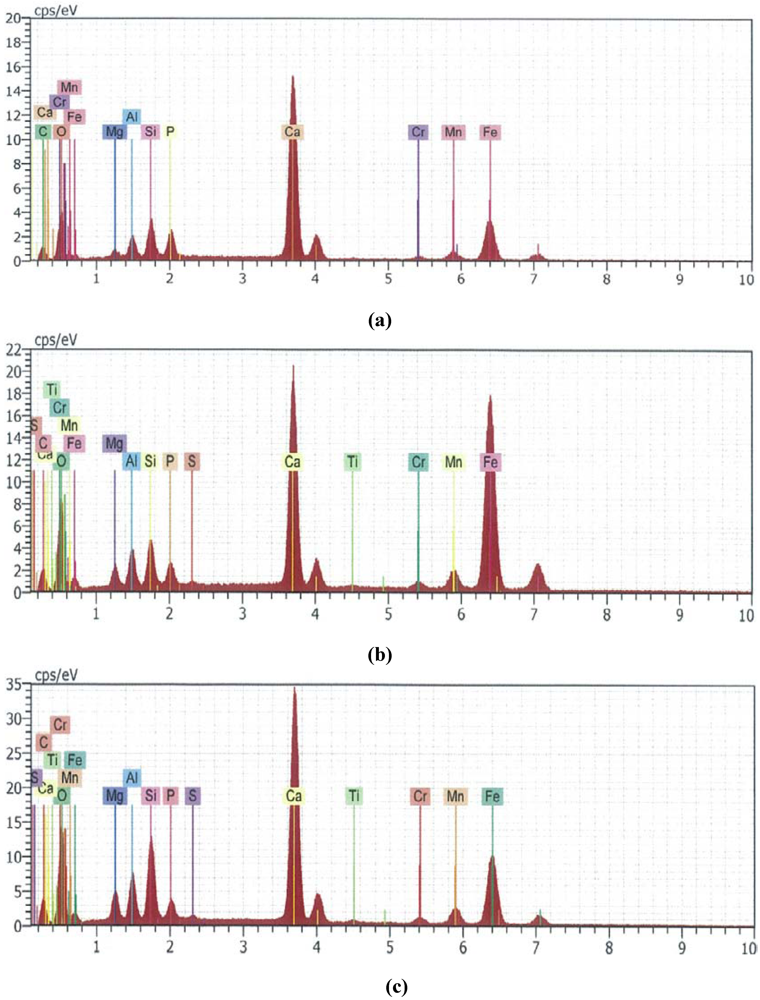
Figure 2.
Semi-quantitative EDS results of spent slag from (a) P-only (P removal: 95%); (b) P + Cu (P removal: 68%); (c) P + Mix (Premoval: 95%).
Figure 2.
Semi-quantitative EDS results of spent slag from (a) P-only (P removal: 95%); (b) P + Cu (P removal: 68%); (c) P + Mix (Premoval: 95%).
The specimen spectra confirm the uptake of P by slag. The data also point to a significant decrease in Fe content of spent slag from P-only and P + Mix, as compared to the Fe content in that from P + Cu experiments. There appears to be correlation between the loss of Fe and the amount of P removed by the slag. Also, the formation of precipitates was visible in the P-only and P + Mix systems at the conclusion of the experiments. It is well-known that P can be removed from wastewater via chemical precipitation by the addition of divalent and trivalent metal salts [38,39,40]. It is thus likely that ferric ions lost from the slag combine with P in solution to form ferric phosphate (FePO4). The basic reaction is:
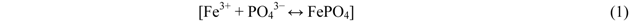
This may explain the increased removal of dissolved P from those systems. For P + Cu, the presence of Cu in the stormwater appeared to generally impede the loss of Fe from slag and thus its eventual consumption for chemical precipitation. This may justify the lower removal of dissolved P detected in P + Cu, as previously observed. Further, the effect of Cu may be explained by the ion-exchange surface model [41]. The model has been used to describe processes occurring at the surface of hydrated oxides and some organic compounds. According to the model, the hydration of aluminum oxide, iron (II) oxide, manganese (IV) oxide, and silicon oxide leads to the formation of amorphous surfaces that contain exchangeable protons. These metals—Al, Fe, Mn and Si—were observed in the slag, which in solution becomes hydrated. Also XRD has confirmed the amorphous nature of the slag surface. Thus, the ion-exchange model may be applicable here. The ion-exchange process in which the adsorbed cation (Mn+) exchanges for a proton (H+) can be represented by the following simplified Equation [41]:
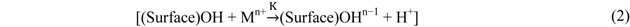
From Equation 2, a theory is proposed that Cu2+ in solution is exchanged for H+ and adsorbed on the surface of the slag. It is likely that Cu2+ forms a highly stable complex with slag, as has been observed in the stable complexes it forms with nitrilotriacetic acid (NTA) and citrate ligands [41]. This stability may be responsible for impeding the loss of Fe from the slag to the solution, and subsequently, the removal of P by precipitation. The lower P uptake from the stormwater in the presence of EAF slag and Cu can thus be explained.
3.2. Effect of Varying Phosphate Concentration
The impacts of varying P concentration are shown in Figure 3. Irrespective of the type of metal constituent(s) present in the stormwater, adsorption density (mg P/g slag) increased with upsurge in P concentration. This result was expected given that there was more P available for sorption in the stormwater solution per unit mass of slag.
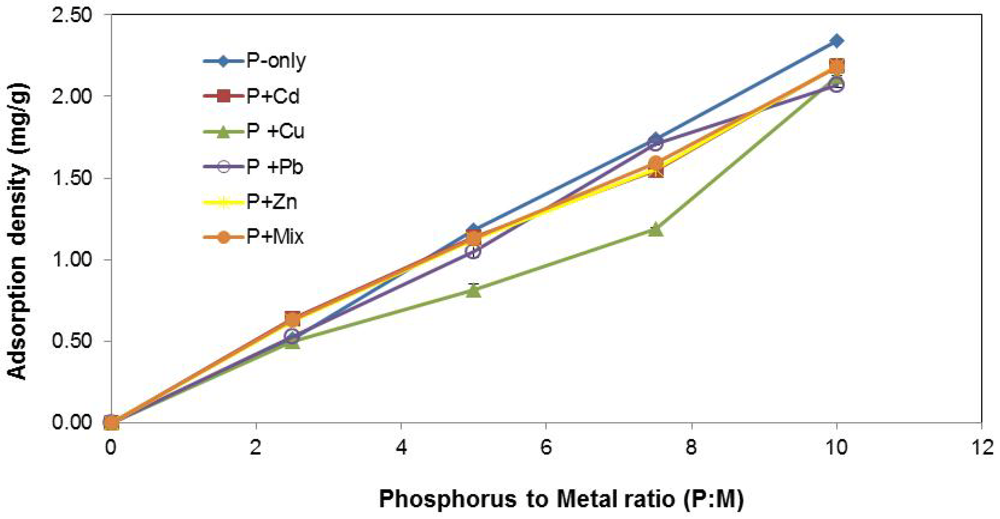
Figure 3.
Influence of P concentration.
Figure 3.
Influence of P concentration.
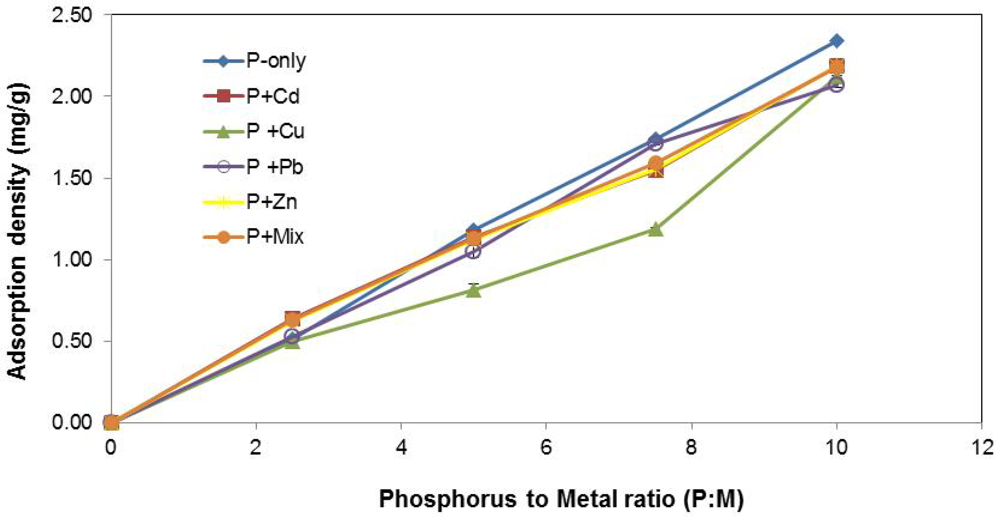
The inhibiting effect of Cu2+ on P removal was once again noted in these experiments. However, P concentration appeared to have an influence on the prominence of the “Cu-effect”. At the lower concentration of the spectrum (i.e., P : M = 2.5), the difference in P-removal (mg/g) was essentially negligible across the stormwater systems, and the presence of Cu2+ in P + Cu did not appear to matter much. One possible explanation may be that at lower P concentrations, physisorption is the principal removal process and greater P-removal advantage enjoyed by the other systems through precipitation via the loss of slag Fe, as proposed previously, may be effectively diminished due to the absence of sufficient P in solution.
At the higher P concentration (i.e., P:M = 10), there appears to be ample P in solution to result in near-saturation or saturation of the slag in all the systems. Therefore, the amount of P removed via varying degrees of physisorption or precipitation, or a combination of these mechanisms in the various systems, evens out and the difference in P uptake that would ordinarily be expected becomes less pronounced.
3.3. Effect of Varying Metal Concentration
The effects of varying heavy metal concentrations in stormwater without the use of slag in the batch adsorption tests are presented in Figure 4. The results show that at higher concentrations, the metal ions, with the exception of Cd2+ (Figure 4a), remove P primarily and increasingly by precipitation.
However, there was a dissimilar precipitation pattern for the different metal constituents. For example, in the case of Cu2+ (Figure 4b), precipitation takes place gradually, reaching equilibrium within the first 24 h. No changes were noticed thereafter. For Zn2+ (Figure 4d), a much slower pattern of precipitation is observed. It is only after considerably long reaction times, and at higher concentrations, that Zn2+ begins to show a tendency to remove P via precipitation. When it comes to Pb2+ (Figure 4c), precipitation is instantaneous, reaching equilibrium immediately. This pattern differs significantly from that of Cd2+ (Figure 4a), which shows that precipitation of P by Cd2+ does not occur with time or over the increasing range of metal concentrations tested. These observed precipitation patterns seem to be related to the electronic structures of the metal ions. The zero to slow precipitation patterns observed in the case of Cd2+ and Zn2+ may be attributed to the stability they derive from having completely filled d-subshells.
Based on the negligible P precipitation observed even at high concentrations, Cd2+ was found not to be a crucial component for further testing with respect to the influence of increasing metal concentrations (M : P). Furthermore, it has already been established that Cd had no detectable effect on the removal of P from simulated stormwater using EAF slag [32]. It was thus eliminated. Only Cu2+, Pb2+ and Zn2+ concentrations were varied to investigate impacts on P removal.
As well, except where mentioned, Cd was discarded for further consideration in this study as it was not a priority metal in urban runoff, unlike Cu, Pb and Zn. These three latter metals are most often detected in urban runoff, with at least 91% of the samples studied in the Nationwide Urban Runoff Program containing measurable concentrations of each [30].
Figure 5 shows the effect of increasing metal concentration on adsorption density (mg P/g slag).
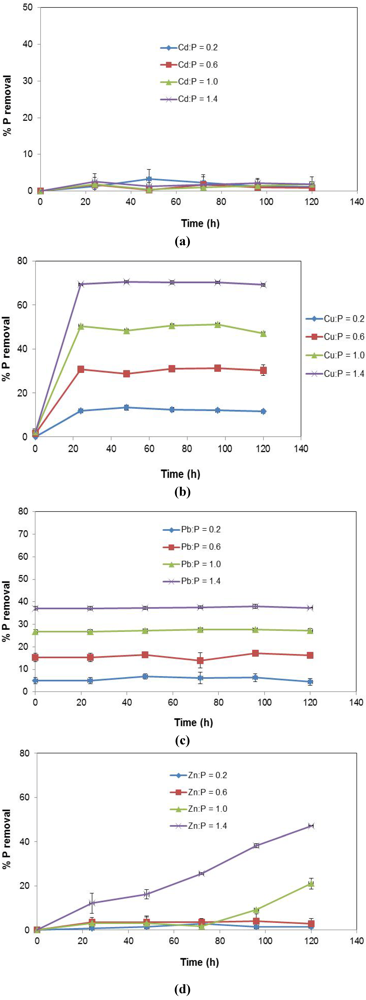
Figure 4.
Precipitation patterns of metal ions of increasing concentration reacting with P in the stormwater systems (no slag) (a) P + Cd; (b) P + Cu; (c) P + Pb; (d) P + Zn.
Figure 4.
Precipitation patterns of metal ions of increasing concentration reacting with P in the stormwater systems (no slag) (a) P + Cd; (b) P + Cu; (c) P + Pb; (d) P + Zn.
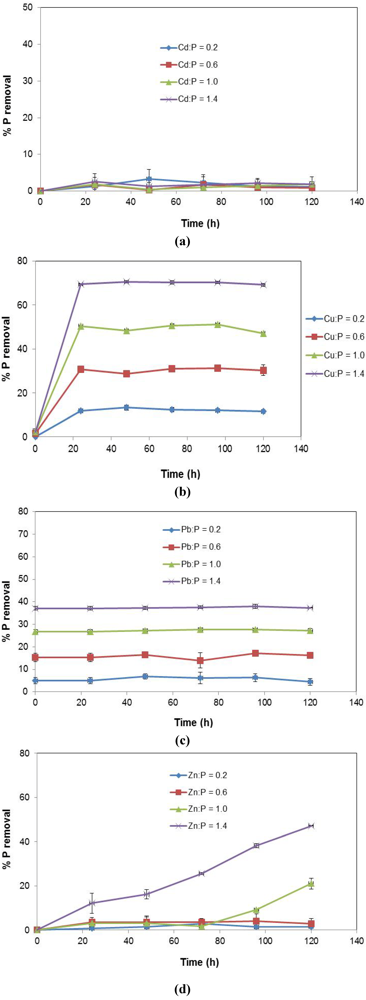
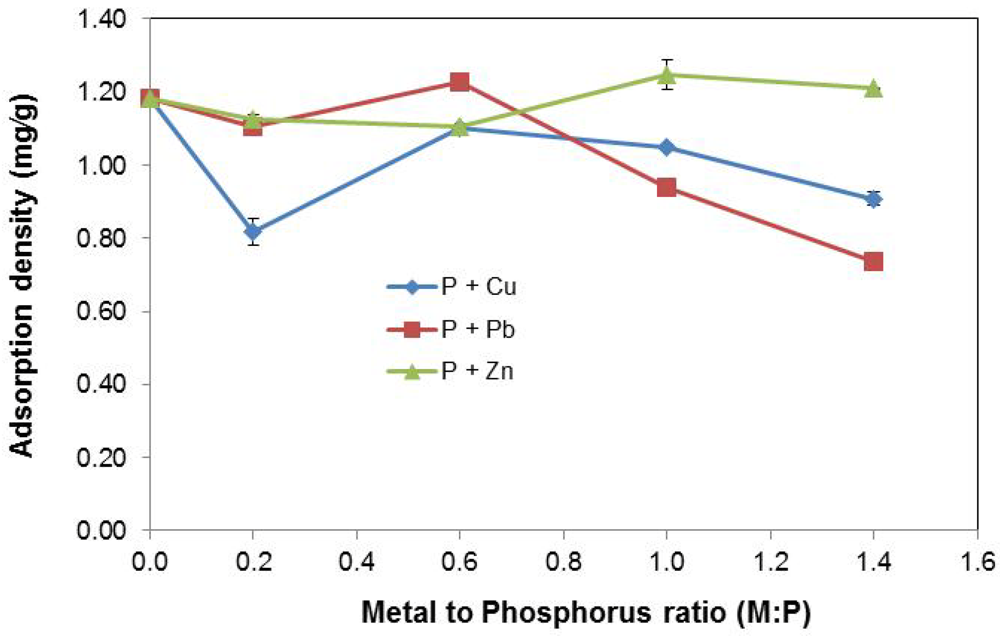
Figure 5.
Effect of varying metal concentration.
Figure 5.
Effect of varying metal concentration.
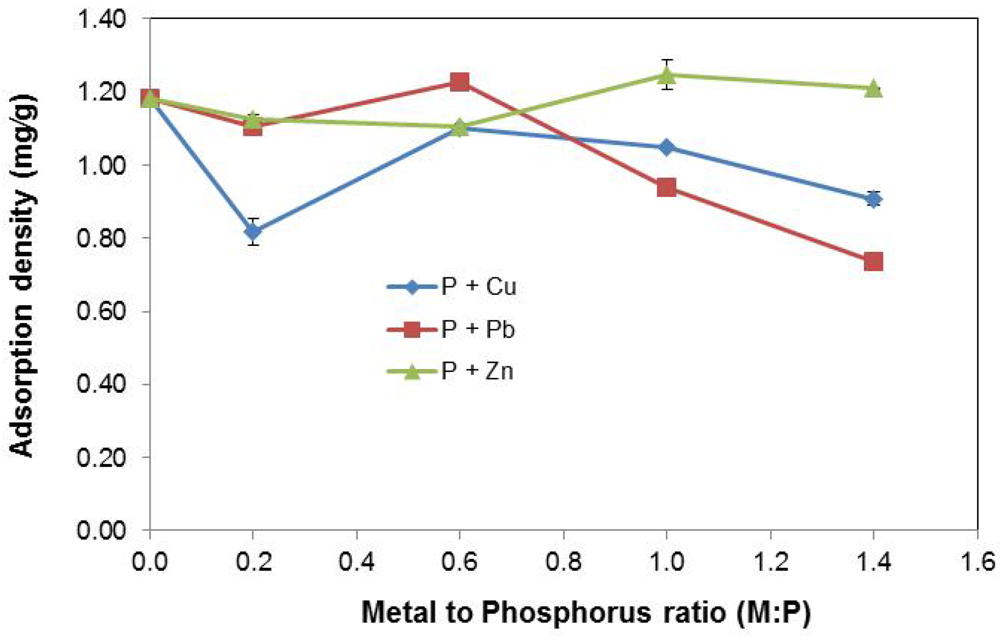
For P + Cu, the introduction of Cu2+ at the lower concentration, Cu:P = 0.2, led to reduced P uptake by the EAF slag substrate as previously explained by the ion-exchange surface model. Increase of Cu2+ concentration at Cu:P = 0.6 resulted in a spike in P removal. From the precipitation profile of P + Cu in Figure 4b, the removal of P by precipitation was intensified at higher concentrations of Cu2+. Thus, the elevated P adsorption density noticed at Cu:P = 0.6 appeared to be as a result of increased P removal by precipitation due to excess Cu2+, in addition to sorption by the slag.
Beyond Cu:P = 0.6, a lesser P adsorption density was noticed. The observed reduction in P removal by slag was considered to be related to the increased removal of P by precipitation at time, t = 0, i.e., prior to the addition of slag to the stormwater system. About 15% to 16 % of P was removed at t = 0, for Cu2+ concentrations at Cu:P = 1.0 and above. This indicated that there was a smaller amount of P available at t = 0 for uptake by the slag; hence the reduction in P adsorption density.
For P + Pb, a sharper decrease in P removal at higher Pb2+ concentrations (Pb:P = 1.0 and above) was observed. At lower Pb2+ concentration (Pb:P = 0–0.6) however, P adsorption density changes were statistically insignificant. This P removal trend was consistent with the pattern of P precipitation by Pb2+ depicted in Figure 4c, where instantaneous removal of P at t = 0 increasingly occurred at higher Pb2+ concentrations. At Pb2+ concentrations of Pb:P = 1.0 and above, between 25% and 40% of P was removed via precipitation at t = 0, leading to less availability of P for removal by EAF slag, after it was introduced into the stormwater solution. The outcome was thus a sharper fall in the measured P adsorption density at higher Pb2+ concentrations than was noticed in the case of higher Cu2+ concentrations, where only ~15% of P was removed at t = 0.
For P + Zn, the effect of increasing Zn2+ concentration on P removal is much less pronounced than for Cu2+ and Pb2+. Even at higher Zn2+ concentrations (Zn:P = 1.0–1.4), there appears to only be a slight increase in P removal due to precipitation. The incremental P uptake at these elevated concentrations is not statistically significant when compared to removal at lower Zn2+ concentrations (Zn:P = 0–0.6). As well, increase in precipitation with higher Zn2+ concentrations occurs gradually and much slower than with Cu2+ and Pb2+, where it is more sudden and faster.
Overall, precipitation of P by the metal ions played a noticeable role in the P removal process, and the P removal patterns appeared to correlate with the precipitation trends presented in Figure 4.
3.4. Effect of Varying Slag Mass
The influence of increased slag mass on P removal is shown in Figure 6. At the lower P concentration (i.e., low concentration), increasing the slag mass has a marginal effect on P conversion. 1 g of EAF slag was sufficient to remove about 90% of P in solution at equilibrium. The incremental advantage of introducing more slag into the solution is negligible. However, at the higher concentration (high concentration), which was three times greater than the low concentration, the presence of more slag results in more P removal, until a certain point, at about 3 g of slag, where the incremental benefit of added slag becomes insignificant. Thus, in terms of adsorption density (Figure 6b), at the low concentration, increasing slag mass, reduces the P removed per gram of slag. Based on the conversion result, this is expected, as using more slag when no significant changes will occur, means reduced adsorption for a unit mass of slag. The same is true at the high concentration. Beyond 2.5 g of slag, no significant, additional adsorption takes place, and therefore, increasing the slag quantity reduces the P adsorption occurring per unit mass of slag.
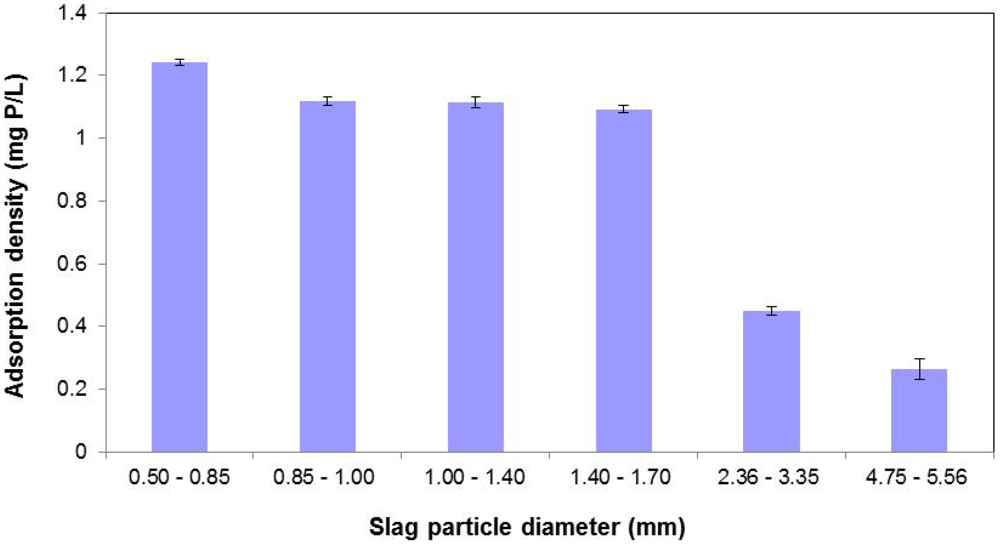
3.5. Effect of Particle Size
Figure 7 depicts the effect of varying slag particle diameter on the P sorption process. As seen, P removal essentially improves with reduced particle size for the same mass of slag. From the BET results presented earlier, surface area was correlated to P removal. Thus, smaller particle diameters result in larger surface areas, and consequently, better P removal efficiencies. However the data provided in Figure 7 clearly show that below a certain particle size (in this case, 1.40–1.70 mm) incremental P removal appears to be negligible.
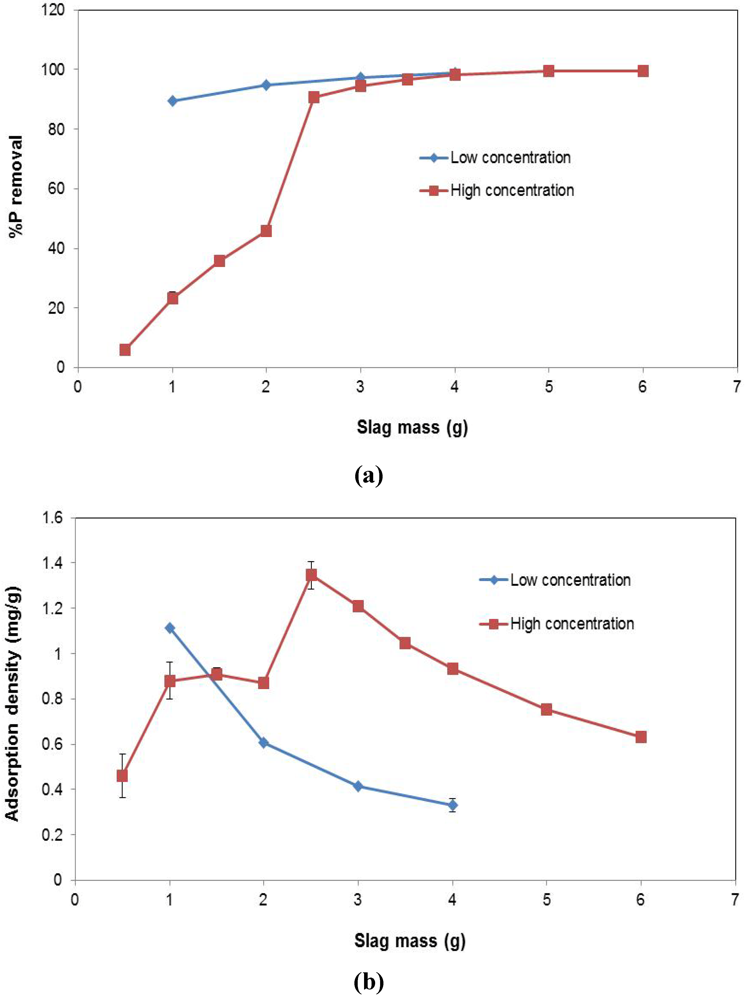
Figure 6.
Effect of varying slag mass across different P concentrations on (a) P conversion (%); (b) Adsorption density (mg P/g).
Figure 6.
Effect of varying slag mass across different P concentrations on (a) P conversion (%); (b) Adsorption density (mg P/g).
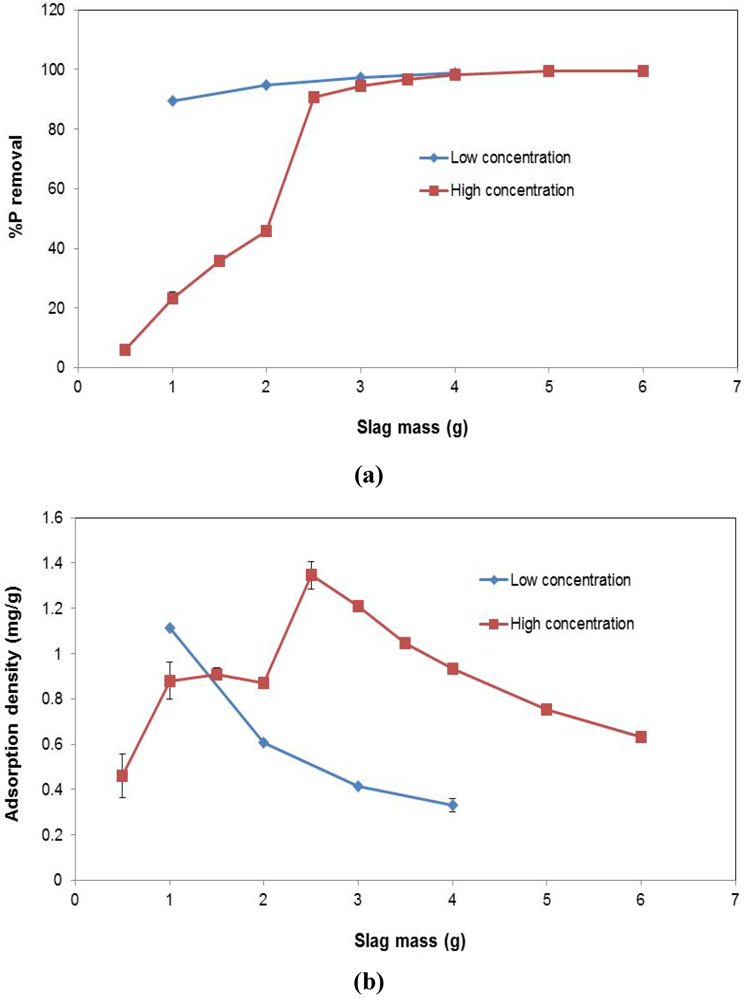
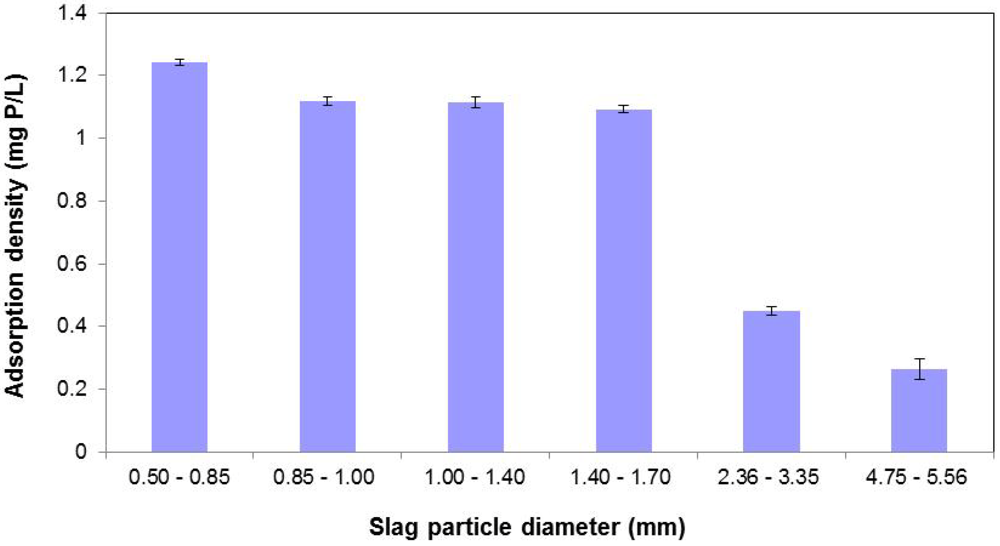
Figure 7.
Effect of slag particle diameter on P removal.
Figure 7.
Effect of slag particle diameter on P removal.
3.6. Effect of Initial pH
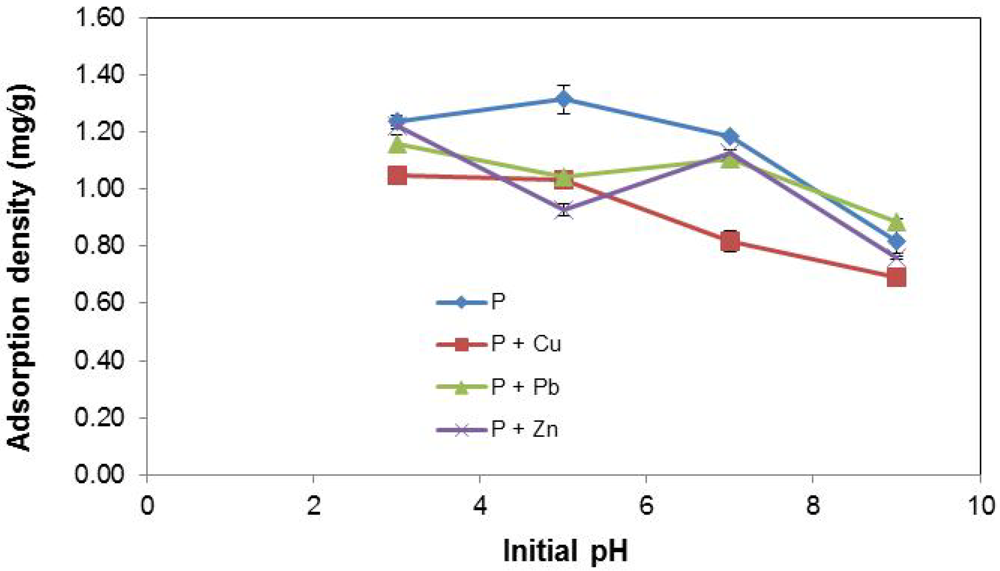
The effects of initial pH on P removal by EAF slag are depicted in Figure 8. For stormwater types P and P + Cu, P adsorption density is enhanced at acidic pH. As initial pH increases (i.e., with a more basic solution), the P removal efficiency drops. Here again, in comparing P with P + Cu, the effect of Cu2+ in impeding the removal of P from stormwater is observed at both low and high pH values. For P + Pb and P + Zn, the P removal pattern as a function of varying initial pH is unclear. Both stormwater types showed a dip in P removal efficiency at pH 5 and then a rebound at pH 7 before a final decline at pH 9. Overall, what is clear is that better performance was obtained at the acidic extreme (pH 3), while at the basic extreme (pH 9), performance was poorest for all stormwater systems.
The findings from this work highlight important complexities noticed in prior similar studies on the effect of initial pH. Numerous investigations have shown that optimal pH levels for P removal vary widely for different adsorbents. For instance, optimal P removal using blast furnace slag has been noticed at a high pH of 10 [42]; the efficiency of P removal using slag has been shown to improve at acidic pH [17]; while in previous a study on EAF slag, it was found that initial pH could not be strongly correlated to the observed P adsorption density [35].
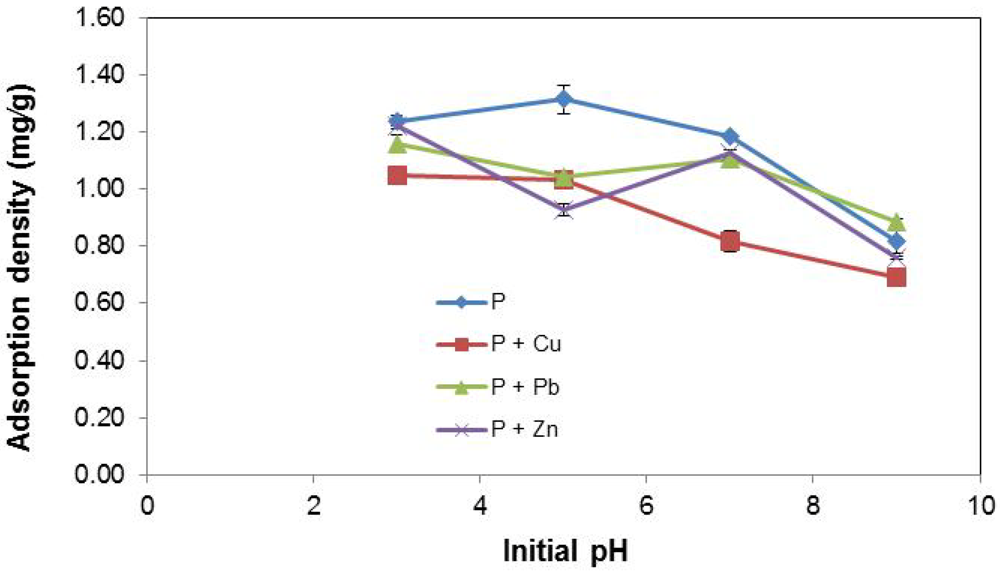
Figure 8.
Effect of initial pH on P removal.
Figure 8.
Effect of initial pH on P removal.
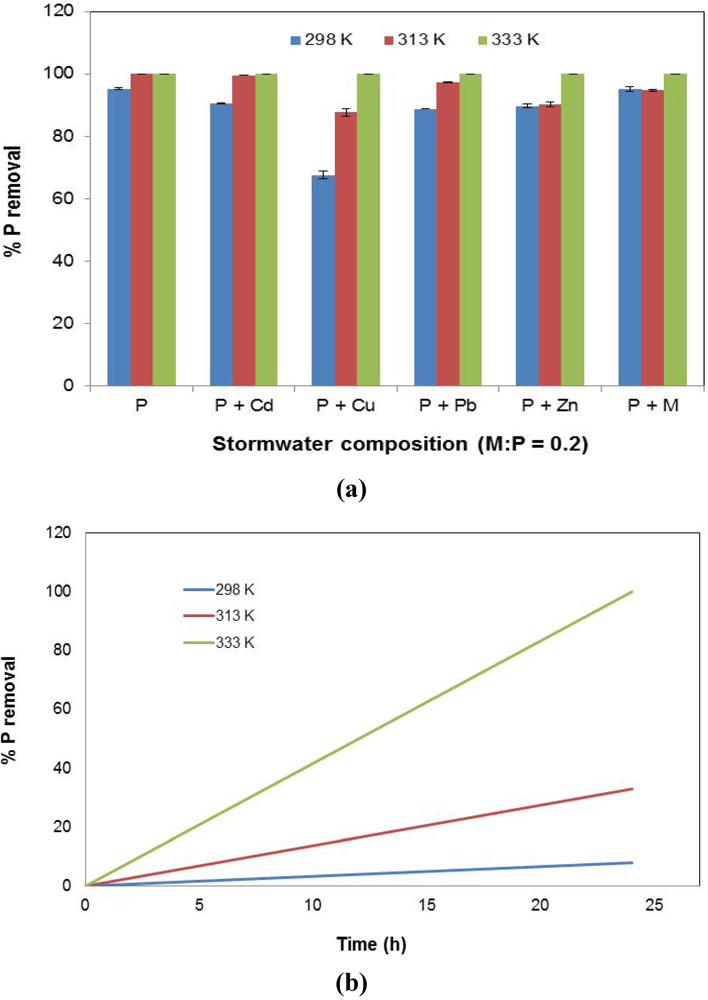
3.7. Effect of Temperature
The sorption behavior of P as a function of solution temperature is presented in Figure 9. It can be seen that P removal increased with temperature (Figure 9a). However, it is the Cu-dominant stormwater (P + Cu) that experienced the greatest improvement with temperature. It is possible that increased temperatures allowed for the weakening of stable bonds formed between Cu2+ with the slag, thereby improving the loss of Fe from the slag into the solution, and consequently, resulting in greater P uptake through precipitation. In addition, at higher temperatures, P removal occurred faster, as shown in Figure 9b. Readings were taken at 0 h and 24 h, with a straight line drawn between those two points. A larger slope signified a quicker decline in solution P concentration with time.
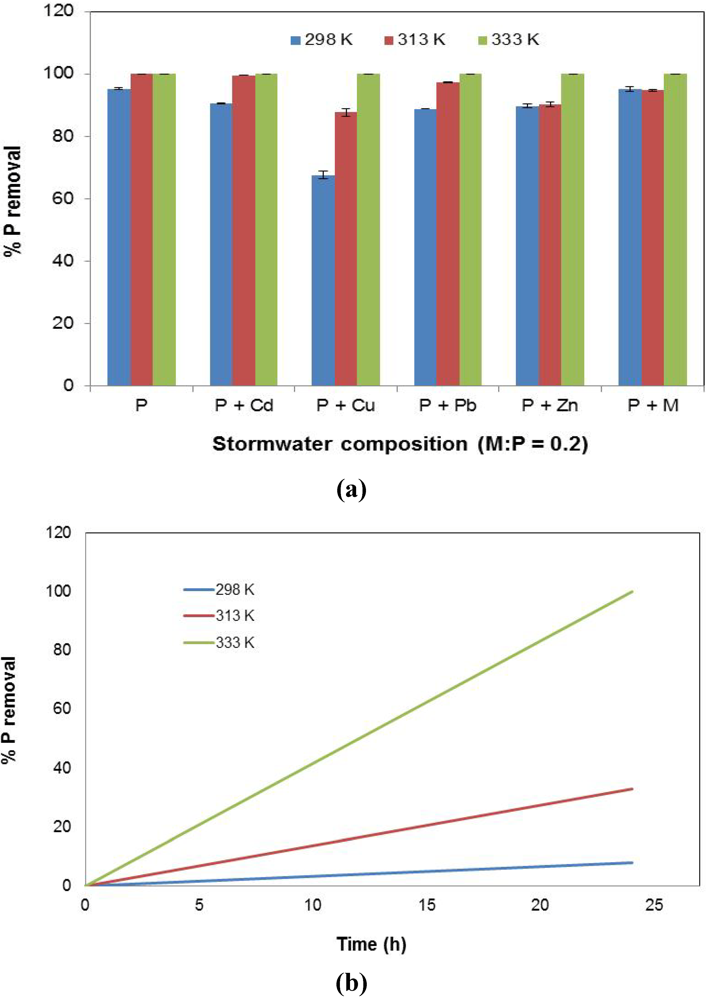
Figure 9.
The effect of temperature on % P removal. (a) P conversion (%); (b) P removal rate from stormwater type P over 24 h (% P removal/h).
Figure 9.
The effect of temperature on % P removal. (a) P conversion (%); (b) P removal rate from stormwater type P over 24 h (% P removal/h).
The distribution coefficient (KD) values at the various temperatures were also calculated. The distribution coefficient of a solute between two phases is calculated as the ratio of the concentration of the solute in one phase to its concentration in the other phase under equilibrium conditions [43]:
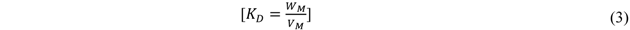
where, WM = amount of metal in adsorbent, mg P/g; VM = amount of metal in solution, mg P/cm3.
The values of KD at the different temperatures are presented in Table 4. KD increases with temperature, which means that sorption process is endothermic. ∆S and ∆H were obtained from a linear plot of ln KD versus 1/T according to the following thermodynamic correlation [43]:
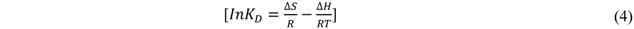
where, KD = Distribution coefficient, cm3/g; ∆S = change in entropy, kJ mol−1 K−1; ∆H = change in enthalpy, kJ mol−1; R = gas constant, kJ mol−1 K, T = absolute temperature, K.
The values of ∆H, ∆S and ∆G, the change in Gibbs free energy (kJ mol-1), are also given in Table 4. ∆G was calculated from the ensuing equation:
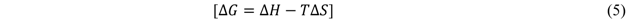

Table 4.
Thermodynamic parameters for P adsorption on EAF slag.
| C0 (mol L−1) | KD (cm3 g−1) | ∆H ( kJ mol−1) | ∆S ( kJ mol−1 K−1) | ∆G ( kJ mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 298 K | 313 K | 333K | 298 K | 313 K | 333K | |||
| 5.26 × 10−5 | 5.03× 103 | 1.34× 106 | 1.28× 107 | 182.1 | 0.69 | −22.9 | −33.2 | −47.0 |
As seen in Table 4, ∆H is positive, which supports the previous conclusion that the sorption process is endothermic. Also, the ∆G values at all three temperatures are negative, decreasing with an increase in temperature. This means that the sorption process is more spontaneous with increasing temperature, and thus occurs more easily at higher temperatures.
3.8. Statistical analysis
Each plotted point on the graphs represented the average value of three readings. The error bars reflected the standard deviation of measurements from their mean. Calculated values for relative standard deviation for all P measurements taken in the course of the study ranged from less than 1% to as much as 20% in one case.
One-way ANOVA showed that there were significant differences in the P removal results obtained across the various slag particle diameters investigated (dfB = 5, dfW = 12, F = 1548.09, p = 2.01E−16). An independent samples t-test revealed that there were significant differences in P removal (df = 4, t = 65.9, p = 4.94E−17) when slag particles in the range 0.50–0.85 mm (Mean = 1.24, SD = 9.59E−03) were used as compared with slag particles of 4.75–5.56 mm (Mean = 0.3, SD = 3.28E−02). Further post-hoc comparisons between P removal results obtained from pairs of different slag particle size ranges were carried out using Scheffé’s test. The results are summarized in Table 5. Statistical significance occurred when F > F’, where F’ is the product of dfB and the critical value of F for dfB and dfW at α = 0.05. If F < F’, then there was no statistical difference between the P removal results obtained from using the different slag particle diameters.
For the P concentration experiments, two-way ANOVA results confirmed that the main effect of stormwater composition was significant (F (5,60) = 579.496, p = 2.4E−49), as was the main effect of P concentration (F (4,60) = 79,199.920, p = 6.5E−111). The interaction effect of these two factors was also significant (F (20,60) = 176.791, p = 8.6E−46).
In the case of metal concentration experiments, the main effect of stormwater composition was significant (F (2,30) = 378.464, p = 5.2E−22), as was the main effect of metal concentration (F (4,30) = 265.673, p = 5.9E−23) and the interaction effect between the stormwater composition and the metal concentration (F (8,30) = 196.032, p = 9.8E−24).

Table 5.
Scheffé’s post-hoc comparisons of P removed using different slag particle sizes (A vs. B).
| No. | A | B | Results |
|---|---|---|---|
| 1 | 0.50–0.85 mm | 2.36–3.35 mm | Significant |
| 2 | 0.50–0.85 mm | 1.40–1.70 mm | Significant |
| 3 | 0.50–0.85 mm | 1.00–1.40 mm | Significant |
| 4 | 0.50–0.85 mm | 0.85–1.00 mm | Significant |
| 5 | 0.85–1.00 mm | 1.00–1.40 mm | Not significant |
| 6 | 0.85–1.00 mm | 1.40–1.70 mm | Not significant |
| 7 | 0.85–1.00 mm | 2.36–3.35 mm | Significant |
| 8 | 0.85–1.00 mm | 4.75–5.56 mm | Significant |
| 9 | 1.00–1.40 mm | 1.40–1.70 mm | Not significant |
| 10 | 1.00–1.40 mm | 2.36–3.35 mm | Significant |
| 11 | 1.00–1.40 mm | 4.75–5.56 mm | Significant |
For pH experiments, the main effect of stormwater composition was significant (F (3,32) = 229.009, p = 1.1E−21), as was the main effect of pH (F (3,32) = 613.512, p = 2.4E−28). The interaction effect of both factors was also significant (F (9,32) = 54.634, p = 6.8E−17).
With respect to the influence of temperature on P removal, the main effect of stormwater composition was significant (F (5,36) = 799.072, p = 8.2E−36). The main effect of temperature (F (2,36) = 2598.005, p = 1.2E−39) was also significant, as was its interaction effect with stormwater composition (F (10,36) = 360.251, p = 5.8E−33).
Experiments on the variation of slag mass at different P concentrations showed that the main effect of P concentration was significant (F (1,16) = 724.369, p = 9.4E−15), as was the main effect of slag mass (F (3,16) = 133.982, p = 1.5E−11) and the interaction effect between P concentration and slag mass (F (3,16) = 287.524, p = 4.0E−14).
From the results of the statistical analysis, it is apparent that the stormwater composition, P concentration, metal concentration, pH, temperature, slag mass and slag particle size have an influence on the extent of P removal. In addition, a combination of the stormwater composition and other environmental factors such as P concentration, metal concentration, pH or temperature affects P removal differently. Also, when considering EAF slag as a treatment system for stormwater remediation, it is also important to note that the slag surface area plays an important role in determining the efficiency of P removal. Larger slag surface areas (i.e., smaller particle sizes) will produce better treatment results. As well, P removal is influenced differently by a combination of P concentration and slag mass.
4. Research Implications
Simulated stormwater was selected to ensure standard compositions of the experimental samples, and to more accurately detect the impacts of varying environmental conditions. Prior studies have shown that P sorption onto an active slag filter was significantly higher for synthetic P solutions than that observed using authentic pond effluent [44]. Thus an important limitation of this research may be the estimation of a higher P adsorption density than would be reflected using actual stormwater. Actual stormwater would imply new complexities such as variation of quality with time and the occurrence of a variety of constituents, e.g. sediments and other chemicals besides heavy metals that could potentially interfere with P sorption. What this research achieves however, is a better understanding that has been absent until now, of how the presence of different heavy metals may affect the removal of P from solution, when EAF slag is used under a number of conditions.
It should also be noted that the use of a batch experimental system instead of a flow-through treatment system has implications that would need to be considered. Batch experiments are well suited to describing static performance conditions and providing important information relating to the potential of a selected adsorbent. However, they are limited in their ability to predict dynamic conditions and should not be extrapolated to field performance [45]. Moreover, in a flow-through system, the slag would most likely be placed as media in a filter, where it will come in contact with the stormwater over a much shorter period of time. Also, a host of issues such as development and maintenance costs, filter breakthrough and media replacement or regeneration amongst others, will require consideration.
5. Conclusions
This study highlights the importance of environmental and treatment system conditions in the removal of P from different stormwater compositions using EAF slag as an adsorbent. Results clearly demonstrate that stormwater composition, P concentration, metal concentration, pH, temperature, slag mass and slag particle size are key factors that determine the effectiveness of EAF slag in removing P from a given stormwater system. Furthermore, it has been revealed that a number of combinations of these factors influence P reduction differently. The findings of this work therefore provide important information for optimizing system performance for P removal by means of EAF slag.
An ongoing study is building on the outcome of this work as input for a full factorial experimental program developed around key environmental factors such as stormwater composition, P concentration, metal concentration and temperature. The completion of that work will provide comprehensive insight into the relative importance of the studied factors and the effect of their interactions on P removal. These findings are intended for application in flow-through experimentation, and ultimately, in the management of stormwater in urban municipalities, where an inexpensive and effective end-of-pipe add-on technology to existing stormwater treatment systems is needed to reduce soluble P currently not captured through those systems.
Acknowledgments
The authors gratefully acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and technical advice from Renata Raina and Rod Kelln, University of Regina (Chemistry and Biochemistry); Darryl Dormuth, National Research Council—Centre for Sustainable Infrastructure Research; and Jim Kells, University of Saskatchewan (Civil Engineering).
The constructive comments of the anonymous reviewers who handled our manuscript were also greatly appreciated and have without doubt, improved the quality and outcome of this work.
References
- USEPA, Nonpoint Source Pollution: The Nation’s Largest Water Quality Problem; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- USEPA, National Water Quality Inventory: 2000 Report; United States Environmental Protection Agency: Washington, DC, USA, 2002.
- BC-MoE, Water Quality—Tackling Nonpoint Source Water Pollution in British Columbia: An Action Plan; British Columbia Ministry of Environment: Victoria, BC, Canada, 1999.
- Corrales, R.A.; Maclean, J.L. Impacts of harmful algae on seafarming in the Asia-Pacific areas. J. Appl. Phycol. 1995, 7, 151–162. [Google Scholar]
- Carpenter, S.R.; Bolgrien, D.; Lathrop, R.C.; Stowe, C.A.; Reed, T.; Wilson, M.A. Ecological and economic analysis of lake eutrophication by nonpoint pollution. Aust. J. Ecol. 1998, 23, 68–79. [Google Scholar]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. R. 2003, 10, 126–139. [Google Scholar]
- Smith, V.H.; Joye, S.B.; Howarth, R.W. Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 2006, 51, 351–355. [Google Scholar]
- Roesner, L.A.; Rowney, A.C. National storm water quality regulations and standards. J. Hydraul. Res. 1996, 34, 841–856. [Google Scholar]
- Bäckström, M. Grassed swales for stormwater pollution control during rain and snowmelt. Water Sci. Technol. 2003, 48, 123–134. [Google Scholar]
- Bavor, H.J.; Davis, C.M.; Sakadevan, K. Stormwater treatment: Do constructed wetlands yield improved pollutant management performance over a detention pond system? Water Sci. Technol. 2001, 44, 565–570. [Google Scholar]
- Shammaa, Y.; Zhu, D.Z.; Gyürék, L.L.; Labatiuk, C.W. Effectiveness of dry ponds for stormwater total suspended solids removal. Can. J. Civil. Eng. 2002, 29, 316–324. [Google Scholar]
- Mallin, M.A.; Ensign, S.H.; Wheeler, T.L.; Mayes, D.B. Pollutant removal efficacy of three wet retention ponds. J. Environ. Qual. 2002, 31, 654–660. [Google Scholar]
- Browning, K.; Greenway, M. Nutrient removal and plant biomass in a subsurface flow constructed wetland in Brisbane, Australia. Water Sci. Technol. 2003, 48, 183–189. [Google Scholar]
- Aryal, R.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Urban stormwater quality and treatment. Korean J. Chem. Eng. 2010, 27, 1343–1359. [Google Scholar]
- Marsalek, J.; Larkin, G.A. Urban Drainage in Cold Climate: Challenges and Progress in Management Practices; National Water Research Institute: Fountain Valley, California, CA, USA, 1998. [Google Scholar]
- Oberts, G.L. Sustainable solutions/best management practices for urban drainage in cold climates. In Proceedings of Urban Drainage in Cold Climates, Technical Documents in Hydrology, 40, UNESCO, Paris, France, 2000; II, pp. 149–169, Chapter 6.
- Agyei, N.M.; Strydom, C.A.; Potgieter, J.H. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cement Concrete Res. 2002, 32, 1889–1897. [Google Scholar] [CrossRef]
- Xu, D.; Xu, J.; Wu, J.; Muhammad, A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 2006, 63, 344–352. [Google Scholar]
- Mortula, M.; Gagnon, G.A. Phosphorus adsorption on oven dried alum residuals solids in fixed bed column experiments. J. Environ. Eng. Sci. 2007, 6, 623–628. [Google Scholar]
- Mortula, M.; Gibbons, M.; and Gagnon, G.A. Phosphorus adsorption in naturally-occurring materials and industrial by-products. J. Environ. Eng. Sci. 2007, 6, 157–164. [Google Scholar]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.L.; Johnson, K.L. Phosphorus removal from waste waters using basic oxygen steel slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar]
- Johansson Westholm, L. The use of blast furnace slag for removal of phosphorus from wastewater in Sweden—A review. Water 2010, 2, 826–837. [Google Scholar]
- Bird, S.C.; Drizo, A. EAF steel slag filters for phosphorus removal from milk parlor effluent: The effects of solids loading, alternate feeding regimes and in-series design. Water 2010, 2, 484–499. [Google Scholar]
- Forget, C. Elimination du Phosphore Dissous des Effluents Piscicoles a L’aide de Materiaux Granulaires Reactifs. Master Thesis, Ecole de Polytechnique, Montreal, Quebec, Canada, 2001. [Google Scholar]
- Soulard, C. Criteres de Selections des Scories D’acieries Pour la Dephosphatation des Eaux Par Lits Filtrants; Ecole de Polytechnique: Montreal, Canada, 2001. [Google Scholar]
- Johansson, W.L. Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Res. 2006, 40, 23–36. [Google Scholar] [CrossRef]
- Chazarenc, F.; Filiatrault, M.; Brisson, J.; Comeau, Y. Combination of slag, limestone and sedimentary apatite in columns for phosphorus removal from sludge fish farm effluents. Water 2010, 2, 500–509. [Google Scholar]
- Weber, D.; Drizo, A.; Twohig, E.; Bird, S.; Ross, D. Upgrading constructed wetlands phosphorus reduction from a dairy effluent using EAF Steel slag filters. Water Sci. Technol. 2007, 56, 135–143. [Google Scholar]
- Drizo, A.; Cummings, J.; Weber, D.; Twohig, E.; Druschel, G.; Bourke, B. New evidence for rejuvenation of phosphorus retention capacity in EAF steel slag. Environ. Sci. Technol. 2008, 42, 6191–6197. [Google Scholar]
- USEPA, Results of the nationwide urban runoff program, Volume I—Final report; United States Environmental Protection Agency: Washington, DC, USA, 1983.
- Makepeace, D.K.; Smith, D.W.; Stanley, S.J. Urban stormwater quality: Summary of contaminant data. Crit. Rev. Env. Sci. Tec. 1995, 25, 93–139. [Google Scholar] [CrossRef]
- Okochi, N.C.; McMartin, D.W. Laboratory investigations of stormwater remediation via slag: Effects of metals on phosphorus removal. J. Hazard. Mater. 2011, 187, 250–257. [Google Scholar]
- APHA; AWWA; WEF, Standard Methods for the Examination of Water and Wastewater, 20th; Clesceri, L.S.; Eaton, A.D.; Greenberg, A.E.; Franson, M.A.H. (Eds.) American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998.
- Drizo, A.; Comeau, Y.; Forget, C.; Chapius, R.P. Phosphorus saturation potential: A parameter for estimating the longevity of constructed wetland systems. Environ. Sci. Technol. 2002, 36, 4642–4648. [Google Scholar]
- Drizo, A.; Forget, C.; Chapius, R.P.; Comeau, Y. Phosphorus removal by electric arc furnace steel slag and serpentinite. Water Res. 2006, 40, 1547–1554. [Google Scholar]
- Engström, F.; Adolfsson, D.; Yang, Q.; Samuelsson, C.; Björkman, B. Crystallization behaviour of some steelmaking slags. Process Met. 2010, 81, 362–371. [Google Scholar]
- Pratt, C.; Shilton, A.; Pratt, S.; Haverkamp, R.G.; Bolan, N.S. Phosphorus removal mechanisms in active slag filters treating waste stabilization pond effluent. Environ. Sci. Technol. 2007, 41, 3296–3301. [Google Scholar]
- Barat, R.; Montoya, T.; Borrás, L.; Ferrer, J.; Seco, A. Interactions between calcium precipitation and the polyphosphate-accumulating bacteria metabolism. Water Res. 2008, 42, 3415–3424. [Google Scholar]
- Szabó, A.; Takács, I.; Murthy, S.; Daigger, G. T.; Licskó, I.; Smith, S. Significance of design and operational variables in chemical phosphorus removal. Water Environ. Res. 2008, 80, 407–416. [Google Scholar]
- Li, C.; Ma, J.; Shen, J.; Wang, P. Removal of phosphate from secondary effluent with Fe2+ enhanced by H2O2 at nature pH/neutral pH. J. Hazard. Mater. 2009, 166, 891–896. [Google Scholar] [CrossRef]
- Pagenkopf, G.K. Introduction to Natural Water Chemistry; M. Dekker: New York, NY, USA, 1978; Volume 3, pp. 1–272. [Google Scholar]
- Sakadevan, K.; Bavor, H.J. Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Water Res. 1998, 32, 393–399. [Google Scholar]
- Oguz, E. Thermodynamic and kinetic investigations of PO43− adsorption on blast furnace slag. J. Colloid Interface Sci. 2005, 28, 62–67. [Google Scholar]
- Shilton, A.N.; Elmetri, I.; Drizo, A.; Pratt, S.; Haverkamp, R.G.; Bilby, S.C. Phosphorus removal by an ‘active’ slag filter—A decade of full scale experience. Water Res. 2006, 40, 113–118. [Google Scholar] [CrossRef]
- Benefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1981; pp. 1–528. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
