A Helping Hand: Fungi, as Well as Bacteria, Support Ecophysiological Descriptors to Depict the Posidonia oceanica Conservation Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Morphometry and Biochemical Analyses
2.3. DNA Extraction, 16S rRNA and ITS2-5.8S Sequencing and Bioinformatic Analysis
2.4. Statistical Analysis
3. Results
3.1. Morphological Parameters
3.2. Biochemical Parameters
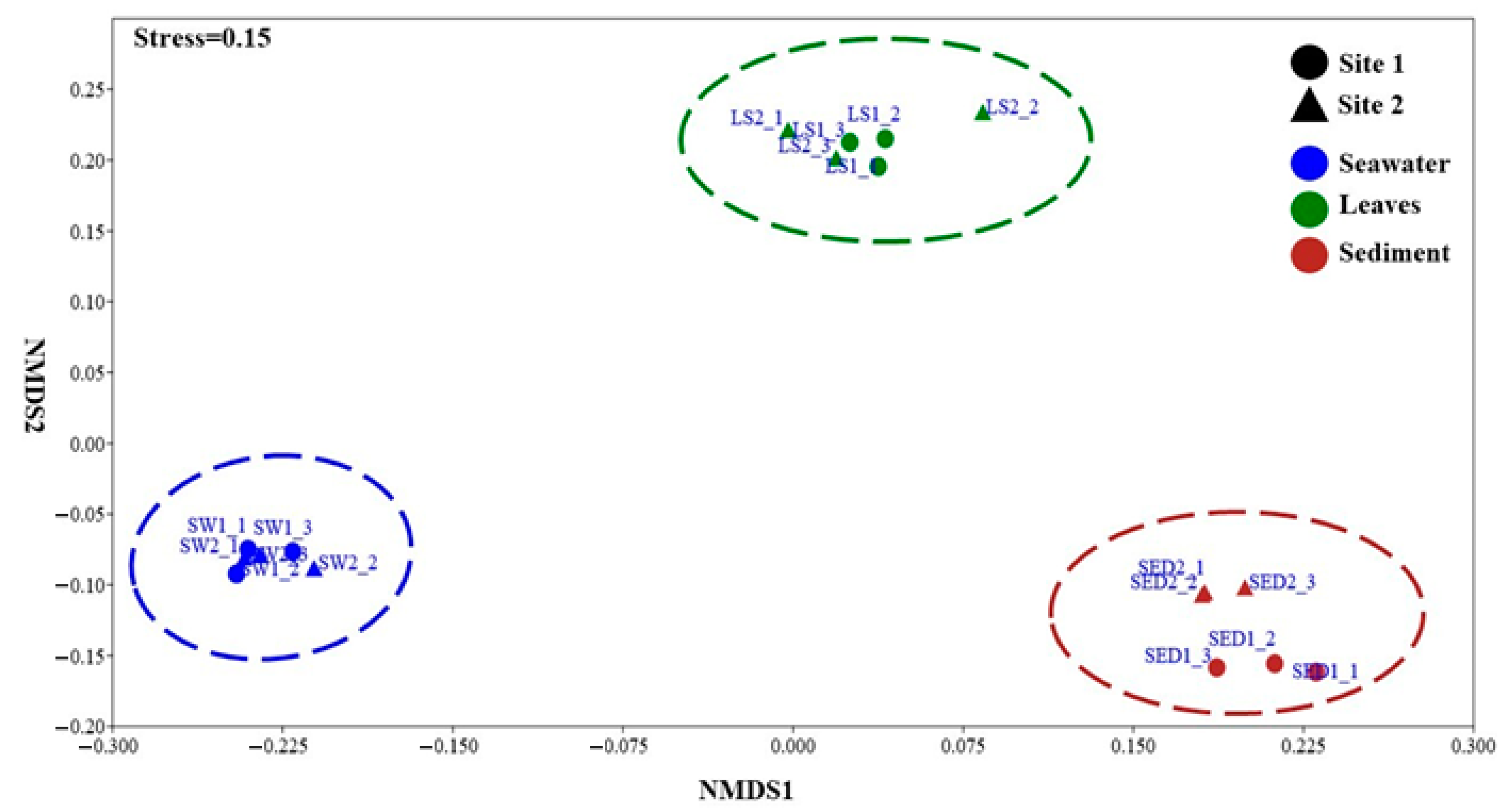
3.3. Microbial Diversity
3.3.1. Bacterial Diversity and Composition
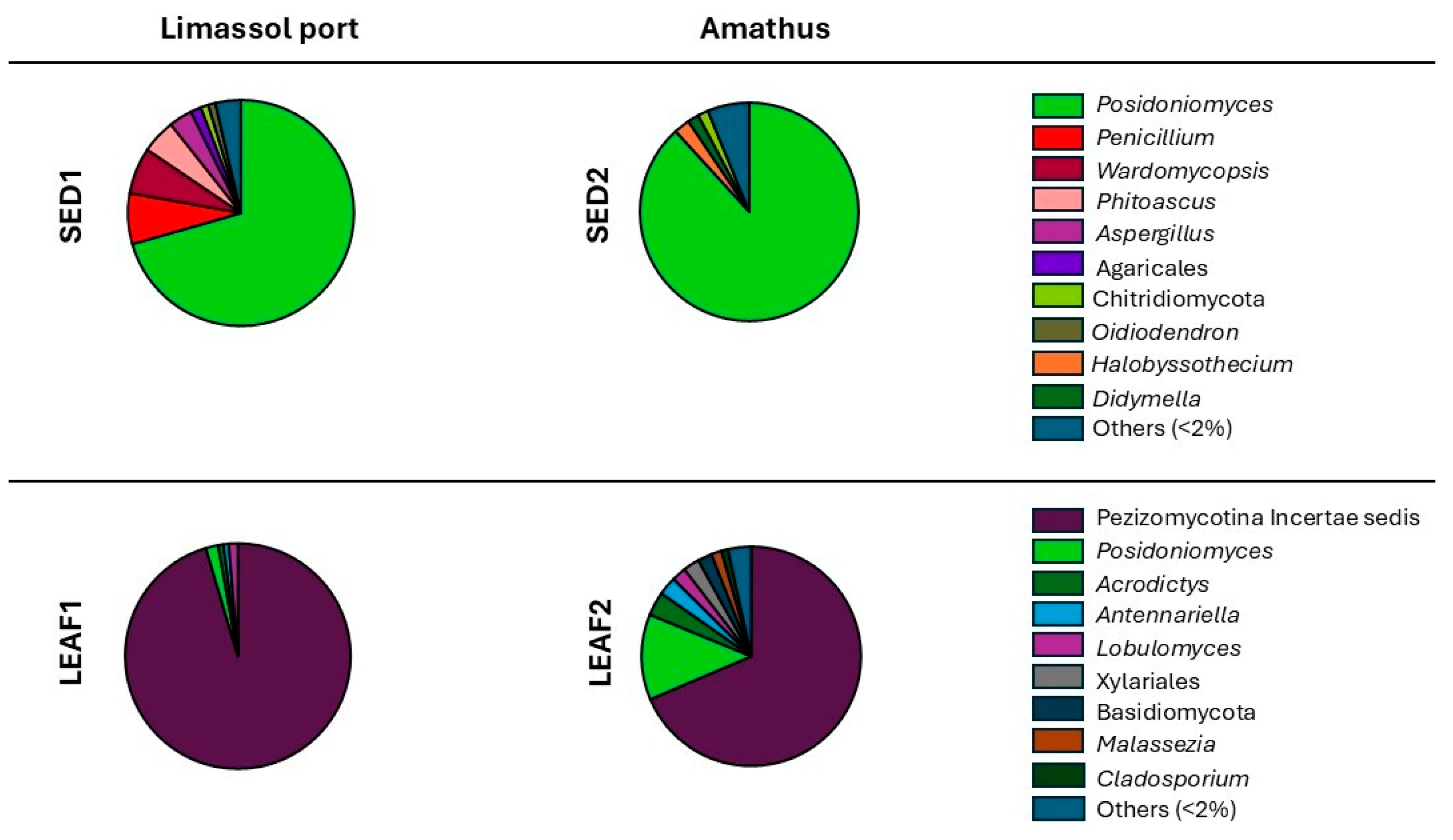
3.3.2. Fungal Diversity and Composition
4. Discussion
4.1. P. oceanica Morphometric and Biochemical Descriptors
4.2. P. oceanica-Associated Bacterial Communities
4.3. P. oceanica-Associated Fungal Communities
| Fungal Taxa | Putative Functions | References |
| Posidoniomyces atricolor | Putative Dark Septate Endophyte (DSE) not reported from other hosts or ecosystems, indicating a specific adaptation to the marine environment and P. oceanica host. It is supposed to be involved in host growth, nutrient acquisition, abiotic stress tolerance, and also in decomposing organic material. It constitutes one of the most important microorganisms by abundance that degrade P. oceanica tissues within the matte | [35,123] |
| Pezizomycotina incertae sedis | Filamentous ascomycetes with septate hypha, known to be saprotrophs involved in the decomposition of plant materials, contributing to the breakdown of complex carbohydrates in leaves. Opportunistic animal or plant pathogens. | [122,124] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The hologenome theory disregards the coral holobiont: Reply from Rosenberg et al. Nat. Rev. Microbiol. 2007, 5, 826–826. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining the functioning of microbial endophytes. MMBR 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Bettenfeld, P.; Canals, J.C.; Jacquens, L.; Fernandez, O.; Fontaine, F.; Van Schaik, E.; Trouvelot, S. The microbiota of the grapevine holobiont: A key component of plant health. J. Adv. Res. 2022, 40, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Zang, Y.; Yang, Z.; Qu, T.; Sun, T.; Liang, S.; Tang, X. Composition and functional diversity of epiphytic bacterial and fungal communities on marine macrophytes in an intertidal zone. Front. Microbiol. 2022, 13, 839465. [Google Scholar] [CrossRef]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019, 10, 862. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Ugarelli, K.; Chakrabarti, S.; Laas, P.; Stingl, U. The seagrass holobiont and its microbiome. Microorganisms 2017, 5, 81. [Google Scholar] [CrossRef]
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 2017, 356, 4501. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.C.; Tisserant, E.; Plassart, P.; Uroz, S.; Griffiths, R.I.; Hannula, S.E.; Lemanceau, P. Soil conditions and land use intensification effects on soil microbial communities across a range of European field sites. Soil Biol. Biochem. 2015, 88, 403–413. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Bettenfeld, P.; Fontaine, F.; Trouvelot, S.; Fernandez, O.; Courty, P.E. Woody plant declines: What’s wrong with the microbiome? Trends Plant Sci. 2020, 25, 381–394. [Google Scholar] [CrossRef]
- Conte, C.; Rotini, A.; Manfra, L.; D’Andrea, M.M.; Winters, G.; Migliore, L. The seagrass holobiont: What we know and what we still need to disclose for its possible use as an ecological indicator. Water 2021, 13, 406. [Google Scholar] [CrossRef]
- Lyu, D.; Zajonc, J.; Pagé, A.; Tanney, C.A.; Shah, A.; Monjezi, N.; Smith, D.L. Plant holobiont theory: The phytomicrobiome plays a central role in evolution and success. Microorganisms 2021, 9, 675. [Google Scholar] [CrossRef]
- Omae, N.; Tsuda, K. Plant-microbiota interactions in abiotic stress environments. MPMI 2022, 35, 511–526. [Google Scholar] [CrossRef]
- Baharum, S.N.; Beng, E.K.; Mokhtar, M.A.A. Marine microorganisms: Potential application and challenges. J. Biol. Sci. 2010, 10, 555–564. [Google Scholar] [CrossRef]
- Batista, D.; Costa, R.; Carvalho, A.P.; Batista, W.R.; Rua, C.P.; de Oliveira, L.; Leomil, L.; Fròes, A.M.; Thompson, F.L.; Coutinho, R.; et al. Environmental conditions affect activity and associated microorganisms of marine sponges. Mar. Environ. Res. 2018, 142, 59–68. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Bessa, L.A. Microbial diversity: The gap between the estimated and the known. Diversity 2018, 10, 46. [Google Scholar] [CrossRef]
- Qiu, Z.; Coleman, M.A.; Provost, E.; Campbell, A.H.; Kelaher, B.P.; Dalton, S.J.; Thomas, T.; Steinberg, P.D.; Marzinelli, E.M. Future climate change is predicted to affect the microbiome and condition of habitat-forming kelp. Proc. Biol. Sci. 2019, 13, 20181887. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Tarquinio, F.; Hyndes, G.A.; Laverock, B.; Koenders, A.; Säwström, C. The seagrass holobiont: Understanding seagrass-bacteria interactions and their role in seagrass ecosystem functioning. FEMS Microbiol. Lett. 2019, 366, fnz057. [Google Scholar] [CrossRef]
- Rajakaruna, O.; Wijayawardene, N.N.; Udagedara, S.; Jayasinghe, P.K.; Gunasekara, S.S.; Boonyuen, N.; Bamunuarachchige, T.C.; Ariyawansa, K.G.S.U. Exploring fungal diversity in seagrass ecosystems for pharmaceutical and ecological insights. J. Fungus 2024, 10, 627. [Google Scholar] [CrossRef]
- Tasdemir, D.; Scarpato, S.; Utermann-Thüsing, C.; Jensen, T.; Blümel, M.; Wenzel-Storjohann, A.; Echelmeyer, V.A. Epiphytic and endophytic microbiome of the seagrass Zostera marina: Do they contribute to pathogen reduction in seawater? Sci. Total Environ. 2024, 908, 168422. [Google Scholar] [CrossRef]
- Jones, E.G. Are there more marine fungi to be described? Bot. Mar. 2011, 54, 343–354. [Google Scholar] [CrossRef]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Gladfelter, A.S. Fungi in the marine environment: Open questions and unsolved problems. MBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Panno, L.; Bruno, M.; Voyron, S.; Anastasi, A.; Gnavi, G.; Miserere, L.; Varese, G.C. Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol. 2013, 30, 685–694. [Google Scholar] [CrossRef]
- Torta, L.; Lo Piccolo, S.; Piazza, G.; Burruano, S.; Colombo, P.; Ottonello, D.; Calvo, S. Lulwoana sp., a dark septate endophyte in roots of Posidonia oceanica (L.) Delile seagrass. Plant Biol. 2015, 17, 505–511. [Google Scholar] [CrossRef]
- Venkatachalam, A.; Govinda Rajulu, M.B.; Thirunavukkarasu, N.; Suryanarayanan, T.S. Endophytic fungi of marine algae and seagrasses: A novel source of chitin modifying enzymes. Mycosphere 2015, 6, 345–355. [Google Scholar] [CrossRef]
- Venkatachalam, A.; Thirunavukkarasu, N.; Suryanarayanan, T.S. Distribution and diversity of endophytes in seagrasses. Fungal Ecol. 2015, 13, 60–65. [Google Scholar] [CrossRef]
- Vohník, M.; Borovec, O.; Kolařík, M. Communities of cultivable root mycobionts of the seagrass Posidonia oceanica in the northwest Mediterranean Sea are dominated by a hitherto undescribed pleosporalean dark septate endophyte. Microb. Ecol. 2016, 71, 442–451. [Google Scholar] [CrossRef]
- Ettinger, C.L.; Eisen, J.A. Fungi, bacteria and oomycota opportunistically isolated from the seagrass, Zostera marina. PLoS ONE 2020, 15, e0236135. [Google Scholar] [CrossRef] [PubMed]
- Vohník, M.; Borovec, O.; Kolaříková, Z.; Sudová, R.; Réblová, M. Extensive sampling and high-throughput sequencing reveal Posidoniomyces atricolor gen. et sp. nov. (Aigialaceae, Pleosporales) as the dominant root mycobiont of the dominant Mediterranean seagrass Posidonia oceanica. MycoKeys 2019, 55, 59–86. [Google Scholar] [CrossRef]
- Vohník, M.; Josefiová, J. Novel epiphytic root-fungus symbiosis in the Indo-Pacific seagrass Thalassodendron ciliatum from the Red Sea. Mycorrhiza 2024, 34, 447–461. [Google Scholar] [CrossRef]
- Wang, X.; Pecoraro, L.; Chen, J.; Tang, Y.; Lee, S.; Chen, S.; Liu, H. Halophilomyces hongkongensis, a novel species and genus in the Lulworthiaceae with antibacterial potential, colonizing the roots and rhizomes of the seagrass Halophila ovalis. J. Fungi 2024, 10, 474. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Mayot, N.; Pergent, G. The outstanding traits of the functioning of the Posidonia oceanica seagrass ecosystem. Biol. Mar. Medit. 2006, 13, 109–113. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia oceanica Meadows; RAMOGE and RAC/SPA: Tunis, Tunisia, 2012. [Google Scholar]
- Boudouresque, C.F.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Thibaut, T.; Verlaque, M. The necromass of the Posidonia oceanica seagrass meadow: Fate, role, ecosystem services and vulnerability. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. Res. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Escolano-Moltó, A.; Flecha, S.; Vaquer-Sunyer, R.; Wesselmann, M.; Marbà, N. Mediterranean seagrasses as carbon sinks: Methodological and regional differences. Biogeosciences 2022, 19, 4619–4637. [Google Scholar] [CrossRef]
- Litsi-Mizan, V.; Efthymiadis, P.T.; Gerakaris, V.; Serrano, O.; Tsapakis, M.; Apostolaki, E.T. Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. New Phytol. 2023, 239, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Vohník, M. Are lulworthioid fungi dark septate endophytes of the dominant Mediterranean seagrass Posidonia oceanica? Plant Biol. 2022, 24, 127–133. [Google Scholar] [CrossRef]
- Vohník, M.; Borovec, O.; Kolařík, M.; Sudová, R. Fungal root symbionts of the seagrass Posidonia oceanica in the central Adriatic Sea revealed by microscopy, culturing and 454-pyrosequencing. Mar. Ecol. Prog. Ser. 2017, 583, 107–120. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Díaz-Almela, E.; Marbà, N.; Álvarez, E.; Santiago, R.; Holmer, M.; Grau, A.; Duarte, C.M. Benthic input rates predict seagrass (Posidonia oceanica) fish farm-induced decline. Mar. Pollut. Bull. 2008, 56, 1332–1342. [Google Scholar] [CrossRef]
- Marbà, N.; Díaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Rotini, A.; Belmonte, A.; Barrote, I.; Micheli, C.; Peirano, A.; Santos, R.O.; Migliore, L. Effectiveness and consistency of a suite of descriptors for assessing the ecological status of seagrass meadows (Posidonia oceanica L. Delile). Estuar. Coast. Shelf Sci. 2013, 130, 252–259. [Google Scholar] [CrossRef]
- Mejia, A.Y.; Rotini, A.; Lacasella, F.; Bookman, R.; Thaller, M.C.; Shem-Tov, R.; Migliore, L. Assessing the ecological status of seagrasses using morphology, biochemical descriptors and microbial community analyses. A study in Halophila stipulacea (Forsk.) Aschers meadows in the northern Red Sea. Ecol. Indic. 2016, 60, 1150–1163. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; van Katwijk, M.M.; Marbà, N.; Romero, J. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Glasl, B.; Webster, N.S.; Bourne, D.G. Microbial indicators as a diagnostic tool for assessing water quality and climate stress in coral reef ecosystems. Mar. Biol. 2017, 164, 1–18. [Google Scholar] [CrossRef]
- Conte, C.; Rotini, A.; Winters, G.; Vasquez, M.I.; Piazza, G.; Kletou, D.; Migliore, L. Elective affinities or random choice within the seagrass holobiont? The case of the native Posidonia oceanica (L.) Delile and the exotic Halophila stipulacea (Forssk.) Asch. from the same site (Limassol, Cyprus). Aquat. Bot. 2021, 174, 103420. [Google Scholar] [CrossRef]
- Rotini, A.; Conte, C.; Winters, G.; Vasquez, M.I.; Migliore, L. Undisturbed Posidonia oceanica meadows maintain the epiphytic bacterial community in different environments. Environ. Sci. Pollut. Res. 2023, 30, 95464–95474. [Google Scholar] [CrossRef] [PubMed]
- Ramos Esplá, A.A. Artificial Reefs in the Amathus Bay (Limassol, Cyprus); Technical Report N°06/2005; Department of Fisheries and Marine Research of Cyprus—University of Alicante: Alicante, Spain; pp. 1–108. [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Intern. 2004, 11, 36–42. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Migliore, L.; Rotini, A.; Randazzo, D.; Albanese, N.N.; Giallongo, A. Phenols content and 2-D electrophoresis protein pattern: A promising tool to monitor Posidonia meadows health state. BMC Ecol. 2007, 7, 1–8. [Google Scholar] [CrossRef]
- Booker, F.L.; Miller, J.E. Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J. Exp. Bot. 1998, 49, 1191–1202. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Abarenkov, K. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. NAR 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Larsson, K.H. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, 2012. [Google Scholar]
- Rivas, L.R. A reinterpretation of the concepts “Sympatric” and “Allopatric” with proposal of the additional terms “Syntopic” and “Allotopic”. Syst. Zool. 1964, 13, 42–43. [Google Scholar] [CrossRef]
- Conte, C.; Apostolaki, E.T.; Vizzini, S.; Migliore, L. A tight interaction between the native seagrass Cymodocea nodosa and the exotic Halophila stipulacea in the Aegean Sea highlights seagrass holobiont variations. Plants 2023, 12, 350. [Google Scholar] [CrossRef]
- Mannino, A.M.; Balistreri, P.; Mancuso, F.P.; Bozzeda, F.; Pinna, M. Searching for the competitive ability of the alien seagrass Halophila stipulacea with the autochthonous species Cymodocea nodosa. NeoBiota 2023, 83, 155–177. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological function of phenolic compounds from Mediterranean fucoid algae and seagrasses: An overview on the genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Casazza, G.; Mazzella, L. Photosynthetic pigment composition of marine angiosperms: Preliminary characterization of Mediterranean seagrasses. Bull. Mar. Sci. 2002, 71, 1171–1181. [Google Scholar]
- Madonia, A.; Caporale, G.; Penna, M.; Bonamano, S.; Marcelli, M. Assessment of the photosynthetic response of Posidonia oceanica (Linneaus) Delile, 1813 along a depth gradient in the Northern Tyrrhenian Sea (Latium, Italy). Geosciences 2021, 11, 202. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Brain, R.A.; Cedergreen, N. Biomarkers in aquatic plants: Selection and utility. Rev. Environ. Contam. 2008, 49–109. [Google Scholar] [CrossRef]
- Banchi, E.; Del Negro, P.; Celussi, M.; Malfatti, F. Sediment features and human activities structure the surface microbial communities of the Venice Lagoon. Front. Mar. Sci. 2021, 8, 762292. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Guo, R.; Ma, X.; Zhang, J.; Liu, C.; Thu, C.A.; Win, T.N.; Wang, P. Microbial community structures and important taxa across oxygen gradients in the Andaman Sea and eastern Bay of Bengal epipelagic waters. Front. Microbiol. 2022, 13, 1041521. [Google Scholar] [CrossRef]
- Roda-Garcia, J.J.; Haro-Moreno, J.M.; Huschet, L.A.; Rodriguez-Valera, F.; López-Pérez, M. Phylogenomics of SAR116 clade reveals two subclades with different evolutionary trajectories and an important role in the ocean sulfur cycle. mSystems 2021, 6, e00944-21. [Google Scholar] [CrossRef]
- Gómez-Pereira, P.R.; Schüler, M.; Fuchs, B.M.; Bennke, C.; Teeling, H.; Waldmann, J.; Amann, R. Genomic content of uncultured Bacteroidetes from contrasting oceanic provinces in the North Atlantic Ocean. Environ. Microbiol. 2012, 14, 52–66. [Google Scholar] [CrossRef]
- Ngugi, D.K.; Stingl, U. High-quality draft single-cell genome sequence of the NS5 marine group from the coastal Red Sea. Genome Announc. 2018, 6, e00565-18. [Google Scholar] [CrossRef]
- Bowman, J.P.; McCammon, S.A.; Brown, J.L.; Nichols, P.D.; McMeekin, T.A. Psychroserpens burtonensis gen. nov., sp. nov., and Gelidibacter algens gen. nov., sp. nov., psychrophilic bacteria isolated from Antarctic lacustrine and sea ice habitats. Int. J. Syst. Bacteriol. 1997, 47, 670–677. [Google Scholar] [CrossRef]
- Williams, T.J.; De Long, E.; Evans, F.; Demaere, M.Z.; Lauro, F.M.; Raftery, M.J.; Ducklow, H.W.; Grzymski, J.J.; Murray, A.E.; Cavicchioli, R. A metaproteomic assessment of winter and summer bacterioplankton from Antarctic Peninsula coastal surface waters. ISME J. 2012, 6, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Krieg, N.R.; Staley, J.R. Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2005. [Google Scholar]
- Thompson, F.L.; Tetsuya, L.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cha, J.; Song, B.; Huang, Y.; Kim, S.; Kim, S.; An, S. Total microbial activity and sulfur cycling microbe changes in response to the development of hypoxia in a shallow estuary. Ocean Sci. 2020, 55, 165–181. [Google Scholar] [CrossRef]
- Ravot, G.; Magot, M.; Fardeau, M.L.; Patel, B.K.; Thomas, P.; Garcia, J.L.; Ollivier, B. Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate-reducing bacterium from an oil-producing well. Int. J. Syst. Evol. Microbiol. 1999, 49, 1141–1147. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.; Stams, A.J. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Brioukhanov, A.L.; Kadnikov, V.V.; Beletsky, A.V.; Savvichev, A.S. Aerotolerant thiosulfate-reducing bacterium Fusibacter sp. strain WBS isolated from littoral bottom sediments of the White Sea—Biochemical and genome analysis. Microorganisms 2023, 11, 1642. [Google Scholar] [CrossRef]
- Pujalte, M.J.; Lucena, T.; Ruvira, M.A.; Arahal, D.R.; Macián, M.C. The family Rhodobacteraceae. In The Prokaryotes Alphaproteobacteria and Betaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Abraham, W.R.; Rohde, M. The family Hyphomonadaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Nielsen, P.H. The family Saprospiraceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, S., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Li, J.; Zheng, L.; Ye, C.; Ni, B.; Wang, X.; Liu, H. Evaluation of an intermittent aeration constructed wetland for removing residual organics and nutrients from secondary effluent: Performance and microbial analysis. Bioresour. Technol. 2021, 329, 124897. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef]
- Kämpfer, P.; Ruppel, S.; Remus, R. Enterobacter radicincitans sp. nov., a plant growth-promoting species of the family Enterobacteriaceae. Syst. Appl. Microbiol. 2005, 28, 213–221. [Google Scholar] [CrossRef]
- Nešvera, J.; Rucká, L.; Pátek, M. Catabolism of phenol and its derivatives in bacteria: Genes, their regulation, and use in the biodegradation of toxic pollutants. Adv. Appl. Microbiol. 2015, 93, 107–160. [Google Scholar] [CrossRef]
- Rotini, A.; Conte, C.; Seveso, D.; Montano, S.; Galli, P.; Vai, M.; Mejia, A. Daily variation of the associated microbial community and the Hsp60 expression in the Maldivian seagrass Thalassia hemprichii. J. Sea Res. 2020, 156, 101835. [Google Scholar] [CrossRef]
- Alexandre, A.; Georgiou, D.; Santos, R. Inorganic nitrogen acquisition by the tropical seagrass Halophila stipulacea. Mar. Ecol. 2014, 35, 387–394. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.; Wei, X. Erosion reduces soil microbial diversity, network complexity, and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, M.; Li, X.; Yu, B.; Wang, H.; Ginawi, A.; Yan, Y. Mechanisms of niche-neutrality balancing can drive the assembling of microbial community. Mol. Ecol. 2021, 30, 1492–1504. [Google Scholar] [CrossRef]
- Mazzola, A.; Favaloro, E.; Sarà, G. Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso, 1810), in submerged cages in the Western Mediterranean Sea. Aquaculture 2000, 181, 257–268. [Google Scholar] [CrossRef]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Chen, J. Transformations from specialists to generalists cause bacterial communities to be more stable than micro-eukaryotic communities under anthropogenic activity disturbance. Sci. Total Environ. 2021, 790, 148141. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, H.; Liu, X.; Wong, M.H.; Xu, F.; Yang, X.; Li, S. Characteristics of spatial and seasonal bacterial community structures in a river under anthropogenic disturbances. Environ. Pollut. 2020, 264, 114818. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Sjøtun, K.; Lanzén, A.; Øvreås, L. Bacterial diversity in relation to secondary production and succession on surfaces of the kelp Laminaria hyperborea. ISME J. 2012, 6, 2188–2198. [Google Scholar] [CrossRef]
- Bockelmann, A.C.; Beining, K.; Reusch, T.B. Widespread occurrence of endophytic Labyrinthula spp. in northern European eelgrass Zostera marina beds. Mar. Ecol. Prog. Ser. 2012, 445, 109–116. [Google Scholar] [CrossRef]
- Bockelmann, A.C.; Tams, V.; Ploog, J.; Schubert, P.R.; Reusch, T.B. Quantitative PCR reveals strong spatial and temporal variation of the wasting disease pathogen, Labyrinthula zosterae, in northern European eelgrass (Zostera marina) beds. PLoS ONE 2013, 8, e62169. [Google Scholar] [CrossRef] [PubMed]
- Michelou, V.K.; Caporaso, J.G.; Knight, R.; Palumbi, S.R. The ecology of microbial communities associated with Macrocystis pyrifera. PLoS ONE 2013, 8, e67480. [Google Scholar] [CrossRef]
- Brakel, J.; Werner, F.J.; Tams, V.; Reusch, T.B.; Bockelmann, A.C. Current European Labyrinthula zosterae are not virulent and modulate seagrass (Zostera marina) defense gene expression. PLoS ONE 2014, 9, e92448. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Kõljalg, U.; Abarenkov, K. Identifying the ‘unidentified’ fungi: A global-scale long-read third-generation sequencing approach. Fungal Divers. 2020, 103, 273–293. [Google Scholar] [CrossRef]
- Priyashantha, A.H.; Dai, D.Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Karunarathna, S.C. Plant–fungi interactions: Where it goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Frasca, S.; Alabiso, A.; D’Andrea, M.M.; Cattaneo, R.; Migliore, L. Diversity and Composition of Posidonia oceanica-Associated Bacterial and Fungal Communities: Effect of Boat-Induced Mechanical Stress in the Villefranche-sur-Mer Bay (France). Diversity 2024, 16, 604. [Google Scholar] [CrossRef]
- Poli, A.; Bovio, E.; Ranieri, L.; Varese, G.C.; Prigione, V. Fungal Diversity in the Neptune Forest: Comparison of the Mycobiota of Posidonia oceanica, Flabellia petiolata, and Padina pavonica. Front. Microbiol. 2020, 11, 933. [Google Scholar] [CrossRef]
- Duarte, B.; Martins, I.; Rosa, R.; Matos, A.R.; Roleda, M.Y.; Reusch, T.B.; Jueterbock, A. Climate change impacts on seagrass meadows and macroalgal forests: An integrative perspective on acclimation and adaptation potential. Front. Mar. Sci. 2018, 5, 190. [Google Scholar] [CrossRef]
- Orsi, W.; Biddle, J.F.; Edgcomb, V. Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS ONE 2013, 8, e56335. [Google Scholar] [CrossRef]
- Kohn, T.; Rast, P.; Kallscheuer, N.; Wiegand, S.; Boedeker, C.; Jetten, M.S.; Jogler, C. The microbiome of Posidonia oceanica seagrass leaves can be dominated by Planctomycetes. Front. Microbiol. 2020, 11, 1458. [Google Scholar] [CrossRef] [PubMed]
- Lunghini, D.; Granito, V.M.; Di Lonardo, D.P.; Maggi, O.; Persiani, A.M. Fungal diversity of saprotrophic litter fungi in a Mediterranean maquis environment. Mycologia 2013, 105, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Maharachchikumbura, S.S.N.; Wang, Y.; Hyde, K.D. An updated account of Fagales-inhabiting Italian Ascomycota and mycogeography, with additions to Pezizomycotina. AJOM 2022, 5, 79–186. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Sung, G.H. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z. 3 Pezizomycotina: Sordariomycetes and Leotiomycetes. In Systematics and Evolution Part B; McLaughlin, D., Spatafora, J., Eds.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Lefebvre, L.; Compère, P.; Gobert, S. The formation of aegagropiles from the Mediterranean seagrass Posidonia oceanica (L.) Delile (1813): Plant tissue sources and colonisation by melanised fungal mycelium. Mar. Biol. 2023, 170, 19. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Raghukumar, S. Fungi in Coastal and Oceanic Marine Ecosystems; Springer: New York, NY, USA, 2017; Volume 378. [Google Scholar]
- Poli, A.; Vizzini, A.; Prigione, V.; Varese, G.C. Basidiomycota isolated from the Mediterranean Sea–Phylogeny and putative ecological roles. Fungal Ecol. 2018, 36, 51–62. [Google Scholar] [CrossRef]
- Poli, A.; Varese, G.C.; Garzoli, L.; Prigione, V. Seagrasses, seaweeds and plant debris: An extraordinary reservoir of fungal diversity in the Mediterranean Sea. Fungal Ecol. 2022, 60, 101156. [Google Scholar] [CrossRef]
- Jones, E.G.; Pang, K.L. Marine Fungi: And Fungal-Like Organisms; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- He, D.; Guo, Z.; Shen, W.; Ren, L.; Sun, D.; Yao, Q.; Zhu, H. Fungal communities are more sensitive to simulated environmental changes than bacterial communities in a subtropical forest: The single and interactive effects of nitrogen addition and precipitation seasonality change. Microb. Ecol. 2023, 86, 521–535. [Google Scholar] [CrossRef]
- Warnasuriya, S.D.; Udayanga, D.; Manamgoda, D.S.; Biles, C. Fungi as environmental bioindicators. Sci. Total Environ. 2023, 892, 164583. [Google Scholar] [CrossRef]




| Site 1 | Site 2 | ||||
| Shannon (H’) | Tot. N Sequences | Shannon (H’) | Tot. N Sequences | Dunn’s post hoc | |
| Seawater | 3.2 ± 0.13 | 31,292 | 3.0 ± 0.07 | 24,327 | p > 0.05 |
| Sediment | 4.0 ± 0.08 | 45,190 | 3.2 ± 0.05 | 29,733 | p = 0.01 |
| Leaves | 3.4 ± 0.28 | 24,813 | 3.1 ± 0.21 | 16,012 | p > 0.05 |
| Site 1 | Site 2 | ||||
| Shannon (H’) | Tot. N Sequences | Shannon (H’) | Tot. N Sequences | Dunn’s post hoc | |
| Sediment | 1.1 ± 0.44 | 50,922 | 0.7 ± 0.22 | 43,637 | p > 0.05 |
| Leaves | 0.3 ± 0.11 | 38,461 | 1.6 ± 0.07 | 24,633 | p = 0.03 |
| Seagrass Descriptors | Limassol (Site 1) | Amathus (Site 2) | ||
| December 2017 # | July 2023 § | December 2017 # | July 2023 § | |
| Leaf area (cm2) | 20.7 ± 4.50 | 30.9 ± 14.10 | 36.7 ± 2.10 | 32.1 ± 16.70 |
| Total phenols (mg g−1 of FW) | 52.11 ± 26.50 | 10.5 ± 3.70 | 20.82 ± 4.49 | 11.2 ± 5.00 |
| Chlorophyll a (mg g−1 of FW) | 0.38 ± 0.10 | 0.41 ± 0.12 | 0.47 ± 0.04 | 0.57 ± 0.11 |
| Chlorophyll b (mg g−1 of FW) | 0.24 ± 0.10 | 0.20 ± 0.07 | 0.29 ± 0.03 | 0.75 ± 0.28 |
| Carotenoids (mg g−1 of FW) | 0.18 ± 0.05 | 0.21 ± 0.06 | 0.21 ± 0.02 | 0.32 ± 0.02 |
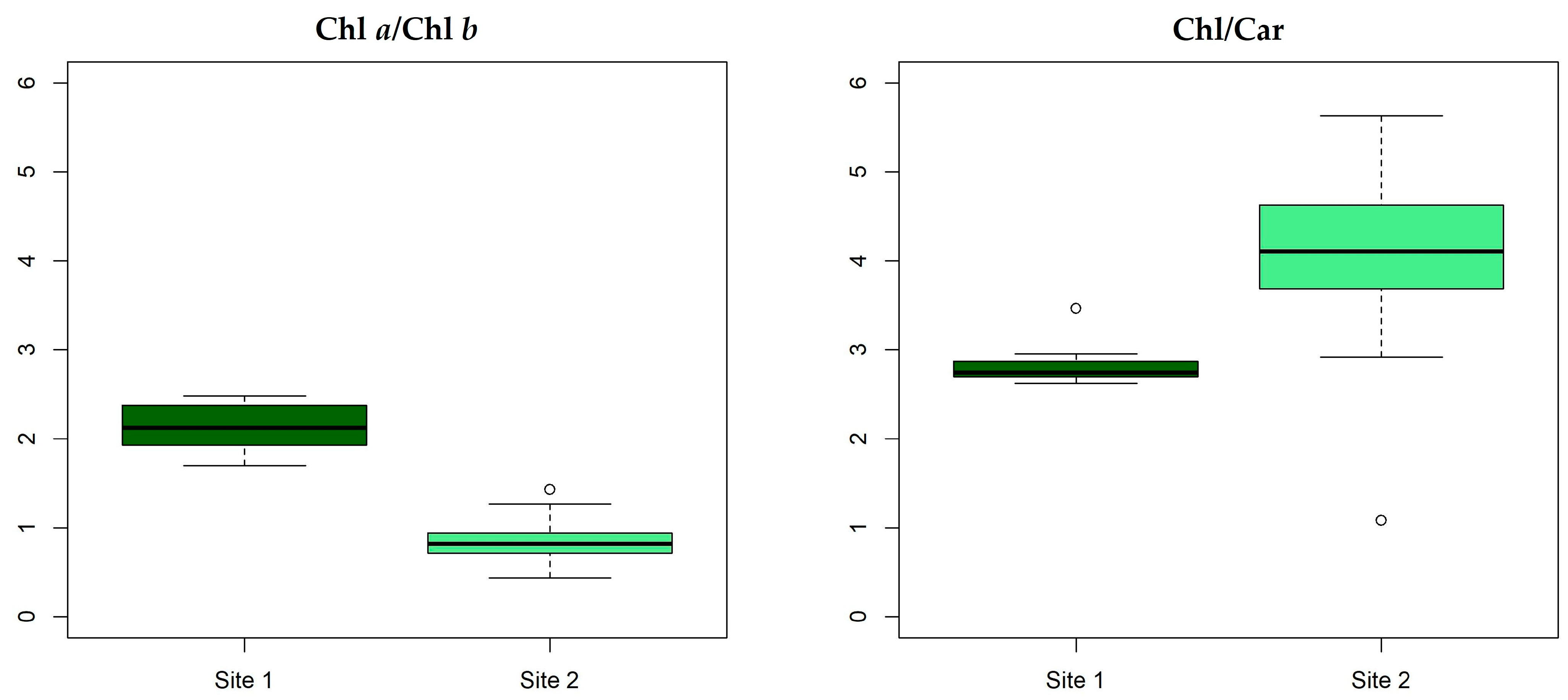
| Total Chl/Car | 3.46 ± 0.49 | 4.0 ± 0.11 | 3.53 ± 0.17 | 2.8 ± 0.20 |
| Chl a/Chl b | 1.62 ± 0.11 | 2.1 ± 0.26 | 1.61 ± 0.03 | 0.9 ± 0.26 |
| Bacterial Taxa | Putative Functions | References |
| SEAWATER | ||
| AEGEAN_169_marine group | Marine bacterioplankton, involved in marine sulfur cycle | [79] |
| SAR86-SAR116 | Heterotrophic bacteria, putative involved in the sulfur cycle | [80] |
| Flavobacteraceae (NS4-NS5) | Marine bacterioplankton, able to degrade high-molecular-weight organic matter, such as proteins and polysaccharides | [81,82] |
| Cryomorphaceae | Chemo-organotrophic bacteria, involved in degradation of high-molecular-weight organic matter, such as polysaccharides, and contributing to carbon cycling in marine ecosystems | [83,84] |
| SEDIMENT | ||
| Vibrionaceae (Vibrio spp.) | Fermentative or aerobic chemoheterotrophs, involved in nitrogen fixation, bioluminescence, and known as pathogens | [85,86] |
| Nitrincolaceae (Amphritea spp.) | Aerobic bacteria, involved in degradation of complex organic compounds and sulfur cycle | [87] |
| Fusibacteraceae (Fusibacter spp.) | Fermentative or chemo-organotrophic obligate anaerobic bacteria, involved in the breakdown of complex organic substrates in anoxic environments; producing hydrogen, acetate, and other fermentation products that serve as substrates for other microorganisms involved in methanogenesis and sulfate reduction | [88,89,90] |
| LEAVES | ||
| Rhodobacteraceae | Heterotrophic bacteria, deeply involved in sulfur and carbon biogeochemical cycling and potentially engaged in mutualistic interactions with aquatic micro- and macro-organisms | [91] |
| Hyphomonadaceae | Chemoheterotrophic bacteria, putatively involved in leaf nitrate supply and biofilm formation | [92] |
| Microtrichaceae (Sva0996 marine group) | Chemoheterotrophic bacteria, involved in nitrification-anammox systems and able to hydrolyze and metabolize complex organic matter | [93,94] |
| Saprospiraceae | Chemoheterotrophic bacteria, able to hydrolyze and metabolize complex organic matter | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasca, S.; Alabiso, A.; Rotini, A.; Manfra, L.; Vasquez, M.I.; Christoforou, E.; Winters, G.; Kaminer, M.; D’Andrea, M.M.; Migliore, L. A Helping Hand: Fungi, as Well as Bacteria, Support Ecophysiological Descriptors to Depict the Posidonia oceanica Conservation Status. Water 2025, 17, 1151. https://doi.org/10.3390/w17081151
Frasca S, Alabiso A, Rotini A, Manfra L, Vasquez MI, Christoforou E, Winters G, Kaminer M, D’Andrea MM, Migliore L. A Helping Hand: Fungi, as Well as Bacteria, Support Ecophysiological Descriptors to Depict the Posidonia oceanica Conservation Status. Water. 2025; 17(8):1151. https://doi.org/10.3390/w17081151
Chicago/Turabian StyleFrasca, Sara, Annamaria Alabiso, Alice Rotini, Loredana Manfra, Marlen I. Vasquez, Eleni Christoforou, Gidon Winters, Moran Kaminer, Marco Maria D’Andrea, and Luciana Migliore. 2025. "A Helping Hand: Fungi, as Well as Bacteria, Support Ecophysiological Descriptors to Depict the Posidonia oceanica Conservation Status" Water 17, no. 8: 1151. https://doi.org/10.3390/w17081151
APA StyleFrasca, S., Alabiso, A., Rotini, A., Manfra, L., Vasquez, M. I., Christoforou, E., Winters, G., Kaminer, M., D’Andrea, M. M., & Migliore, L. (2025). A Helping Hand: Fungi, as Well as Bacteria, Support Ecophysiological Descriptors to Depict the Posidonia oceanica Conservation Status. Water, 17(8), 1151. https://doi.org/10.3390/w17081151













