Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Biomass Collection and Culture
2.2. Biochar Synthesis and Characterization
2.3. Phosphate Quantification
2.4. Adsorption Study
3. Results and Discussion
3.1. Spectroscopic Characterization
3.2. Morphological Characterization
3.3. Study Adsorption Parameters
3.4. Results of Adsorption Study
| Biochar Source | Maximum Adsorption Capacity (mg g−1) 1 | Kinetic Model 1 | Isotherm Model 1 | Ref. |
|---|---|---|---|---|
| Peanut shell | 3.01 | PSS | Langmuir | [47] |
| Marine macroalgae | 5.22 | PSS | Freundlich | [54] |
| Fe2O3/Bamboo | 3.08 | Intraparticle | Langmuir | [55] |
| Sewage sludge | 0.7–1.2 | Not reported | Not reported | [24] |
| Pine sawdust | 2.0 | Not reported | Not reported | [35] |
| Pineapple | 3.70 | PSS | Langmuir | [56] |
| Cyanobacteria | 5.51 | PSS | Langmuir | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. State of the World’s Drinking-Water: Executive Summary; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar]
- Ruiz, Y.; Medina, L.; Borusiak, M.; Ramos, N.; Pinto, G.; Valbuena, O. Biodegradation of polyethoxylated nonylphenols. ISRN Microbiol. 2013, 2013, 284950. [Google Scholar] [CrossRef]
- Matsubae, K.; Yamasue, E.; Inazumi, T.; Webeck, E.; Miki, T.; Nagasaka, T. Innovations in steelmaking technology and hidden phosphorus flows. Sci. Total Environ. 2016, 542, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and Clinical Application of Phosphorus-Containing Drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Haffner, D.G. Plateau Lake Water Quality and Eutrophication: Status and Challenges. Water 2023, 15, 337. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Angulo, B.; Patiño, K.; Hernández, V.; Vallejo, W.; Gallego-Cartagena, E.; Romero Bohórquez, A.R.; Zarate, X.; Schott, E. Cyanobacterial Biomass as a Potential Biosorbent for the Removal of Recalcitrant Dyes from Water. Water 2021, 13, 3176. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Review of phosphate removal from water by carbonaceous sorbents. J. Environ. Manag. 2021, 287, 112245. [Google Scholar] [CrossRef]
- Nan, L.; Zhang, Y.; Liu, M.; Zhu, Y.; Zhao, L. Modification of Biochar and Its Removal Mechanism of Phosphorus. In Water Resources Management and Water Pollution Control; Environmental Science and Engineering; Springer: Cham, Switzerland, 2024; pp. 61–70. [Google Scholar]
- Moharami, S.; Jalali, M. Effect of TiO2, Al2O3, and Fe3O4 nanoparticles on phosphorus removal from aqueous solution. Environ. Prog. Sustain. Energy 2014, 33, 1209–1219. [Google Scholar] [CrossRef]
- Gao, D.; Ji, H.; Li, R.; Tajammal Munir, M.; Wu, X.; Huang, Y.; Li, B. Advancing sustainable phosphorus removal and recovery with Metal-Organic frameworks (MOFs). Chem. Eng. J. 2023, 475, 145949. [Google Scholar] [CrossRef]
- Tu, Y.J.; You, C.F.; Chang, C.K.; Chen, M.H. Application of magnetic nano-particles for phosphorus removal/recovery in aqueous solution. J. Taiwan Inst. Chem. Eng. 2015, 46, 148–154. [Google Scholar] [CrossRef]
- Liu, R.; Chi, L.; Wang, X.; Sui, Y.; Wang, Y.; Arandiyan, H. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water. J. Environ. Chem. Eng. 2018, 6, 5269–5286. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Wang, C.; Chen, Z.; Tao, Q.; Wei, W.; Cho, K.; Yuan, P.; Frost, R.L.; Ni, B.J. Comprehensive review of modified clay minerals for phosphate management and future prospects. J. Clean. Prod. 2024, 447, 141425. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.E.; Al-Shannag, M. Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2020, 19, 557–582. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Azam, R.; Kothari, R.; Singh, H.M.; Ahmad, S.; Ashokkumar, V.; Tyagi, V.V. Production of algal biomass for its biochemical profile using slaughterhouse wastewater for treatment under axenic conditions. Bioresour. Technol. 2020, 306, 123116. [Google Scholar] [CrossRef]
- Nobaharan, K.; Novair, S.B.; Lajayer, B.A.; Hullebusch, E.D. van Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Melia, P.M.; Busquets, R.; Hooda, P.S.; Cundy, A.B.; Sohi, S.P. Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars. Sci. Total Environ. 2019, 675, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: A review on challenges, commercialization, and future perspectives. Chemosphere 2022, 286, 131490. [Google Scholar] [CrossRef]
- Patiño-Camelo, K.; Diaz-Uribe, C.; Gallego-Cartagena, E.; Vallejo, W.; Martinez, V.; Quiñones, C.; Hurtado, M.; Schott, E. Cyanobacterial biomass pigments as natural sensitizer for TiO2 Thin Films. Int. J. Photoenergy 2019, 2019, 7184327. [Google Scholar] [CrossRef]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-p phosphorus. In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; American Public Health Association Press: Washington, DC, USA, 2021. [Google Scholar]
- Dijkstra, M.L.; Auer, M.T.; Kuczynski, A.; Lambert, R. Determination of bioavailable phosphorus in water samples using bioassay methods. MethodsX 2020, 7, 100807. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Efficient removal of Rhodamine B dye using biochar as an adsorbent: Study the performance, kinetics, thermodynamics, adsorption isotherms and its reusability. Chemosphere 2024, 354, 141702. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Naima, A.; Ammar, F.; Abdelkader, O.; Rachid, C.; Lynda, H.; Syafiuddin, A.; Boopathy, R. Development of a novel and efficient biochar produced from pepper stem for effective ibuprofen removal. Bioresour. Technol. 2022, 347, 126685. [Google Scholar] [CrossRef]
- Ghorbani, E.; Nowruzi, B.; Nezhadali, M.; Hekmat, A. Metal removal capability of two cyanobacterial species in autotrophic and mixotrophic mode of nutrition. BMC Microbiol. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Chang, S.X. Pyrolysis temperature and steam activation effects on sorption of phosphate on pine sawdust biochars in aqueous solutions. Chem. Pollut. Bioavailab. 2016, 28, 42–50. [Google Scholar] [CrossRef]
- Xia, S.; Liang, S.; Qin, Y.; Chen, W.; Xue, B.; Zhang, B.; Xu, G. Significant Improvement of Adsorption for Phosphate Removal by Lanthanum-Loaded Biochar. ACS Omega 2023, 8, 24853–24864. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616–617, 1242–1260. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, N.; Hu, W.; Feng, C.; Zhang, B.; Ning, Q.; Xu, B. Phosphate removal from aqueous solution by an effective clay composite material. J. Solution Chem. 2013, 42, 691–704. [Google Scholar] [CrossRef]
- Han, R.; Han, P.; Cai, Z.; Zhao, Z.; Tang, M. Kinetics and isotherms of Neutral Red adsorption on peanut husk. J. Environ. Sci. 2008, 20, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sudha, S.; Chand, S.; Srivastava, V.C. Phosphate Removal from Aqueous Solution Using Coir-Pith Activated Carbon. Sep. Sci. Technol. 2010, 45, 1463–1470. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Park, H.; Kwak, C.; Yang, J.; Kim, J.; Ryu, S.Y.; Lee, J. Role of electrostatic interactions in the adsorption of dye molecules by Ti3C2-MXenes. RSC Adv. 2021, 11, 6201–6211. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of pb(ii) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Ahn, K.H.; Ok, Y.S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2020, 18, 3273–3294. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.; Wu, Z.; Xu, F.; Yang, D.; He, Q.; Li, G.; Chen, Y. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresour. Technol. 2019, 289, 121600. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Ortiz, J.; Duran, F.; Vallejo, W.; Fals, J. Methyl Orange Adsorption on Biochar Obtained from Prosopis juliflora Waste: Thermodynamic and Kinetic Study. ChemEngineering 2023, 7, 114. [Google Scholar] [CrossRef]
- He, D.; Ma, H.; Hu, D.; Wang, X.; Dong, Z.; Zhu, B. Biochar for sustainable agriculture: Improved soil carbon storage and reduced emissions on cropland. J. Environ. Manag. 2024, 371, 123147. [Google Scholar] [CrossRef]
- Chen, Y.; Van Zwieten, L.; Xiao, K.; Liang, C.; Ren, J.; Zhang, A.; Li, Y.; Dong, H.; Sun, K. Biochar as a green solution to drive the soil carbon pump. Carbon Res. 2024, 3, 44. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, C.P.; Zhu, Y.; Wei, W.; Qin, H. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water. J. Water Process Eng. 2018, 25, 96–104. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef] [PubMed]
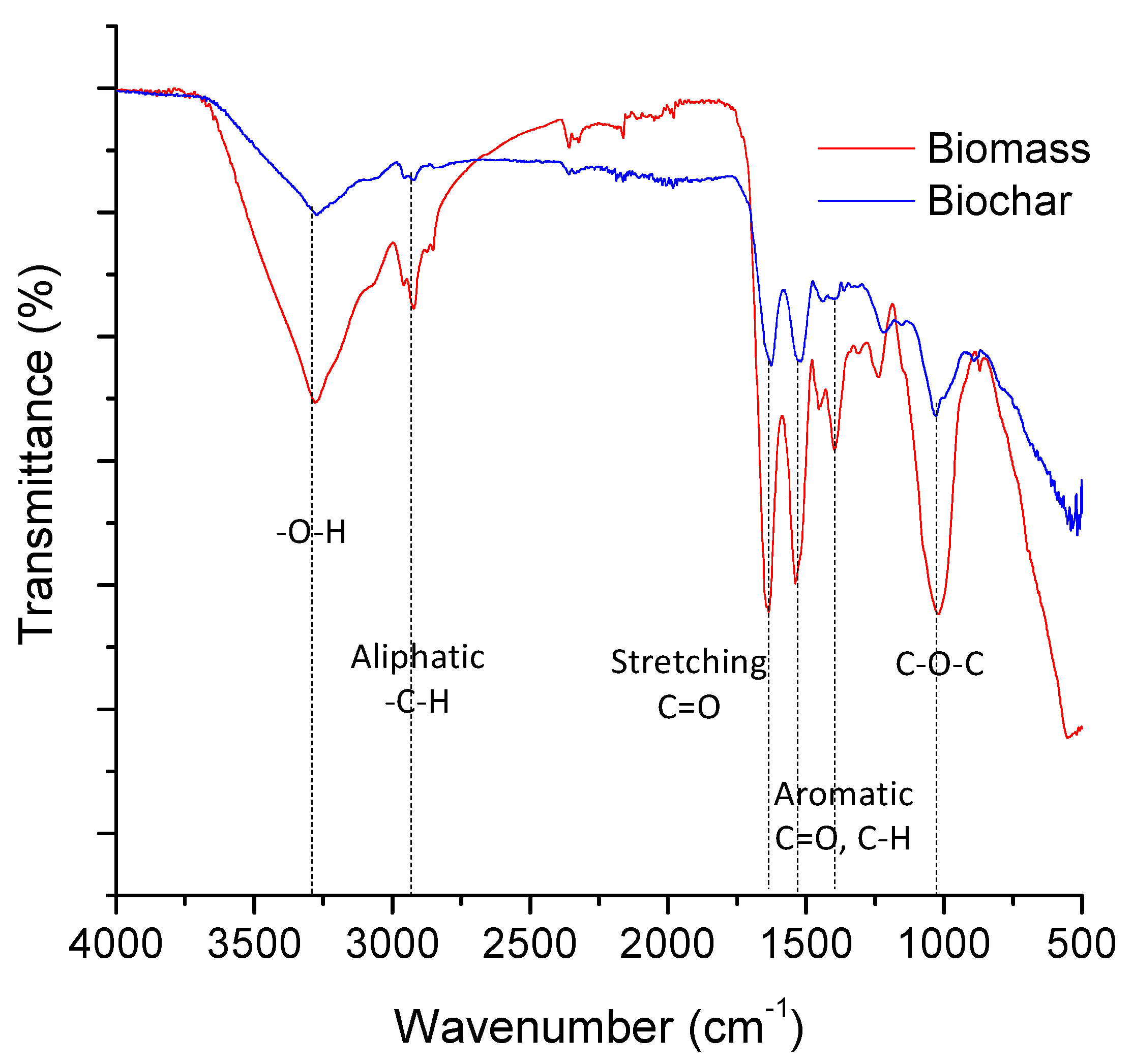
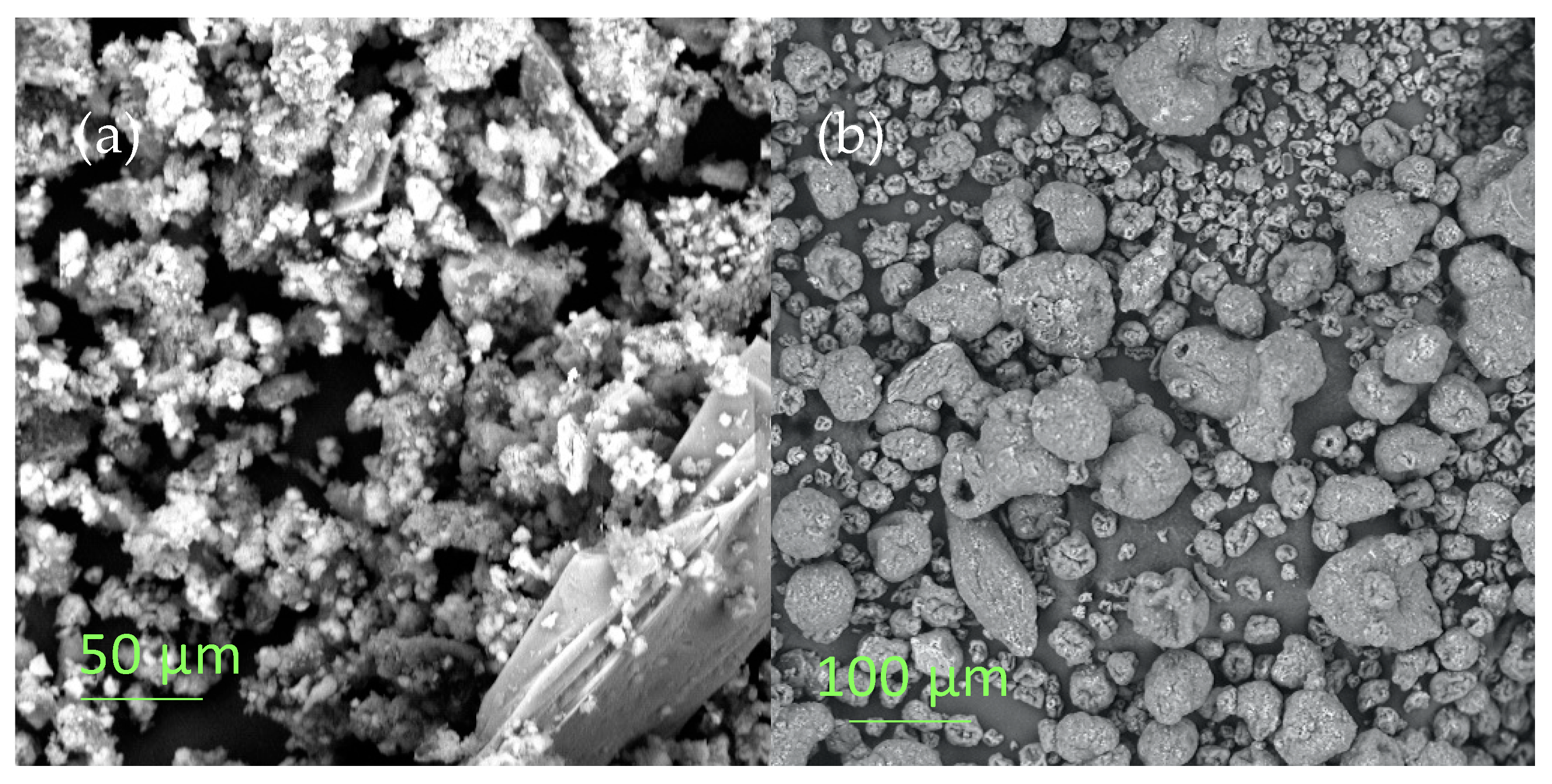
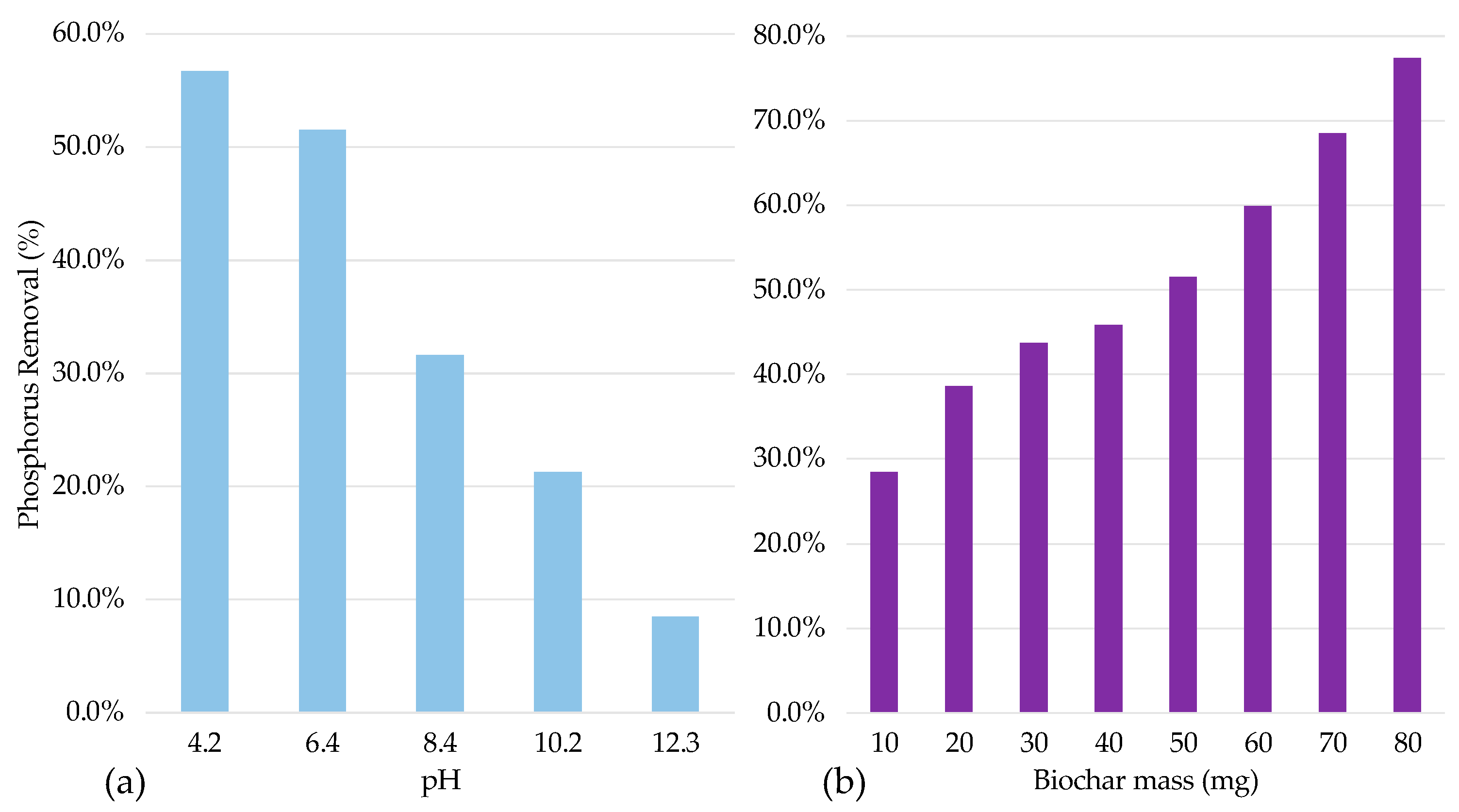
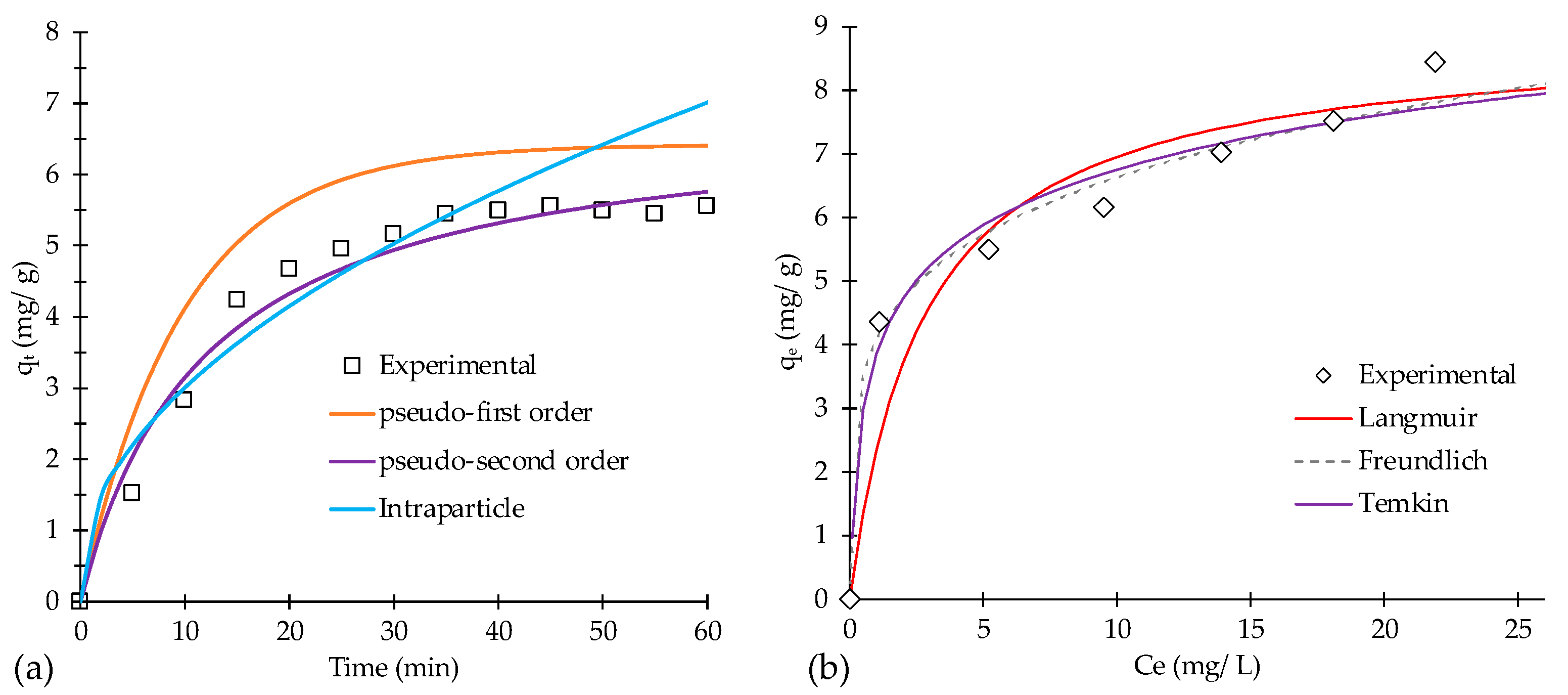
| Sample | C (%) 1 | O (%) 1 |
|---|---|---|
| Biomass | 5.0 | 53.6 |
| Biochar | 53.70 | 21.50 |
| Model | Parameters 1 | ||
|---|---|---|---|
| Pseudo-first-order | qe (mg g−1) | k1 (min−1) | R2 |
| 6.49 | 0.103 | 0.892 | |
| Pseudo-second-order | qe (mg g−1) | k2 (g mg−1 min−1) | R2 |
| 5.51 | 0.084 | 0.977 | |
| Intraparticle | C (mg g−1) | kid (gmg−1 min−1) | R2 |
| 0.244 | 0.874 | 0.892 | |
| Model | Parameters 1 | ||
|---|---|---|---|
| Langmuir | qmax (mg g−1) | KL (L mg−1) | R2 |
| 8.89 | 0.360 | 0.973 | |
| Freundlich | KF (mg g−1) (L mg−1)1/n | 1/n | R2 |
| 4.09 | 0.209 | 0.949 | |
| Temkin | Bt (L mg−1) | KT (L g−1) | R2 |
| 3.86 | 21.63 | 0.895 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Uribe, C.; Monterrosa, F.; Simons, V.; Duran, F.; Florian, V.; Vallejo, W.; Castellanos, K.; Diosa, J.E.; Mosquera-Vargas, E. Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass. Water 2025, 17, 1287. https://doi.org/10.3390/w17091287
Diaz-Uribe C, Monterrosa F, Simons V, Duran F, Florian V, Vallejo W, Castellanos K, Diosa JE, Mosquera-Vargas E. Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass. Water. 2025; 17(9):1287. https://doi.org/10.3390/w17091287
Chicago/Turabian StyleDiaz-Uribe, Carlos, Flor Monterrosa, Vanessa Simons, Freider Duran, Vicente Florian, William Vallejo, Karina Castellanos, Jesús E. Diosa, and Edgar Mosquera-Vargas. 2025. "Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass" Water 17, no. 9: 1287. https://doi.org/10.3390/w17091287
APA StyleDiaz-Uribe, C., Monterrosa, F., Simons, V., Duran, F., Florian, V., Vallejo, W., Castellanos, K., Diosa, J. E., & Mosquera-Vargas, E. (2025). Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass. Water, 17(9), 1287. https://doi.org/10.3390/w17091287







