1. Introduction
In the Earth’s crust, manganese is an evenly distributed element found with iron ore. It is the twelfth most common element when ranked among those present in the lithosphere (about 0.2% weight). Manganese is most often present in metamorphic sediments, soil and some parts of dead plants. It is among the macronutrients that together with cobalt, copper and molybdenum are required by most organisms.
Manganese enters water from manganese ore via leaching. The most frequent manganese ores are burel or pyrolusite (MnO2), braunite (Mn2O3), hausmannite (MnO·Mn2O3 or Mn3O4), manganite (MnO(OH)), and dialogite (MnCO3). Manganese oxide and manganese carbonate (rhodochrosite) minerals are the most important Mn ore resources globally. A problem in mining activities (e.g., pyrite mining) is the creation of acidic conditions during mining that solubilize Fe and Mn into the environment.
An increase in the manganese concentration in underground water may occur at the temporal elevation of the underground water level. In such a period, sulfur ores are oxidized by atmospheric oxygen while sulfuric acid is formed, and manganese is leaked. Manganese occurs in the surface waters of water reservoirs in the summer due to stratification (temperature zonation) and the release of Mn from sediments. Most often manganese is present in natural water at a level below 1.0 mg/L. A higher occurrence can be seen in water with humic substances, while high concentrations of Mn can only be found in acidic mining waters [
1,
2,
3].
Manganese (Mn) is an essential nutrient for human life, and numerous enzymes utilize the redox properties of this element. However, high levels of Mn in the water supply stain porcelain and cause an undesirable taste in beverages. Manganese is also toxic to humans when present in excessive concentrations in water. Emerging health studies have shown that its half-life in bones is about 8–9 years, once Mn is absorbed by humans. Even worse, Mn can be transported directly to the brain, leading to nerve toxicity. A disease called manganism causes anxiety, ataxia and dementia [
4].
Given the adverse effects of Mn on human health, the World Health Organization in 2004 suggested a guidance value of 0.2 mg/L in freshwater. Many countries have also set limits for Mn concentration in water bodies. For example, the European Commission and the United States Environmental Protection Agency have set the Mn level in drinking water at 0.05 mg/L [
5,
6].
1.1. Forms of Mn Presence in Water
Manganese in water can be found either in soluble form as Mn(II) or in the more insoluble forms Mn(III) and Mn(IV). It can also be found in colloidal form (bound to humic substances). The form of its presence depends on the concentration of oxygen, solubility of manganese substances in water, pH, redox potential, hydrolysis, presence of complexing inorganic and organic compounds, water temperature and the composition of the water itself (e.g., the carbon dioxide content) [
7,
8].
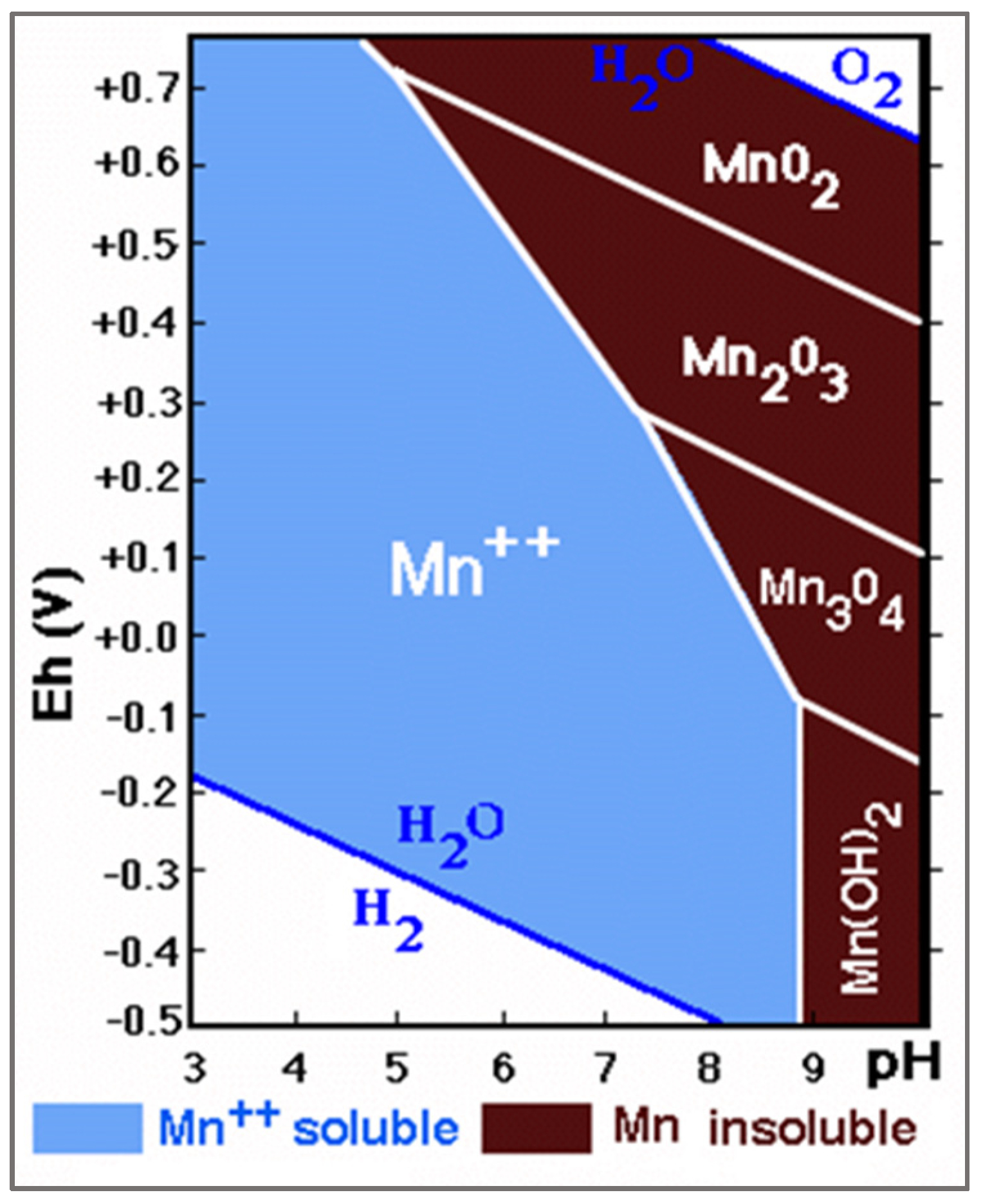
Various graphical representations of the dependence of pH and oxidation-reduction potential (ORP) for substances present in water can be found in the literature.
Figure 1 summarizes the presence of various forms of Mn in relation to the ORP (Eh) and pH of water [
9].
1.2. Mechanism of the Oxidation of Mn
Manganese in the oxidation state of Mn(II) in waters containing dissolved oxygen under certain conditions is unstable, however the oxidation of Mn to form MnO2 is very slow (half-life of 200–300 days). Oxidation using oxygen only reaches an appreciable rate when the pH rises above 8.0. Mn2+ oxidation using oxygen depends on temperature, alkalinity, water composition and solubility of Mn(II).
In alkaline conditions manganese is rapidly oxidized and hydrolyzed to form the less soluble oxides of manganese in the higher oxidation state Mn(IV):
Since the reaction produces H+ ions, the oxidation is started in an alkaline medium.
Alkalinity and pH have a marked effect on the solubility of Mn(II). This solubility is governed by the formation of manganese carbonate. Manganese hydroxide has a much higher solubility. At pH values of 8 and higher, the calculated solubility of Mn(II) is very limited (1–2 mg/L or lower) even at low alkalinity (1.2 mmol/L) [
10].
The mechanism of the oxidation of Mn(II) in an actual rock environment is complicated. This is a set of the interconnected processes of oxidation, catalysis, sorption, ion exchange, and biological oxidation. The composition of the final products of oxidation, which is partially secreted in a colloidal form, depends on factors such as the pH, temperature, oxidation-reduction potential, reaction time, and rocks. The general scheme of Mn
2+ oxidation by oxygen dissolved in water can be represented as follows:
The relationship between iron and manganese under increasing pH and redox potential (pE) suggests that ferrous iron (Fe
2+) normally occurs in the area with lower redox potential (<200 mV) and within the pH range of 5.5 to 8.2. This also means that Fe
2+ is more easily and rapidly oxidized than Mn
2+. The latter often occurs with Fe
3+ under pH values larger than 8 and redox potential between 420 to 790 millivolts. Above this redox potential, the stable form of MnO
2 is found [
11].
Chemical oxidation is a convenient method to separate manganese from water by transforming soluble Mn(II) into insoluble Mn oxides, which can be easily removed by subsequent sedimentation and filtration. To enhance the removal of Mn(II), some strong oxidants such as chlorine (HOCl), chlorine dioxide (ClO
2), ozone (O
3), ferrate (Fe(VI)), and permanganate (Mn(VII)) are introduced into water treatment [
12,
13].
The chemical reactions of oxidation of dissolved manganese in water with the oxidizing agent KMnO
4 or ClO
2 can be written as follows:
Although ClO
2 and O
3 are efficient for the oxidation of Mn(II) with half-lives of a few seconds, relatively complex onside generation was required for ClO
2 and O
3 production, and regulated byproducts (e.g., toxic chlorate, chlorite and bromate ions) may be generated. Mn(II) could be rapidly oxidized by Fe(VI) and Mn(VII). However, overdosing on colored Fe(VI) and Mn(VII) may lead to their entrance into the distribution system, causing an aesthetic problem [
12].
1.3. Effect on Water Quality and Distribution System
In concentrations found in natural waters, manganese is harmless to health. However, it significantly affects the sensory properties of water, similarly to iron. The taste of water can be adversely affected even at concentrations above 0.1 mg/L. Materials in contact with water, unlike iron, can be colored even at lower concentrations. This certainly also applies to the occurrence of Mn bacteria in water. Therefore, the manganese content in drinking water and in water used in various industrial sectors is strictly limited, as it is more harmful than iron. Decree No. 91/2023 Coll. prescribes a maximum concentration of 0.05 mg/L for manganese in drinking water and a recommended value of 0.3 mg/L for surface water (Government Decree No. 269/2010 Coll. establishing requirements for achieving good water status).
The negative impact of Mn in higher concentrations in drinking water can be summarized in a few points [
14,
15]:
(a) Ions of manganese are oxidized chemically in drinking water distribution systems into their higher states, causing suspensions of hydroxides to be formed that cause unacceptable turbidity of water; furthermore, the water’s color turns brownish-black, which can lead to customer complaints.
(b) Manganese bacteria can be present in distribution networks, such that the water quality changes (e.g., in terms of odor) and pipe clogging can occur.
(c) When the ions of manganese reach the customer, oxidation and manganese secretion occur in places providing conditions suitable for the process (e.g., in boilers and washing machines). Thus, manganese can have a negative impact on customer economy when the functionality of such equipment is not optimal.
(d) Manganese and insoluble particles of manganese can cause fittings to become clogged, can settle on surfaces, can form coatings on filtration materials, and can dye laundry.
It follows that higher concentrations of manganese in the water source are a cause of technological problems and failures in the operation of distribution systems and of deterioration of water quality if the water is slightly over-oxygenated; in particular, adverse incrustations that reduce the flow profile of the pipelines can form [
14].
The values for Mn and MnO
2 present in the distribution system (kg/year) for various flows are listed in
Table 1 alongside the limit concentration of Mn in drinking water (0.05 mg/L Mn) and for the value 0.151 mg/L as the average concentration found in the well in the locality of Jelka during the experiments which are described in the next chapter.
1.4. Presence of Manganese in Ground Water in Slovakia
According to the Report on the Status of the Environment in the Slovak Republic from 2022, 1333 samples of ground water from 703 monitoring objects exceeded the iron limit of 0.2 mg/L in more than 29.1% of analyzed samples, while the manganese limit of 0.05 mg/L in drinking water was exceeded in more than 41.6% of ground water samples. In 2023, 1309 samples of ground water within the basic monitoring program exceeded the limits, with 369 analyzed samples (28.2%) exceeding the limit for iron, while the manganese limit was exceeded in 537 analyzed samples (41.0%). Limits are listed by the Decree of the Ministry of Health of the Slovak Republic No. 91/2023 Col., which sets the parameters and limit values for drinking water and the quality parameters of warm water, the procedure for monitoring drinking water, risk management for the distribution system and distribution systems in households. The presence of Mn in ground water in Slovakia is shown in
Figure 2.
1.5. Jelka’s Water Source
Jelka’s Water source is located in the north-east of the protected water management area known as Žitný ostrov. It is a source for the group distribution system comprising Jelka–Galanta–Nitra. It consists of seven wide-diameter drilled wells marked HJ-1 up to HJ-7 with a recommended summary water offtake of 720 L/s and a well depth of 40 to 65 m. Ground water is moderately mineralized and disinfected using chlorine dioxide.
The manganese concentration after water from all wells is mixed is below 0.05 mg/L on a long-term horizon. However, the average manganese concentration in HJ-6 is 0.151 mg/L. The amount of water taken from this well is 80 L/s, representing a mass flow of manganese of 382 kg per year. Manganese oxidizes in the pipelines and, little by little, is excluded from water in pipelines or accumulation tanks, where the annual mass of MnO2 sedimentation per year reaches up to 604 kg per year. This significant amount of sedimented MnO2 causes long-term problems, especially when the water intakes are sudden and sediments are excluded from the drinking water. At the same time, they degrade the sensory characteristics of water and can cause clogging of armatures.
1.6. Methods of Removing Mn from Water
The removal of manganese from drinking water sources is a long-known and quite common requirement for water treatment facilities. Various technologies are used to remove manganese from water according to the water source quality, the concentration of Mn in raw water, and the required concentration of Mn in water after treatment.
Manganese is removed using physical, chemical, and biological processes or a combination of these methods. To remove manganese from ground and surface water, the following methods can be used [
15,
16,
17,
18,
19,
20]:
Oxidation via aeration;
Various oxidizing agents (O2, Cl2, O3, and KMnO4);
Alkalization using lime;
Chemical precipitation;
Contact filtration;
Ion exchange;
Adsorption;
Membrane processes;
Electrochemical treatment;
Biological filtration;
The in situ method.
The principle in most of these methods is that the dissolved manganese is initially present in oxidation state II and is transformed into the insoluble compound MnO
2 or Mn(OH)
2. It is then possible to remove these particles via one- or two-step separation. The oxidation rate and hydrolysis of manganese are performed under strict conditions related to water properties (pH, ORP, water temperature, and the concentration of Mn and Fe, organic carbon, humic acid, and alkalinity) and the type of removal equipment [
4,
14,
17].
The oxidation of Mn(II) using atmospheric oxygen, chlorine, chlorine dioxide, and ozone is most common, where the reaction time of oxidation and the oxidation agent dose are crucial. The required theoretical doses of the oxidation agents are presented in
Table 2. Eliminating manganese using ion exchangers and membrane separation is much rarer management praxis. As a single step separation with previous aeration, and slow or contact filtration can be used to remove manganese in lower contents from water [
14,
18].
Iron and manganese complexed with other substances, such as humic acid, may require higher oxidant amounts and longer contact times to destroy the complexes and complete the oxidation and (iron or manganese) precipitation processes. Water quality characteristics, such as pH, temperature, concentrations of other oxidant-demanding substances, and the solubility of the oxidizing agent in the water all impact the practical amounts of the oxidizing agents necessary to achieve effective oxidation [
7,
21].
Table 2.
Stoichiometric amounts of oxidizing agents required for the oxidation of 1 mg of soluble Fe and Mn [
22,
23].
Table 2.
Stoichiometric amounts of oxidizing agents required for the oxidation of 1 mg of soluble Fe and Mn [
22,
23].
| Oxidation Agent | Dose Necessary for Oxidation 1 mg [mg] |
|---|
| Fe | Mn |
|---|
| Oxygen O2 | 0.14 | 0.29 |
| Hydrogen peroxide H2O2 | 0.32 | 0.62 |
| Ozone O3 | 0.43 | 0.88 |
| Chlorine Cl2 | 0.62 | 1.27 |
| Potassium permanganate KMnO4 | 0.94 | 1.92 |
| Chlorine dioxide ClO2 | 1.21 | 2.45 |
Although the physical and chemical removal processes have been studied for decades there are still some knowledge gaps. The finding of adverse byproducts when certain oxidants are used in water treatment impacts the physicochemical methods of manganese removal. Our understanding of the microorganisms present in the systems used for the biological removal of Mn has increased over the last decade as the designed methods have become more sophisticated, leading to the use of bio-filtration in manganese removal. The removal method needs to be integrated into the complete design and operation of equipment for water treatment [
4,
14].
Commonly used adsorbents for Mn removal include activated carbon, zeolites, kaolinite clay, nanoparticles, polymers, and a wide range of natural and artificial solids, agricultural and industrial wastes, and bio-sorbents [
4,
17].
In our pilot plant experiment, we aimed to remove manganese from drinking water by combining of manganese pre-oxidation using selected oxidation agents (ClO2, and KMnO4) and separation of formed particles MnO2 with ultrafiltration (UF). The efficiency of manganese removal from water was monitored at different retention (oxidation) times and flow rates.
The most suitable type of membrane filtration for this technology is ultrafiltration. In comparison with contact filtration (material with a layer of MnO
2 on the surface; e.g., Greensand, Birm, Pyrolusite, Pyrolox, Cullsorb M, and Klinopur-Mn), UF comes with a significantly lower sensitivity for Mn concentration changes in raw water, which is one of the advantages of using this method. When the concentration of Mn changes, a simple change in the dose of oxidation agent ensures the required treatment efficiency [
14].
The major issue in using membrane technology is fouling due to the deposition of particles on the membrane sur-face or within the pores. The accumulation of particles and microorganisms can clog the membrane’s pores, dimin-ishing its effectiveness. This leads to increased pressure requirements, higher energy consumption, more frequent cleaning, and the need for membrane replacement, thereby inflating operational costs and reducing the membrane’s lifespan. Fouling is not merely a decline in performance; it is a complex problem that intertwines with the chemi-cal and physical properties of the materials involved.
The disadvantages of membranes can be their fragility, mechanical and chemical resistance, high capital and operating costs. Therefore, a balanced approach to adopting this technology is indispensable, weighing up its advantages and disadvantages.
2. Membrane Methods for Manganese Removal
It has been demonstrated that ultrafiltration technology has great potential in various water treatment areas due to its characteristics of low energy demand, simple construction, small built-up areas, easy operation and automation, and so on. It is also possible to use it to remove manganese from water.
For ground water treatment at high concentrations of Fe
2+ and Mn
2+ and low temperatures, the process of a gravity-driven membrane (GDM) was implemented with two layers—a biological membrane and an ultrafiltration (UF) membrane. The results show that flow stabilization was observed during the long-term GDM filtration with the average stabilized flow within the range of 3.6–5.7 L·m
−2·h
−1. Process GDM created an efficient removal of Fe
2+ and Mn
2+ with an efficiency of 95%. Adding manganese oxides (MnOx) could shorten the time needed to incorporate the biolayer from 50 to 30 days while contributing to the removal of Mn
2+ and improvement in the flow. The presence of Mn
2+ in treated water made the formation of the heterogeneous structure of the biofilter layer easier. The biological layer contributed the most to the removal of Mn
2+, while the UF membrane’s contribution was secondary. Furthermore, the autocatalytic chemical oxidation derived from MnOx particles plays a crucial role [
24].
In [
25], the removal of iron and manganese in different concentrations from lake water using doses of chlorine was examined using various ultrafiltration systems (UF) joined with the in-line step of pre-chlorination. For feeding water containing 1.0 mg/L of iron and/or 0.5 mg/L of manganese, significant removal of iron was achieved without the need for chlorine addition due to the oxidation of Fe
2+ to Fe
3+ via dissolved oxygen and subsequent formation of the less soluble particles of ferric hydroxide. In the absence of chlorine, a negligible removal of manganese occurred. However, with the chlorine dose, the removal efficiency increased significantly. It reached over 80% (corresponding to the concentration of 0.1 mg/L of manganese in treated water) when the dose of chlorine as Cl
2 was 3 mg/L. With a higher dose (5 mg/L of Cl
2), no significant increase in metal ion removal occurred, but serious membrane contamination did occur. The removal efficiency of turbidity and natural organic mass (NOM) increased, as the oxides of metals formed by chlorination could have acted as adsorbents.
The low-pressure ultrafiltration membrane (LPM) is an ideal technology for decentralized water supply in remote areas. It is applicable in the removal of dissolved iron (Fe
2+), manganese (Mn
2+) and ammonia (NH
4+). In [
26], the LPM membrane was compared with an MnO
2 pre-loaded membrane (Mn-LPM). The Mn-LPM membrane allowed higher water flux and, at the same time, increased the removal efficiency of Fe
2+, Mn
2+, and NH
4+. The removal efficiency of Mn
2+ immediately reached 99.6% on the first day of treatment. The MnO
2 preload also increased the capacity of NH
4+ removal. The results indicate that NH
4+ led to the bio-layer growing thicker, corresponding to the contaminant removal efficiency and the decrease in stabilized flow.
The content of humic substances (HS) can have a significant impact on the removal efficiency of Fe and Mn from water. In [
27], oxidation with subsequent separation of particles via ultrafiltration and nanofiltration in the presence of a greater humic substance concentration in water was used. This study focused on the performance of ultrafiltration (UF) and nanofiltration (NF) preceded by coagulation, flocculation and sedimentation processes to remove iron, manganese, and HS. At the same time, two different water sources with a high humic substance content were used (expressed via dissolved organic carbon and UV absorbance at 254 nm). Coagulation, flocculation, and sedimentation, in combination with UF/NF, efficiently removed the dissolved iron, HS, color, and turbidity. The results of dissolved iron were lower than 0.01 mg/L for both water sources. The described water treatment system was not sufficient (less than 50%) in removing dissolved manganese, and a significant decrease in the HS concentration was reached (approx. 80% after the UF > 90% after NF), measured as DOC. It was shown that classic water treatment effectively removes the highly molecular HS, which minimizes membrane clogging.
Iron and manganese removal from ground water using potassium permanganate (PP), sedimentation, and membrane filtration are described in [
28]. The obtained results show that PP provides a good result in terms of iron and manganese removal. Using a half-dose of the theoretical dose of PP, up to 100% of Fe and 90% of Mn were removed at different tested concentrations at pH = 7.0. Increasing the filtration ratio has an impact on the process of Mn
2+ removal. Sedimentation is needed if the iron and manganese concentrations are higher than 5.0 mg/L such that fast clogging of the filter can be reduced.
The effect of pH on iron and manganese removal from model water was examined in [
29] on the basis of permeate quality and performance of membranes. In this study, two commercially available polyamide nanofiltration and ultrafiltration membranes (PA-NF and PA-UF) were tested alongside their ability to treat modeled ground water. The experimental results show that a pH in the range of 3–11 significantly increased the performance of the membranes in terms of Fe and Mn removal.
In [
30], the long-term removal of dissolved Mn via membrane filtration using sodium permanganate (NaMnO
4) as an oxidant in drinking water treatment was evaluated. Pre-oxidation with 0.67 mol/L NaMnO
4 for 10 min removed 91% of dissolved Mn from raw water. In addition, increasing the pH improved the removal efficiency, as did changing the temperature (83% to 89% for 5.1 to 25 °C). The additional use of aluminum chloride as a coagulant helped to remove dissolved Mn and organic matter from water. The use of NaMnO
4 played a significant role in the removal of dissolved Mn based on membrane filters using both pressure and submerged membrane filters.
The rate of decrease in manganese oxides and organic substances from water using methods of pre-oxidation and coagulation/sedimentation with various chemicals has been presented in [
31]. The efficiency of NaOCl in organic mass removal was approximately 11%, while for manganese it was 12%. The rate of organic compound reduction was just 7% when chlorine dioxide was used, and in the case of manganese reduction it was 29%. Potassium permanganate (KMnO
4) removed the organic mass and Mn more efficiently, with a rate ranging from 18 to 71%.
In [
32], research was performed on manganese compound removal from ground water with a high manganese content (5 mg/L of chemical oxygen consumption of manganese); the study was performed on ODM-2F sorbent filters with a fraction of 0.7–1.5 mm.
It was found that conventional technologies with oxidation and subsequent filtration are not capable of reaching a level below 0.1 mg/L. Innovative technology for manganese removal based on oxidation with continual dosing of catalytic reaction agents has been designed. This technology efficiently alkalizes up to a pH of 8.8–9.0. Potassium permanganate was used as the catalytic agent, sodium hypochlorite was used as the oxidation agent, and sodium hydroxide was used as the alkalizing agent.
Membrane filtration after pre-chlorination removed 70% of the iron and manganese. The oxidizing agents used were air, NaClO, ClO
2, and KMnO
4. The concentrations of iron and manganese decreased by more than 90% after oxidation with KMnO
4, and, after membrane filtration, the values decreased below the detection limit. In terms of economy (based on the investment and operational costs after 15 years of depreciation) of a fast sand filtration system and membrane filtration system with low capacity (300 m
3/day) and moderate capacity (10.000 m
3/d), the total costs of the membrane filtration system were almost the same as those of the fast sand filtration system with both capacities [
33].
3. Materials and Methods
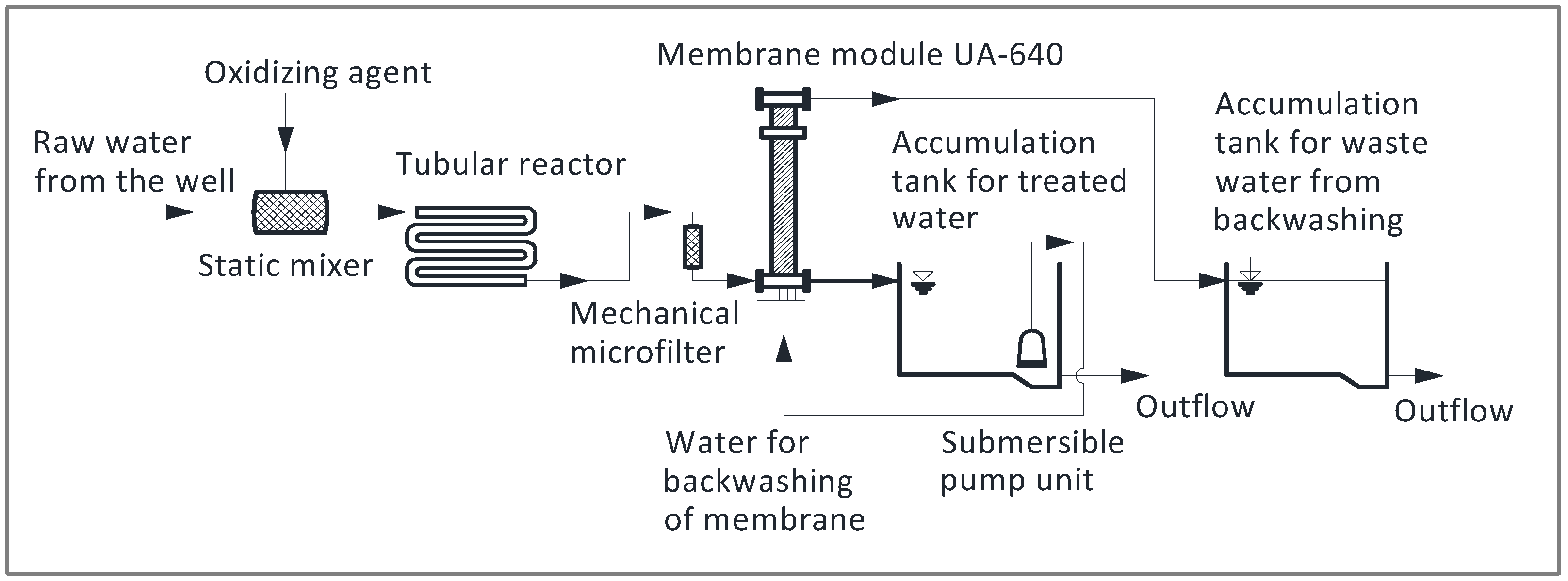
The equipment used for the pilot plant experiment (
Figure 3) consisted of the following devices: (1) a dosing pump to dose the oxidizing agent, which was injected into the pipeline at the entry point to (2) a static mixer to homogenize the oxidizing agent with raw water. The next step represented the (3) tubular reaction engaged to reach the retention time sufficient for manganese oxidation. (4) Before the water entered ultrafiltration, a small 50 µm mechanical filter was used to protect the UF from clogging the membrane module with small sand particles, as well as particles of MnO
2. The last water treatment step represented (5) ultrafiltration as the separation step to remove the oxidized manganese. Water after treatment accumulated in the (6) tank with a pump unit for backwashing the membrane module. Backwashing was carried out using the previously mentioned pump unit. (7) Wastewater from the backwashing of the membrane accumulated in the second tank and could be treated further or discharged to the sewer.
Fully automated ultrafiltration equipment was applied to remove manganese from drinking water. The equipment consisted of a membrane module UA-640 (Microdyn-Nadir, Wiesbaden, Germany) with a regulation system, measurement of trans-membrane pressure, and backwashing of the membrane using water and air with chemical regeneration. The automat switched between filtration and backwash at regular intervals. Basic data on the membrane module are listed in
Table 3.
In removing manganese from water, it is necessary to transform the manganese from its insoluble to soluble form. We used a 10% solution of chlorine dioxide or 4% of potassium permanganate for oxidation. To increase the oxidation and formation of insoluble particles of MnO2, we used a tubular reactor.
The tubular reactor was equipped with a static mixer to inject the chemicals. The static mixer was designed to allow for the intense mixing of water with chemicals, and the diameter of the reactor was DN25. The PVC tubes became wider up to DN70 and were longer than 6.0 m. In this way, the volume of the tubular reactor of 30 L (approx.) was reached. At the flow rate of 1.3 m3/h, a retention time above 1 min was ensured, and slightly turbulent conditions impacted colloidal particles that agglomerated to adequate flocks. The tubular reactor was fixed to a stainless steel construction.
A Hach DR 2800 spectrophotometer with reagents from Hach (Loveland, CO, USA) was used to analyze the monitored parameters (Mn, Cl2, and ClO2). At the same time, the following parameters were monitored: pH, conductivity, turbidity and color.
To determine the manganese content in water, Hach method 8149 and a DR 2800 spectrophotometer were used. This PAN method was in the concentration range of 0.006 to 0.700 mg/L Mn (LR). For the analysis of ClO2, we used method 8109 and DPD powder reagents for free and residual chlorine, measuring the range of 0.02–2.00 mg/L. Mn and ClO2 analysis was performed immediately after sampling.
The chemical composition and microstructure were determined using an SU 3500 Hitachi Scanning Electron Microscope (Hitachi, Tokyo, Japan).
4. Results and Discussion
Figure 4 illustrates the manganese concentration changes in raw water during the pilot plant experiments.
Table 4 summarizes the results related to using ClO
2 as the oxidizing agent.
At a flow of 60 L/h and detention time of about 30 min the efficiency of manganese removal from water reached 74.31%, and the manganese concentration in water after treatment was 0.037 mg/L. We also decided to use chlorine dioxide, which is used at the Jelka WTP (ProMinent GmbH, Heidelberg, Germany) to disinfect drinking water.
A high water detention time in a tubular reactor was necessary to reach the manganese limit in treated water as the reaction time was high. The ClO2 dose corresponds to the limit value of ClO2 for drinking water; therefore, we tested its concentration in the range of 0.2 to 0.4 mg/L. Residual chlorine was determined at the end of the technological line in samples at the outlet of the ultrafiltration system (i.e., in treated water).
Table 5 presents the results of the experiments with the doses of the oxidation agent KMnO
4 at different flow rates (from 400 up to 600 L/h). At the same time, ultrafiltration was used, and the retention time in the tubular reactor ranged from 3 to 9 min. Mn input represents the concentration Mn in raw water, Mn + KMnO
4 represents the actual concentration of Mn after the addition of KMnO
4 and oxidation in the tubular reactor before entering the ultrafiltration module, and Mn output is the concentration in treated water after ultrafiltration.
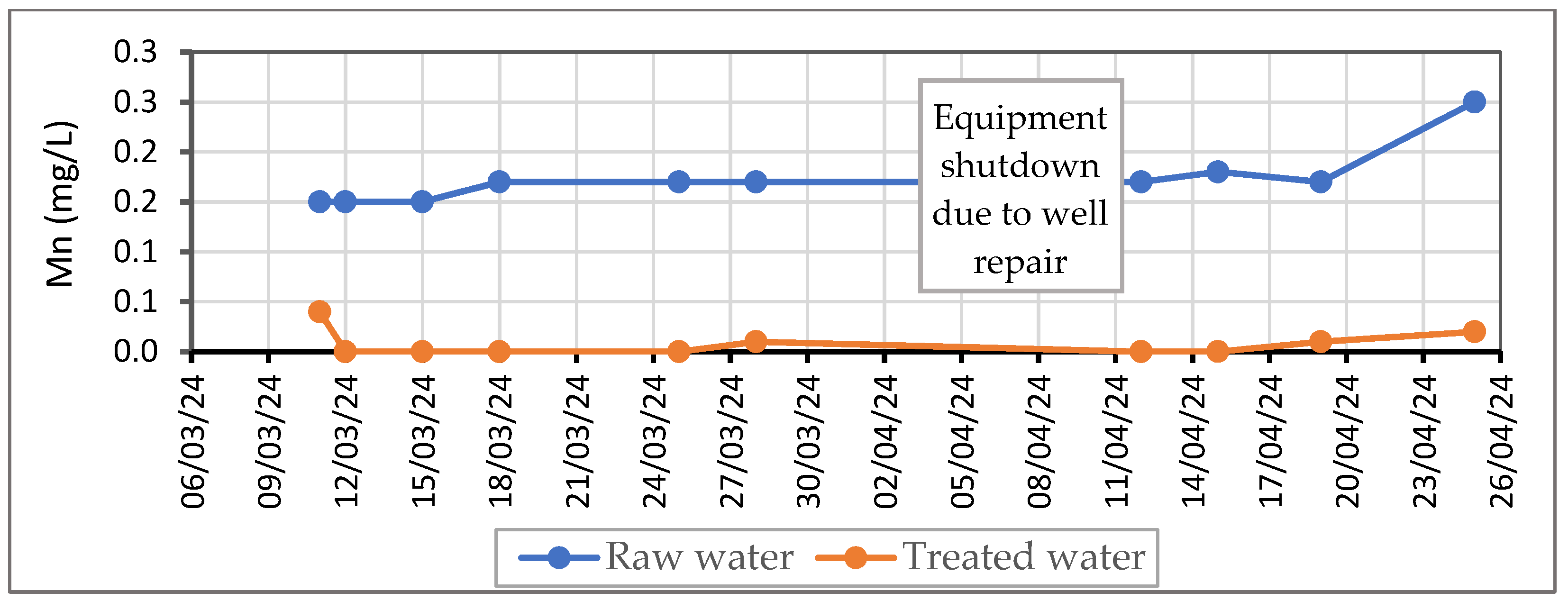
Table 6 consists of test results with the dose of oxidizing agent KMnO
4 in continual ultrafiltration at the flow from 350 to 800 L/h. The experiment lasted for 42 days, but we needed to take a break for 8 days due to the scheduled well drilling that we used as the source of raw water for the experiment. The results are shown in
Figure 5 and
Figure 6.
A summary of the results of the continual experiments:
We decided to use KMnO4 as the oxidizing agent, due to its high efficiency in the previous phase of our research and the short reaction time.
After day 1, when we found out that the dose of KMnO4 was not sufficient for the complete removal of Mn from the water, to support its oxidation, the flow rate was slightly modified so the final concentration of Mn in water after treatment would fall below the limit for drinking water, in most cases below the detection limit of the measurement device.
The detention time needed for the oxidation of Mn ranged between 2.3 and 5.1 min in the tubular reactor. The results indicated that the retention time of 2.8 min is appropriate for the given technological line.
Pressure loss in UF expressed by the transmembrane pressure (TMP) was linearly dependent on the flow rate, which is readable in
Figure 5.
During the pilot plant experiment, no consecutive increase in TMP was observed, so there was no need for chemical washing. The load of substances on the membrane was low, and the input concentration of Mn ranged from 0.150 to 0.250 mg/L. There is a mechanical filter with a 50 µm lining at the output of the tubular reactor. Its primary goal was to protect the UF from bigger insoluble substances. It is obvious that some of the oxidized Mn was removed by this mechanical filter.
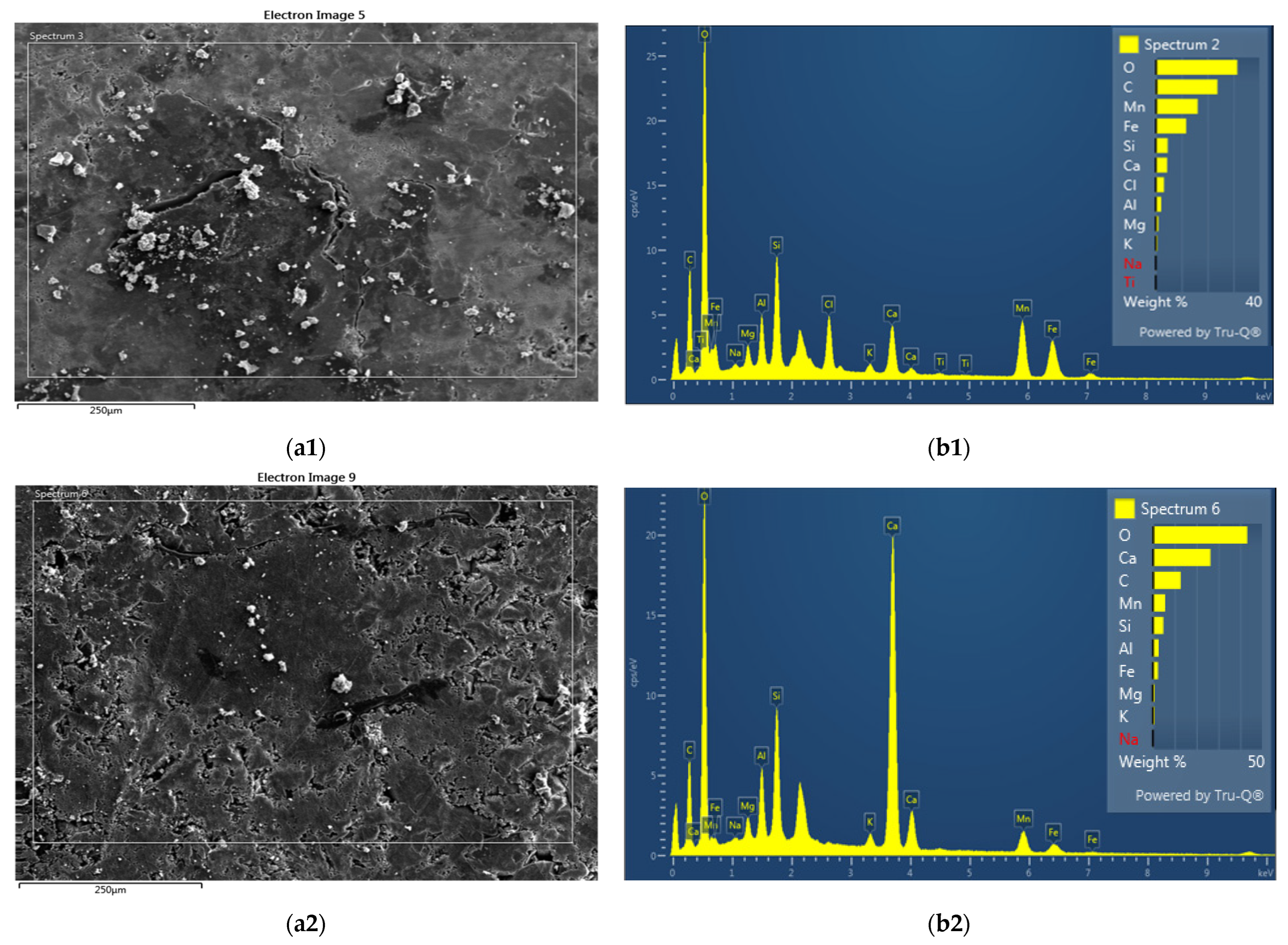
The chemical composition of the captured deposits on mechanical filter 1 (input UF) was determined using X-ray microanalysis, SEM, and X-ray phase analysis. The results are listed in
Table 7 and shown in
Figure 7.
Regarding the load of substances in raw water, it was possible to run the ultrafiltration for 90 min, after which a pre-wash with air lasted for the next 20 s, and washing with the water after treatment lasted for 2 min. The flow rate of the backwash was two times higher than the flow rate during filtration. Therefore, we reached 95.55% system utilization.
Samples of wastewater from backwashing in ultrafiltration were taken step by step in three containers: (1), (2), and (3). Sample (1) was taken during washing with air in combination with washing with water; therefore, the concentration of Mn in the first sample was not the highest. For this reason, the profile for Mn, turbidity, and color in wastewater is described in
Table 8.
In our experiments, we also dealt with the possibility of recirculating wastewater back into the process of water treatment. We performed clarification of wastewater (average of three samples mixed at the same ratio) samples without pH change and at an increased pH by adding NaOH to reach 9.41, and we took samples of clarified water over time (
Table 9). Even after just 1 h of water retention, we approached the limit value for Mn for drinking water. So, after clarification, water can be reintegrated into the water treatment process with no increase in the substance load of raw water. Regarding this, wastewater represents just 4.45% of the total water amount, and the pH of clarified wastewater has no considerable impact on the pH of treated water.
The wastewater from the membrane washing passed through a mechanical filter 2 (WR9 filter cartridge, with a porosity of 60 microns). It was either discharged into the sewer or returned before ultrafiltration. The composition of the sediments on the mechanical filter 2 is shown in
Table 7. Among the compounds, CaCO
3 and SiO
2 predominated, and low content was measured for MnO
2 and KCl.
5. Conclusions
Pilot plant experiments confirmed the efficiency of ultrafiltration, demonstrating the possibility of decreasing the manganese concentration below the limit for drinking water using the considered method.
When ClO2 was applied, the limiting factor was its maximal permitted concentration in drinking water. Therefore, we performed tests in the concentration range from 0.2 to 0.4 mg/L. With the use of KMnO4, we worked with concentrations ranging from 0.215 to 0.431 mg/L (converted to manganese value). Aside from the concentration of the oxidizing agent, we tested the effects of retention in a tubular reactor on manganese removal efficiency from raw water.
At the flow 60 L/h and detention time of about 30 min the efficiency of manganese removal from water reached 74.31% and the manganese concentration in water after treatment was 0.037 mg/L. To achieve the desired efficiency, it is necessary to either increase the concentration of the added oxidizing agent, or increase the oxidation time, or both conditions. Increasing the dose of oxidizing agent did not achieve the desired effect. Only by increasing the oxidation time (decreasing the flow rate) did an efficiency of 74.31% occur. This reaction time (oxidation time) is very high; therefore, we do not recommend using this oxidizing agent.
To achieve high efficiency in removing Mn from water, we recommend using a fresh 4% KMnO4 solution. For the given technological line and KMnO4 dose the detention time was 2.8 min at flow rate 650 L/h. Under these conditions 100% efficiency of removing Mn from water was achieved.
There was a mechanical pre-filter at the input into the ultrafiltration (
Figure 3). Its primary goal is to protect the UF from bigger insoluble substances (for example sand). It is obvious that some of the oxidized Mn was removed by this mechanical filter. During the pilot plant experiment, no consecutive increase in the transmembrane pressure (TMP) was observed, so there was no need for chemical washing.
Wastewater from backwashing of ultrafiltration contains a high value of Mn (0.7 mg/L). Sedimentation and pH adjustment can be used to remove Mn from wastewater. At pH 9.4 and a sedimentation time of 1 h, the manganese concentration was reduced to 0.062 mg/L. Such water can be returned before ultrafiltration, or discharged into the sewer.