Abstract
Drawing upon an understanding of the distribution characteristics of groundwater in the Taoshan rock mass within the Yushan Uplift region of southern Jiangxi, this study utilizes mathematical statistics, ion ratio coefficients, factor analysis, and mineral dissolution equilibrium methods to characterize in detail the hydrochemical features of groundwater in humid mountainous areas. Furthermore, the study delves into the lithological source control and the primary natural mechanisms that underlie these characteristics. The results indicate that the average pH of groundwater in the study area is 7.13, classifying it as weakly alkaline. The dominant cations are Ca2+ and Na+, accounting for 61% and 26% of the total cations, respectively, while the dominant anion is HCO3−, constituting 91% of the total anions. The total dissolved solids (TDS) range from 37.93 mg/L to 228.16 mg/L, indicating low mineralization. The groundwater types are primarily HCO3-Ca·Na and secondarily HCO3-Ca. The groundwater type is mainly controlled by rock weathering, with the primary ion sources influenced by the weathering and dissolution of silicate rocks, supplemented by contributions from carbonate rock dissolution. Ion ratio analysis further confirms that the major ions in groundwater predominantly originate from the weathering of silicate minerals, with minimal influence from human activities. Na+, K+, and H2SiO3 are primarily derived from the weathering and dissolution of silicate rocks, while the weathering and dissolution of carbonate rocks (e.g., calcite) significantly contribute to Ca2+ and Mg2+. TDS shows significant positive correlations with Mg2+, SO42−, HCO3−, Na+, and Ca2+, with the most pronounced correlations observed between TDS and Ca2+ and HCO3−, exhibiting a correlation coefficient of 0.89. Factor analysis reveals that the first principal component has relatively high loadings for TDS, Ca2+, HCO3−, Mg2+, and SO42−. Additionally, among 45 natural spring water samples, 36 exhibit metasilicic acid (H2SiO3) concentrations exceeding 30 mg/L, meeting the standards for metasilicic acid mineral water and demonstrating significant potential for development and utilization.
1. Introduction
Water is a primary source of essential elements for maintaining and enhancing human health. The distribution, migration, and enrichment of various inorganic and organic substances, gases, and microorganisms in water—whether harmful or beneficial to humans—through hydrological and geological cycles have garnered increasing attention from scholars. Groundwater composition is primarily influenced by natural factors such as lithology, geological structures, and topography, as well as human activities like production and daily life [1,2]. Within groundwater systems, water–rock interactions represent a complex process of material and energy exchange, characterized by spatiotemporal evolution [3].
Zhao et al., through the analysis of 97 groundwater samples from Yudu County, Jiangxi Province, found that Ca2+ and HCO3− account for over 80% of the total cations and anions, respectively. The hydrochemical processes in shallow groundwater are predominantly controlled by rock weathering and dissolution, with limited influence from cation exchange and human activities [4]. Na+ and K+ primarily originate from the weathering and dissolution of silicate minerals such as albite and potassium feldspar, while Ca2+ and Mg2+ are mainly derived from the dissolution of carbonate rocks (e.g., calcite and dolomite) [5]. Liu et al., analyzing 33 karst water and 12 pore water samples from Shunping County, Hebei Province, concluded that the weathering of carbonate and silicate rocks is the primary source of substances in karst and pore water, respectively. Human activities have a significant impact on Cambrian–Ordovician karst water. Analysis of 42 groundwater samples from the Kali watershed (Bulandshahr and Aligarh districts) by Arina et al. demonstrated that SiO2 concentrations (18–52 mg/L) showed no significant correlation with Cl− or TDS, confirming silicate mineral weathering as the primary source of SiO2. In contrast, Cl− enrichment was linked to anthropogenic inputs (e.g., agricultural runoff and wastewater). These results underscore the distinct roles of natural weathering and human activities in shaping groundwater chemistry, highlighting the spatial variability of geogenic versus anthropogenic controls under diverse hydrogeological conditions [6].
Humid mountainous regions are characterized by abundant rainfall, well-developed vegetation, complex groundwater movement through rock and soil, and strong water–rock interactions. The hydrochemical processes in these areas encompass dissolution, concentration, cation exchange adsorption, and the influence of human activities [7]. Research on the hydrochemical characteristics of groundwater in humid mountainous regions has revealed notable variations across different water-bearing structures. The observed hydrochemical types, including HCO3-Ca, HCO3-Ca-Na, and HCO3-SO4-Na, demonstrate a strong correlation with groundwater runoff depth, suggesting this hydrological parameter plays a governing role in water–rock interaction processes. Sun et al. [8] explored the development patterns and health-related functions of mineral water under these hydrochemical characteristics, suggesting that mineral water from different aquifers is influenced by dissolution and cation exchange adsorption processes. Studies have shown that the distribution patterns of pH in hydrochemistry are closely related to the types of aquifer media and generally exhibit a gradual increasing trend with greater groundwater depth. Gong et al. investigated the distribution patterns of total dissolved solids (TDS), noting a general correlation with elevation, reflecting the pathways and duration of groundwater movement. Current research commonly employs methods such as mathematical statistics [9], Piper trilinear diagrams [10], Gibbs diagrams [11], ion ratio coefficients [12], and isotope tracing [13].
Building on the understanding of groundwater distribution characteristics in the Taoshan rock mass of the Yushan Uplift in southern Jiangxi, this study employs mathematical statistics, ion ratio coefficients, and factor analysis to summarize the hydrochemical characteristics of groundwater in humid mountainous areas. The study also examines the influence of lithological sources and primary natural mechanisms. By doing so, it contributes to a deeper comprehension of the hydrochemical distribution patterns in the region. Moreover, it offers valuable reference insights for groundwater research in similar areas.
2. Materials and Methods
2.1. Study Area
The study area is located in the mid-subtropical monsoon humid zone, character-ized by distinct seasons and synchronous rainfall and heat, with an average annual precipitation of approximately 1928.4 mm [14,15]. Situated in Jiang Xi province, the study area spans a latitude range of 115°46′12″ E to 116°06′00″ E and a longitude range of 26°30′00″ N to 26°54′00″ N. The region is entirely mountainous, with well-developed vegetation and a general topography that is higher in the north and lower in the south, featuring predominantly north–south-oriented valleys.
The Taoshan rock mass is situated in the northern part of the eastern segment of the Nanling Mountains, at the junction of the Yangtze Plate and the Cathaysia Plate, within the northern end of the Yushan Uplift Belt. It is bordered by the Ganzhou Basin to the left and the Yudu–Ningdu Depression to the right, exhibiting a northeast-trending tongue-shaped distribution. It spans approximately 40 km in length from north to south and 28 km in width from east to west, covering an area of about 1100 km2 [16,17]. The regional structure of the Taoshan rock mass is influenced by the NNE-trending Yingtan–Anyuan deep fault and the NE-trending Dayu–Nancheng deep fault zone, with predominant development of NE-trending and EW-trending faults, primarily characterized by brittle faulting and localized brittle–ductile deformation. The Taoshan Fault, the largest fault structure in the study area, is mainly thrust in nature and exhibits multiphase activity, often intersected by later NNE-trending or NW-trending faults and filled with dikes, contributing to the enrichment of minerals in groundwater.
The rock types are predominantly Early Cretaceous Kuzhushan sequence biotite monzogranite and Middle Jurassic Daguzhai sequence two-mica syenogranite [18], rich in SiO2. Influenced by climate, vegetation, structure, and rock composition, the weathering crust is well developed, reaching thicknesses of over a hundred meters, forming widely distributed bedrock fissure water. The ZK22 hydrogeological borehole, with a depth of 201.9 m, is artesian, with a confined water level 3.2 m above the ground surface, a discharge rate of 290 m3/day, and a water temperature of 30.5 °C, primarily due to radioactive heating. The hydrochemical type is HCO3-SO4-Na, rich in H2SiO3 (80.55 mg/L) and F− (7.80 mg/L), meeting the criteria for natural mineral water with therapeutic benefits, as defined in Chinese National Standard for Geological Exploration of Natural Mineral Water Resources [19].
2.2. Research Data
This study is based on 45 groundwater samples collected by the Hydrogeological and Environmental Geological Survey Center of the China Geological Survey between August and October 2017. At each sampling site, three types of water samples—raw, acidified (pH < 2), and alkalized (pH > 12)—were collected simultaneously and transported to the laboratory for analysis within 24 h. The sampling locations were strategically selected based on topography, geological formations, and hydrogeological characteristics, with all samples sourced from bedrock-dominated mountainous regions. Sampling primarily focused on springs and civilian wells, most of which were expanded from springs, with well depths generally less than 10 m. The distribution of sampling points is illustrated in Figure 1.
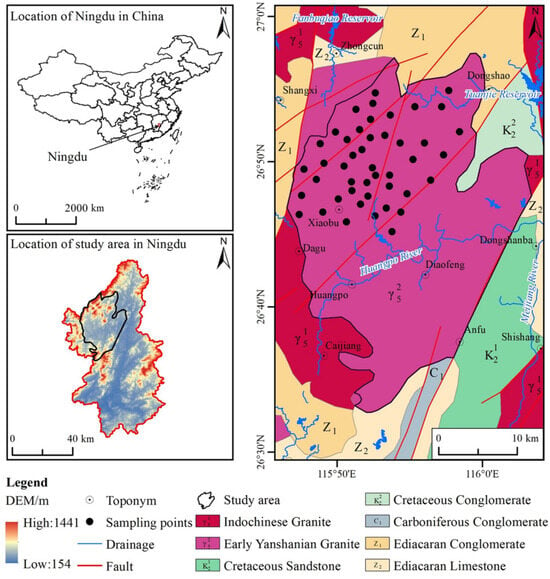
Figure 1.
Study area and sample distribution [20,21].
During sample collection, sampling bottles were rinsed with distilled water and then flushed three times with the original water to ensure that the samples accurately reflected the true conditions of the groundwater at the sampling sites. All sampling points were georeferenced using GARMIN GPS devices. The collected water samples were transported and stored according to standard protocols and sent to the Nanchang Mineral Resources Supervision and Testing Center of the Ministry of Natural Resources (Jiangxi Institute of Geological Survey) for analysis. The analysis was conducted in accordance with the “Testing Methods for Drinking Natural Mineral Water ” [22].
The analytical parameters included Cl−, SO42−, HCO3−, K+, Na+, Ca2+, Mg2+, CO32−, NO3−, F−, NO2−, Br, I, Li, Sr, Zn, Se, Cu, Hg, Cd, Ba, Cr, Pb, Co, V, Mo, Mn, Ni, As, Ag, Al3+, CN−, Fe2+, Fe3+, NH4+, H2SiO3, pH, color, turbidity, odor and taste, visible particulates, free CO2, total hardness, total dissolved solids (TDS), coliform bacteria, permanganate index, total bacterial count, boric acid (as B), and volatile phenols, among others. The samples were analyzed by the Experimental and Testing Center of Jiangxi Institute of Geological Survey. The lab followed standard groundwater quality testing methods. A flame atomic absorption spectrophotometer (contrAA 300, Jena, Germany) measured K+, Na+, Ca2+, and Mg2+ concentrations, with detection limits of 0.05, 0.01, 0.1, and 0.1 mg/L, respectively. HCO3− and CO32− were quantified through acid–base indicator titration, both exhibiting a method detection limit of 5 mg/L. An ion chromatography (Metrohm 883 Basic IC Plus, Herisau, Switzerland) was employed to analyze SO42−, Cl−, NO3−, and F− concentrations, showing method detection limits of 0.1, 0.05, 0.02, and 0.03 mg/L, respectively. NO2− concentration was measured using the TU-1901 UV spectrophotometer (Beijing Puxi General Instrument, Beijing, China) with a method detection limit of 0.02 mg/L. The ionic charge balance error of the measured water samples was controlled within 5%.
2.3. Methodology
The study utilized SPSS 26 and Excel 2019 software to perform statistical descriptive analysis on various ion indicators in groundwater, determining the basic composition of groundwater and analyzing the distribution of each component. The hydrochemical software RockWare AQQA 1.5.0 was employed to construct Piper trilinear diagrams, elucidating the evolution of groundwater hydrochemical types. Gibbs diagrams were used to analyze the controlling factors of major ions in groundwater from three perspectives: atmospheric precipitation control, rock weathering control, and evaporation–concentration control. The ion ratio coefficient method was applied to explore the hydrochemical characteristics of groundwater, while correlation analysis and factor analysis were conducted to investigate the sources of ions. A detailed methodological flow chart for groundwater hydrochemical analysis is provided in Figure 2.
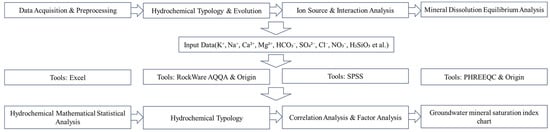
Figure 2.
Methodological flow chart.
3. Results and Discussion
3.1. Statistical Characteristics of Groundwater Components
As shown in Table 1, the groundwater in the Taoshan rock mass is predominantly neutral, with a mean pH value of 7.13, classifying it as weakly alkaline. The coefficient of variation for pH is only 0.04, indicating that pH remains highly stable in the groundwater environment. The total dissolved solids (TDS) range from a minimum of 37.93 mg/L to a maximum of 228.16 mg/L, categorizing the groundwater as low-mineralization water. This is attributed to the humid climate of the region, where soil and rocks are subject to leaching processes, preventing the accumulation of soluble salts.

Table 1.
Statistical characteristics of major groundwater components in the study area [mg·L−1].
Among the major cations (K+, Na+, Ca2+, Mg2+), the concentrations generally follow the order Ca2+ > Na+ > K+ > Mg2+. Ca2+ exhibits the highest concentration, with an average of 12.28 mg/L and a maximum of 53.20 mg/L. Among the anions (Cl−, SO42−, HCO3−, NO3−), HCO3− has the highest concentration, averaging 67.74 mg/L and reaching a maximum of 220.41 mg/L, following the order HCO3− > Cl− > NO3− > SO42−. The coefficients of variation for K+, Na+, Ca2+, and HCO3− are relatively small, all below 1, indicating that the concentrations of these cations in groundwater are relatively stable. In contrast, the coefficients of variation for SO42− and NO3− are larger, with the former related to the depth of groundwater runoff in the granite weathering crust and the activity of rock veins, and the latter primarily influenced by human activities.
The elevated metasilicic acid (H2SiO3) concentrations in regional groundwater (mean: 34.15 mg/L) are intrinsically linked to the weathering of silicate minerals (e.g., quartz, feldspars) within the fracture-controlled aquifer system, where prolonged water–rock interactions under CO2–H2O conditions drive the dissolution of biotite–monzogranite and two-mica syenogranite widely distributed in the study area. According to the standards for drinking natural mineral water, water with metasilicic acid (H2SiO3) concentrations between 25.0 and 30.0 mg/L and a temperature above 25 °C can be classified as metasilicic acid mineral water [23]. In this study area, nine samples did not meet the criteria, as their H2SiO3 concentrations were below 30.0 mg/L and temperatures were under 25 °C. However, 36 samples exhibited H2SiO3 concentrations ranging from 30.72 to 48.56 mg/L, with no exceedances in other hydrochemical indicators, meeting the standards for classification as natural metasilicic acid mineral water.
3.2. Characteristics of Hydrochemical Types
The Piper trilinear diagram provides an intuitive representation of the evolutionary patterns of groundwater chemical composition [24]. As previously mentioned, the concentration of major cations in the groundwater of the study area follows the order Ca2+ > Na+ > K+ > Mg2+, while the concentration of major anions follows the order HCO3− > Cl− > NO3− > SO42−. Among these, HCO3− and Ca2+ dominate in terms of concentration. As shown in Figure 3, the groundwater sample points are clustered near the HCO3− endmember and distant from the SO42− and Cl− endmembers. In the cation triangle, the sample points are located near the Ca2+ endmember and also close to the Na+ and K+ endmembers, indicating that Ca2+ and Na+ are the predominant cations. This suggests that the sampled groundwater is minimally influenced by human activities, with ions primarily derived from the weathering and dissolution of granite and metamorphic rocks. The weathering of silicate rocks not only releases abundant HCO3− but also dissolves Na+ and K+ into the water. Consequently, the groundwater types in the study area are predominantly HCO3-Ca·Na (accounting for 69%) and HCO3-Ca (accounting for 15%).
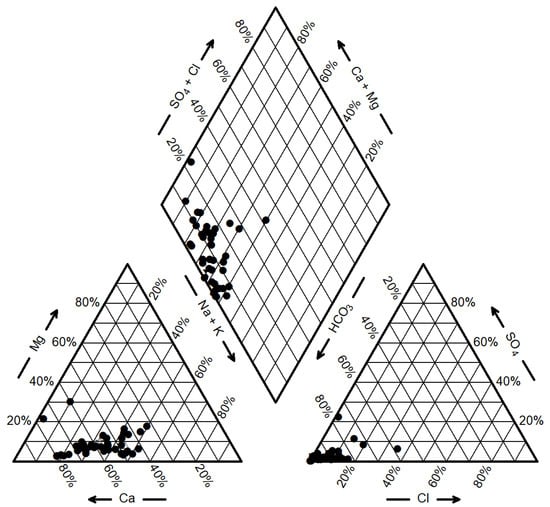
Figure 3.
Ternary diagrams for groundwater.
3.3. Analysis of Hydrochemical Genesis
3.3.1. Control Analysis of Ion Composition
The Gibbs diagram, which plots Na+/(Na+ + Ca2+) against Cl−/(Cl− + HCO3−), categorizes the controlling factors of groundwater formation into three types: atmospheric precipitation control, rock weathering control, and evaporation–concentration control. In the Gibbs diagram, samples with low TDS (<10 mg/L) and high Na+/(Na+ + Ca2+) ratios fall within the atmospheric precipitation control zone, indicating dominant influence from atmospheric precipitation. Samples with moderate TDS and low ratios are located in the rock weathering control zone, primarily influenced by rock weathering. Samples with high TDS and high ratios are situated in the evaporation–crystallization zone, mainly affected by evaporation–concentration processes [25].
As shown in Figure 4, all groundwater samples from the study area fall within the TDS range of 38–229 mg/L, with Na+/(Na+ + Ca2+) ratios mostly below 0.5 and Cl−/(Cl− + HCO3−) ratios below 0.38. The sample points are clustered on the left side of the Gibbs diagram, with a few points leaning toward the precipitation control zone. This indicates that the chemical composition of the groundwater is primarily controlled by rock weathering, with secondary influence from precipitation, and minimal impact from evaporation–concentration processes. The water–rock interaction, a long-term and continuous natural process, plays a significant role in the study area. Groundwater, recharged by atmospheric precipitation, migrates through the well-developed weathering crust of granite under a hot and rainy climate. As groundwater flows through fractures, ion concentrations gradually accumulate, resulting in the current ion distribution characteristics.
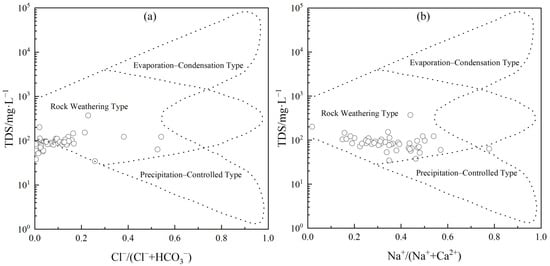
Figure 4.
Plots of the major ions within the Gibbs boomerang envelope: (a) Cl−/(Cl−+ HCO3−) versus TDS; (b) Na+/(Na+ + Ca2+) versus TDS.
Groundwater composition is typically influenced by the weathering of evaporite salts, silicate rocks, and carbonate rocks. The ratios of Ca2+/Na+ and Mg2+/Na+ to HCO3−/Na+ are commonly used to study the interactions between water and different rock types [26]. As shown in Figure 5, only two sampling points exhibit a shift toward carbonate rock control. Field investigations reveal that these points are located in karst-developed areas, where groundwater continuously reacts with limestone, resulting in elevated concentrations of Ca2+ and HCO3−. The remaining sampling points cluster near the silicate rock control endmember, with a few samples approaching the carbonate rock endmember. This indicates that the groundwater in the study area is primarily controlled by the weathering and dissolution of silicate rocks, with a secondary contribution from carbonate rock weathering.
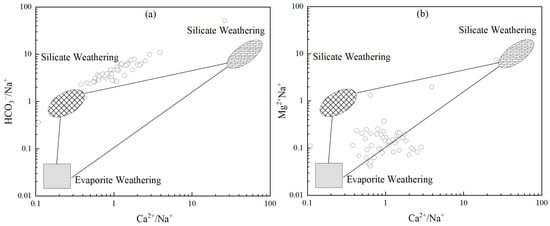
Figure 5.
Bivariate relationships of ionic ratios in groundwater: (a) Ca2+/Na+ versus HCO3−/Na+; (b) Ca2+/Na+ versus Mg2+/Na+.
The triangular diagrams of anions and cations can be used to distinguish the material composition of different weathering source areas [27]. In the anion triangular diagram, weathering products of carbonate rocks, which contain almost no Si, are clustered near the HCO3− peak. Weathering products of evaporite salts are located near the (Cl− + SO42−) end, while weathering products of silicate rocks, which contain both HCO3− and Si, are generally positioned in the upper part of the triangle. In the cation triangular diagram, weathering products of carbonate rocks are located near the Mg2+ and Ca2+ lines, evaporite salt weathering products are clustered near the (Na+ + K+) peak, and silicate mineral weathering products are positioned along the Mg2+–Ca2+ line toward the (Na+ + K+) end.
As shown in Figure 6, in the cation triangular diagram, the sample points are located along the Mg2+–Ca2+ line toward the (Na+ + K+) end. In the anion triangular diagram, the sample points are clustered in the upper left, near the Si end. This indicates that the chemical composition of groundwater in the study area reflects the control of silicate rock weathering, which is consistent with the widespread distribution of granite in the region.
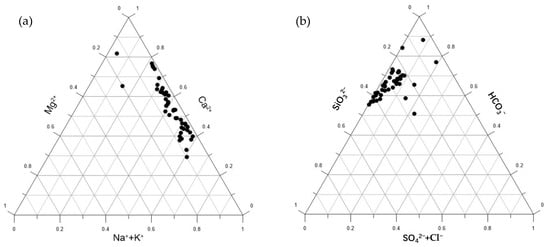
Figure 6.
Ternary diagram of major ion composition: (a) Cationic components (Na+ + K+ versus Ca2+ versus Mg2+); (b) Anionic components (SiO32− versus HCO3− versus (SO₄2− + Cl−)).
This analysis further confirms that silicate rock weathering is the dominant process influencing groundwater chemistry in the study area, with a minor contribution from carbonate rock weathering.
3.3.2. Analysis of Ion Proportionality Coefficients
- γNa/γCl
Groundwater recharge is generally sourced from atmospheric precipitation, with ions such as Na+ and K+ typically originating from the dissolution of atmospheric precipitation and the weathering of silicate rocks. The Na+/Cl− ratio in atmospheric precipitation and seawater is approximately 0.86 [28].
As shown in Figure 7, only one data point in the study area groundwater has a Na+/Cl− ratio less than 0.86, with a mean value of 21.54, which is significantly higher than the Na+/Cl− ratio in seawater. This indicates that the groundwater is not influenced by marine aerosols. The ratios are predominantly above the 1:1 line, with Na+ concentrations far exceeding those of Cl−. This suggests that the sources of Na+ and K+ ions in the groundwater are not solely from atmospheric precipitation and the dissolution of rock salt, but rather largely from the weathering dissolution of rocks.
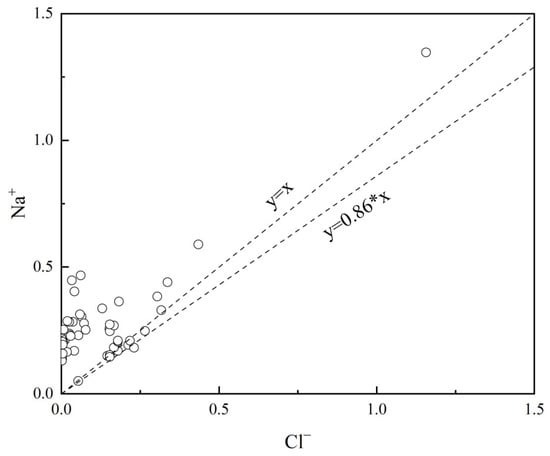
Figure 7.
Relationship between Na+ and Cl− in groundwater.
As depicted in Figure 8, only one data point for the groundwater Na++K+/Cl− ratio is below the 1:1 line, while the others are above it, with a mean Na++K+/Cl− ratio of 26.16. The significantly higher concentrations of Na++K+ compared to Cl− indicate that the excess Na++K+ content is primarily derived from the weathering dissolution of silicate rocks such as potassium feldspar and sodium feldspar. The contributions from atmospheric precipitation and the dissolution of evaporite rocks are minimal.
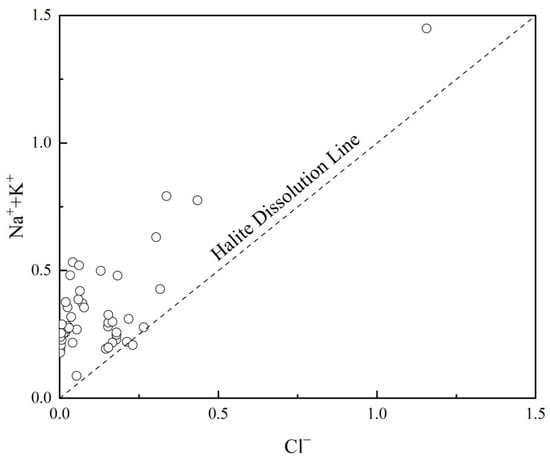
Figure 8.
Relationship between Na++K+ and Cl− in groundwater.
- 2.
- SO42−/Ca2+ and NO3−/Ca2+
When studying the impact of human activities on groundwater, the ratios of SO42−/Ca2+ and NO3−/Ca2+ are commonly used to identify the primary influencing factors. If SO42−/Ca2+ < NO3−/Ca2+, the groundwater is considered to be significantly influenced by agricultural activities and domestic wastewater. Conversely, if SO42−/Ca2+ > NO3−/Ca2+, industrial and mining activities are the dominant influences [29]. As shown in Figure 9, the average concentrations of SO42− and NO3− in the groundwater of the study area are only 2.26 mg/L and 2.86 mg/L, respectively, indicating that the groundwater in the bedrock mountainous area is largely unaffected by human activities. Only a few sampling points show minor influences from agricultural activities and domestic wastewater.
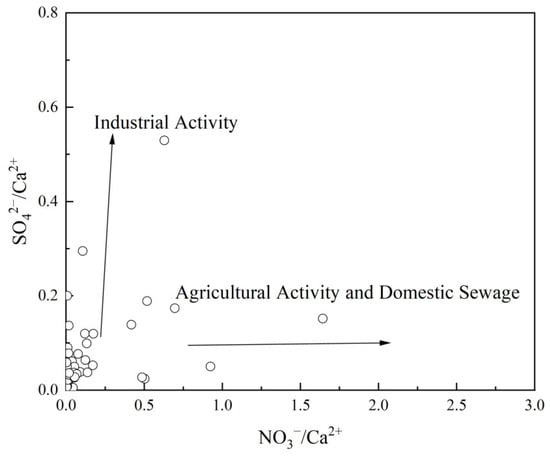
Figure 9.
Relationship between SO42−/Ca2+ and NO3−/Ca2+ in groundwater.
- 3.
- Ca2+/Mg2+
Ca2+ and Mg2+ in groundwater primarily originate from the weathering and dissolution of evaporite minerals. A Ca2+/Mg2+ ratio between 1 and 2 suggests that Mg2+ and Ca2+ are mainly derived from the weathering of carbonate rocks, while a Ca2+/Mg2+ ratio greater than 2 indicates that the dissolution of evaporite minerals (e.g., gypsum) and silicate minerals plays a dominant role [30]. As shown in Figure 10, only two sampling points fall within the 1–2 range, while the remaining 43 points have Ca2+/Mg2+ ratios greater than 2, with an average value of 9.89. This demonstrates that the groundwater in the study area is primarily influenced by the weathering and dissolution of silicate rocks and gypsum. However, the average SO42− concentration is only 2.26 mg/L, suggesting that the dissolution of gypsum is limited, and the dominant source of ions is the dissolution of silicate minerals.
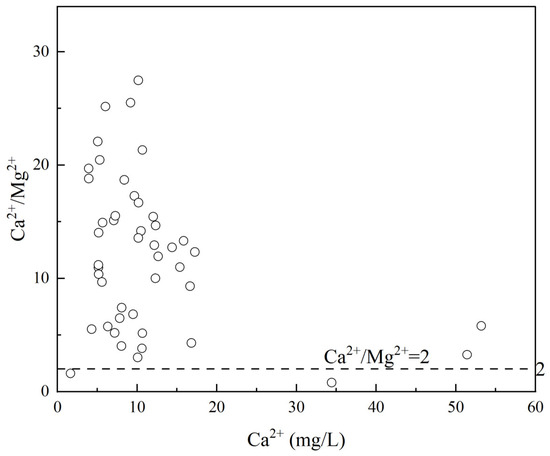
Figure 10.
Relationship of the Ca2+/Mg2+ and Ca2+ in groundwater.
- 4.
- Mg2+/Ca2+ and HCO3−
The ratio of Mg2+/Ca2+ in relation to HCO3− is commonly used to assess the contribution of dolomite or calcite to the material sources in groundwater within carbonate rock weathering zones. In the study area, the average Mg2+/Ca2+ ratio in groundwater is 0.16 (Figure 11), with most values clustered around 0.16. Only two sampling points exceed 0.5, indicating that the weathering and dissolution of calcite dominate in the study area. These two points, however, reflect the combined influence of calcite and dolomite weathering and dissolution. This further supports the conclusion that calcite dissolution is the primary process controlling groundwater chemistry in the region, with limited contributions from dolomite.
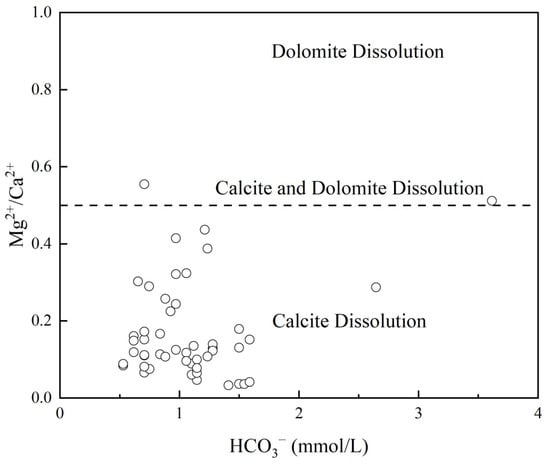
Figure 11.
Carbonate rock weathering in the Ningdu county.
- 5.
- (Mg2++Ca2+) and (HCO3−+SO42−)
As shown in Figure 12, only two points have (Mg2+ + Ca2+) > (HCO3− + SO42−). Field investigations revealed that these two points are located in strata of Carboniferous dolomitic limestone, which are distinctly controlled by carbonate rocks. The remaining points are all below the 1:1 line, indicating that additional cations such as K+ and Na+ are required to balance the anions to achieve equilibrium. These cations, K+ and Na+, primarily originate from the weathering and dissolution of silicate rocks, suggesting that the dissolution of silicate rocks is widespread and dominant in the study area. Only two points have (Mg2+ + Ca2+) < (HCO3− + SO42−). Field surveys indicated that these two points are exposed in strata of Carboniferous dolomitic limestone, which are significantly influenced by carbonate rocks. The other points are all on the 1:1 line. In conjunction with Figure 8, this suggests that additional anions, such as silicate ions, are needed to balance the charges. The SiO32− mainly comes from the weathering and dissolution of minerals like quartz, feldspar, and mica, indicating that the dissolution of silicate rocks is common and plays a significant dominant role in the study area.
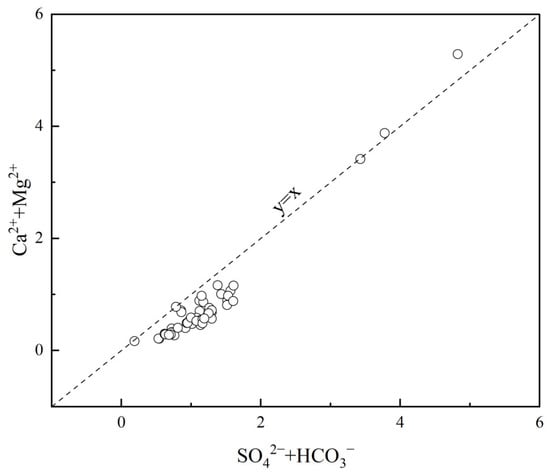
Figure 12.
(Ca2++ Mg2+) versus (SO42−+ HCO3−) concentrations in the samples.
3.3.3. Correlation Analysis of Ion Sources
- 6.
- Correlation Analysis
As shown in Figure 13, the statistical analysis software SPSS was used to calculate the Pearson correlation coefficients for Cl−, SO42−, HCO3−, NO3−, K+, Na+, Ca2+, Mg2+, NO3−, TDS, and H2SiO3 in the groundwater of the study area.
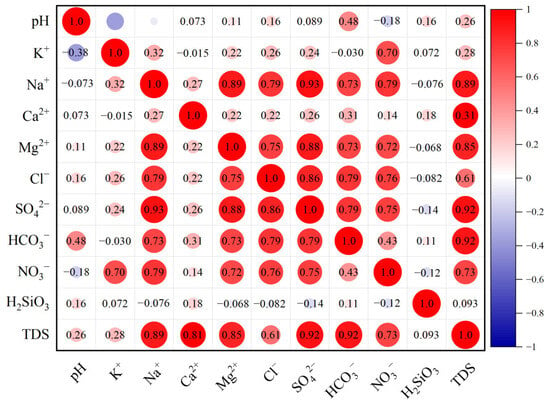
Figure 13.
Groundwater water chemical correlation chart.
It can be observed that TDS has significant positive correlation with Mg2+, SO42−, HCO3−, Na+, Ca2+, and others, indicating that these components all make a significant contribution to TDS. Among them, the correlation between TDS and HCO3− is the most significant, with a correlation coefficient of 0.92. The correlation coefficient between Ca2+ and HCO3− is as high as 0.89, suggesting that Ca2+ and HCO3− have the same origin. HCO3− has significant correlation with both Ca2+ and Mg2+, with correlation coefficients all above 0.80, indicating that these three have the same material source, likely from the weathering and dissolution of granite. Na+ and K+ have correlation coefficients of 0.79 and 0.70 with NO3−, respectively, suggesting that these three ions may be affected by human activities. The correlation coefficient between SO42− and Mg2+ is 0.99, indicating that the dissolution of gypsum may provide SO42− and Mg2+ to the groundwater. H2SiO3 has no significant correlation with other ions, suggesting that in addition to the weathering and dissolution of silicate rocks, H2SiO3 should also be related to the weathering and dissolution of quartz minerals.
- 7.
- Factor Analysis
Factor analysis is a method of data dimensionality reduction that uses a few factors to describe the correlation patterns observed in multiple variables, with the principal factors exerting a primary influence on the subject of study [30] Utilizing SPSS software and employing principal component extraction with varimax orthogonal rotation factor analysis, the results presented in Figure 14 were obtained. Factors with eigenvalues greater than 1 were selected based on the cumulative variance contribution rate, with a cumulative contribution rate of 87.698%, which can reflect 87.698% of the information in the samples; three principal components were obtained. The projection values of each variable onto the first, second, and third principal components represent their loading values. The evaluation criteria define factor loading values as follows: values greater than 0.90 are classified as “Extremely High Positive”, values between 0.75 and 0.90 as “High Positive”, values between 0.60 and 0.75 as “Moderate Positive”, values between 0.45 and 0.60 as “Low Positive”, and values below 0.45 as “Very Low Positive”. This classification quantitatively reflects the strength of correlations between variables and latent factors in statistical analyses.
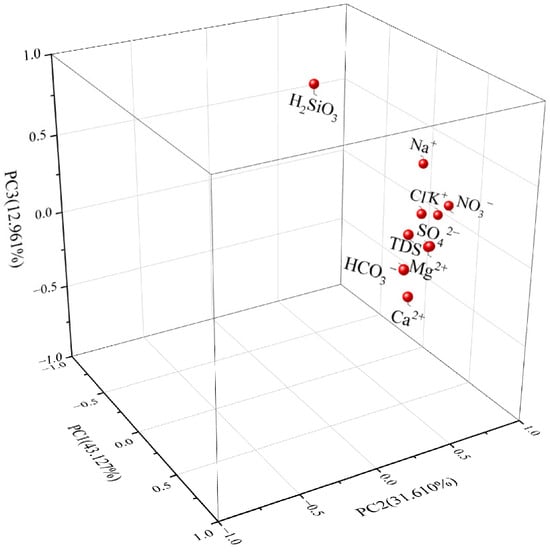
Figure 14.
Analysis of principal components of groundwater ions.
As illustrated in Figure 14, the first principal component (PC1) exhibits high-to-extremely high positive loadings (>0.75) for hydrochemical indicators including TDS, Ca2+, HCO3−, Mg2+, and SO42−, with strong inter-correlations among these parameters. PC1 accounts for 43.127% of the total variance, signifying its dominant role in governing groundwater chemistry. This component is predominantly attributed to dissolution processes involving silicate minerals (e.g., aluminosilicates), calcite, and sulfate-bearing minerals, which continuously release soluble constituents (Ca2+, Mg2+, HCO3−, SO42−) into groundwater through water–rock interactions. Such processes collectively drive the evolution of hydrochemical facies, ultimately manifesting as the HCO3–Ca water type characteristic of this aquifer system.
The second principal component (PC2) explains 31.610% of the total variance. This component exhibits high positive loadings (>0.75) for NO3− and K++Na+, suggesting a hydrogeochemical regime influenced by shallow groundwater circulation with rapid recharge–discharge cycles dominated by meteoric precipitation. The elevated loadings likely reflect anthropogenic inputs (e.g., domestic wastewater infiltration) into localized flow paths, which alter groundwater quality through nitrate enrichment and ionic composition shifts. Such processes highlight the system’s enhanced vulnerability to surface-derived contaminants, consistent with observed deviations in sodium/potassium ratios and nitrate anomalies across sampling sites.
The third principal component (PC3) accounts for 12.961% of the total variance, with high positive loadings (>0.75) observed for H2SiO3 and Na+. These loadings are attributed to the hydrolysis of sodium-rich silicate minerals (e.g., albite), which release H2SiO3 and Na+ into groundwater through weathering processes. This mechanism not only governs the hydrochemical evolution of the aquifer but also serves as a primary factor in the formation of metasilicic mineral water, characterized by elevated H2SiO3 concentrations (>30 mg/L) and Na+-enriched compositions.
3.3.4. Mineral Dissolution Equilibrium
The dissolution dynamics of minerals during water–rock interactions are critical for deciphering geochemical cycling and solute acquisition mechanisms in aquifer systems. To assess these processes, we employed PHREEQC to simulate saturation indices (SI) of key minerals (gypsum, calcite, dolomite, quartz, and clinopyroxene/clinostatite) across groundwater samples from the study area. As illustrated in Figure 15, the boxplot statistically synthesizes SI distributions derived from hydrogeochemical datasets, with the interquartile range (IQR: 25th–75th percentiles) delineating the central 50% of mineral saturation states. The median line represents equilibrium tendencies, while whiskers (1.5 × IQR) define natural variability thresholds; outliers beyond this range signify extreme SI anomalies attributable to localized geochemical perturbations (e.g., evaporite dissolution hotspots or kinetic inhibition zones). This visualization quantifies both equilibrium constraints (e.g., near-saturation medians for gypsum, SI ≈ 0) and disequilibrium drivers (e.g., quartz supersaturation across all samples), providing a robust framework to differentiate lithologically controlled weathering patterns from anthropogenically altered solute regimes.
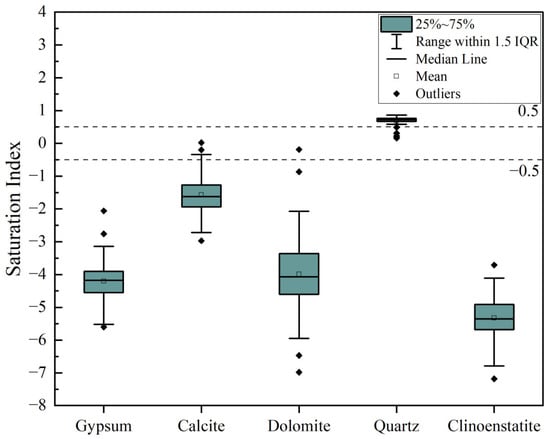
Figure 15.
Groundwater mineral saturation index chart.
Gypsum (CaSO4·2H2O), as a typical soluble mineral, exhibits a significant undersaturation state in fracture water. Its dissolution reaction primarily releases Ca2+ and SO42−, significantly increasing the concentrations of these two ions in fracture water. This indicates that, in the early stages of water–rock interaction, the dissolution of gypsum plays a dominant role in adjusting the chemical composition of fracture water. However, over time, the gradual dissolution of gypsum leads to a decrease in its concentration, thereby affecting the concentrations of Ca2+ and SO42− in the water. It is worth noting that the dissolution process of gypsum is also regulated by environmental factors, such as the pH value, temperature, and CO2 concentration in the water, which can significantly alter the rate of mineral dissolution and the ultimate equilibrium state.
Compared with gypsum, carbonate minerals (such as calcite and dolomite) typically exhibit more complex dissolution–precipitation equilibrium characteristics in water. Studies have shown that the saturation index (SI) of calcite is close to zero, indicating that the water is in a state of dissolution–precipitation equilibrium with calcite. Dolomite, with an SI value close to that of gypsum, is significantly undersaturated. The concentrations of Ca2+ and CO32− ions in the water are balanced within a certain range, determining the dissolution or precipitation behavior of these two carbonate minerals. Particularly for dolomite, its dissolution rate is usually slow and is mainly controlled by the Mg2+ concentration in the water and the partial pressure of CO2. Due to the significant variations in CO2 and HCO3− concentrations in the water, the dissolution behavior of carbonate minerals is particularly critical in the evolution of water bodies. They not only affect the hardness of the water but also participate in interactions with other minerals.
The dissolution kinetics of silicate minerals are even more complex. Quartz is generally supersaturated in fracture water, with a high SI value, indicating that it may precipitate in the form of amorphous silicates or secondary clay minerals over long-term water–rock interactions. Clinoenstatite, as a magnesium silicate mineral, is compositionally similar to biotite and is significantly undersaturated. However, it plays an important role in the release of Mg2+ during weathering processes. In summary, the mineral dissolution equilibrium in fracture water during the weathering of aquifer media exhibits diversity and complexity. The dissolution behavior of different minerals is influenced by their chemical composition and solubility, and the hydrochemical environment.
4. Conclusions
The groundwater in the study area exhibits a weakly alkaline nature, with an average pH of 7.13. The cation composition is dominated by Ca2+ and Na+, which account for 61% and 26% of the total cation concentration, respectively. HCO3− is the predominant anion, making up 91% of the total anion concentration. The Total Dissolved Solids (TDS) levels range from 37.93 mg/L to 228.16 mg/L, indicating low mineralization of the water. Among the 45 natural spring water samples analyzed, 36 have metasilicate content exceeding 30 mg/L, meeting the standard for metasilicate mineral water. This suggests significant potential for the development and utilization of these water resources.
The groundwater type in the study area is primarily HCO3–Ca·Na, with HCO3–Ca as the secondary type. The groundwater composition is mainly controlled by rock weathering. The primary sources of ions are influenced by the weathering and dissolution of silicate rocks, and there are also contributions from the dissolution of carbonate rocks such as calcite. Ion ratio analysis further confirms that the main sources of ions in the groundwater are silicate rock-weathering minerals, with minimal influence from human activities. Na+, K+, and H2SiO3 mainly originate from the weathering and dissolution of silicate rocks. The weathering and dissolution of carbonate rocks like calcite significantly contribute ions such as Ca2+ and Mg2+.
There is a significant positive correlation between TDS and Mg2+, SO42−, HCO3−, Na+, and Ca2+. The correlation between TDS and Ca2+, HCO3− is particularly strong, with a correlation coefficient as high as 0.89. Factor analysis reveals that the first principal component has relatively high loadings for TDS, Ca2+, HCO3−, Mg2+, and SO42−. Overall, these findings provide a comprehensive understanding of the hydrochemical characteristics and the factors influencing the groundwater quality in the study area.
Author Contributions
Conceptualization, X.W., Y.W. (Yan Wang). and G.G.; methodology, M.L., K.M. and Z.S.; software, H.A.; validation, Y.L., Y.W. (Yan Wang), and X.W.; formal analysis, X.W and Z.S.; investigation, X.W.; resources, X.W., Y.W. (Yibing Wang), and J.Y.; data curation, L.G., M.S. and Y.L.; writing—original draft preparation, X.W.; writing—review and editing, X.W; supervision, X.W.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project of China Geological Survey titled “Investigation of lakes in the northern part of the plain lake region in eastern China”(DD20230505, DD20179262), and the Ministry of Natural Resources Provincial Cooperation Project (2024ZRBSHZ028).
Data Availability Statement
The data used in this study are available upon request from the corresponding authors.
Conflicts of Interest
Author Zhijie Sun was employed by the company Sanshandao Gold Mine of Shandong Gold Mining (Laizhou) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ren, C.; Zhang, Q. Groundwater Chemical Characteristics and Controlling Factors in a Region of Northern China with Intensive Human Activity. Int. J. Environ. Res. Public Health 2020, 17, 9126. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Xiao, J.; Li, S.; Li, Z. Analysis of Hydrochemical Characteristics and Their Controlling Factors in the Fen River of China. Sustain. Cities Soc. 2020, 52, 101827. [Google Scholar]
- Khan, A.; Khan, H.H.; Umar, R. Impact of land-use on groundwater quality: GIS-based study from an alluvial aquifer in the western Ganges basin. Appl. Water Sci. 2017, 7, 4593–4603. [Google Scholar]
- Zhao, X.; Xiao, P.; Li, Y.; Shao, C. Hydrochemical Characteristics and Genesis of Shallow Groundwater in Water Shortage Area of Southern Jiangxi Province—Taking Yinkeng Sheet as an Example. South China Geol. 2021, 37, 418–426. [Google Scholar]
- Zhao, X.; Xiao, P.; Song, W.; Li, Y.; Liu, Q. Hydrochemical Characteristics and Genetic Analysis of Groundwaterin Red-Bed Area of South Jiangxi Province. Sci. Technol. Eng. 2023, 23, 14112–14122. [Google Scholar]
- Khan, A.; Umar, R.; Khan, H.H. Hydrochemical characterization of groundwater in lower Kali watershed, Western Uttar Pradesh. J. Geol. Soc. India 2015, 86, 195–210. [Google Scholar]
- Liu, J.; Wang, X.; Xu, W.; Song, M.; Gong, L.; Zhang, M.; Li, L.; He, L. Hydrogeochemistry of Fluorine in Groundwater in Humid Mountainous Areas: A Case Study at Xingguo County, Southern China. J. Chem. 2021, 2021, 1–11. [Google Scholar]
- Sun, Z.; Gao, Z.; Wang, X.; Lin, H.; Song, M. Exploration of Mineral Water Outcropping Pattern in the Mountainous Area of South Jiangxi. Acta Geosci. Sin. 2018, 39, 565–572. [Google Scholar]
- Chen, W.; Wu, Y.; Zhang, H.; Liu, H. Hydrochemical Characteristics and Formation Mechanism Of groundwater in the Western Part of Hepu Basin. Environ. Sci. 2024, 45, 194–206. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water-analyses. Neurochem. Int. 1984, 6, 27–39. [Google Scholar]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [PubMed]
- Wei, S.; Ding, G.; Yuan, G.; Wang, L.; Nie, Y.D. Hydrochemical Characteristics and Formation Mechanismof Groundwater in Yinan Area of Dongwen River, Shandong Province. Acta Geol. Sin. 2021, 95, 1973–1983. [Google Scholar]
- Meng, L. Recharge Source and Evolution Process of Karst Groundwater in Tai’an Urban Area Based on Hydrochemistry and Hydrogen and Oxygen Lsotopes. Environ. Sci. 2024, 45, 2096–2106. [Google Scholar]
- Sun, Y.; Ji, H.; Luo, J.; Jiang, Y.; Li, T. Hydro-Geochemistry and Chemical Weathering Processes of Small Water Sheds in the Southern Jiangxi Province. Environ. Chem. 2006, 25, 550–557. [Google Scholar]
- Sun, Z. Chemical Characteristics and Genesis of Mineral Water in Southern Jiangxi Mountainous Areas. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2019. [Google Scholar]
- Zhang, S.; Xiao, F.; Zou, Y. Discovery of Geothermal Associated Helium Resources and Exploration of Their Anomalous Origins in the Southern Jiangxi Region. Nat. Gas Geosci. 2023, 35, 495–506. [Google Scholar]
- Liu, X.; Luo, H.M.; Jia, F. Geological Characteristics of Rock Mass and Uranium Ore Exploration Sign in Taoshan Area. Zhejiang Geological Society. In Proceedings of the 2015 Academic Annual Meeting of Zhejiang Geological Society, Lishui, China, 13 November 2015; Zhejiang Nuclear Industry 262nd Brigade; In Memory of the 120th Anniversary of the Birth of Geologist Zhu Tinghu. Zhejiang Huzhou Geological Technology Development Company: Huzhou, China, 2015; p. 6. [Google Scholar]
- Xu, X.S.; Zhang, H.; Wu, Y.; Dong, X.Y. The Disintegration of the Taoshan Composite Granite Body in Southern Jiangxi and Its Geological Significance. China Nuclear Society. In Progress Report on Nuclear Science and Technology in China (Volume 6), Proceedings of the 2019 Academic Annual Meeting of China Nuclear Society Volume 1 (Uranium Geology Section (Part 1)). Nuclear Industry 270th Institute, Beijing, China, 20–23 August 2019; China Atomic Energy Press: Beijing, China, 2019; p. 10. [Google Scholar]
- GB/T 13727-2016; Code of Geologic Exploration of Natural Mineral Water Resources. General Administration of Quality Supervision: Beijing, China; Inspection and Quarantine of China: Beijing, China; China Standards Press: Beijing, China, 2016.
- Zhao, K.; Jiang, S.; Dong, C.; Chen, W.; Chen, P.; Ling, H.; Zhang, J.; Wang, K. Uranium-Bearing and Barren Granites from the Taoshan Complex, Jiangxi Province, South China: Geochemical and Petrogenetic Discrimination and Exploration Significance. J. Geochem. Explor. 2011, 110, 126–135. [Google Scholar]
- Sun, T.; Liao, Z.; Wu, K.; Chen, L.; Liu, W.; Yi, C. Fractal Distribution Characteristics and Geological Significances of Fracturestructure in Southern Jiangxi. J. Jiangxi Univ. Sci. Technol. 2017, 38, 48–54. [Google Scholar]
- GB 8537-2018; National Food Safety Standard—Drinking Natural Mineral Water. National Health Commission of the People’s Republic of China: Beijing, China; State Administration for Market Regulation: Beijing, China; China Standards Press: Beijing, China, 2019.
- Zhao, H. Groundwater Recharge, Discharge, Runoff Characteristics and Hydrochemical Evolution of Hamatong River Basin in Sanjiang Plain. Master’s Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Xiao, J.; Jin, Z.D.; Zhang, F.; Wang, J. Solute Geochemistry and Its Sources of the Groundwaters in the Qinghai Lake Catchment, NW China. J. Asian Earth Sci. 2012, 52, 21–30. [Google Scholar]
- Liu, H.; Song, Y.; Li, Y.; Wei, W.; Zhao, G.; Wang, X.; Huang, J. Hydrochemical Characteristics and Control Factors of Shallow Groundwaterin Anging Section of the Yangtze River Basin. Environ. Sci. 2024, 45, 1525–1538. [Google Scholar]
- Edmond, J.M.; Palmer, M.R.; Measures, C.I.; Brown, E.T.; Huh, Y. Fluvial Geochemistry of the Eastern Slope of the Northeastern Andes and Its Foredeep in the Drainage of the Orinoco in Colombia and Venezuela. Geochim. Et Cosmochim. Acta 1996, 60, 2949–2974. [Google Scholar]
- Zhu, B.; Yu, J.; Qin, X.; Rioual, P.; Zhang, Y.; Liu, Z.; Mu, Y.; Li, H.; Ren, X.; Xiong, H. Identification of Rock Weathering and Environmental Control in Arid Catchments (Northern Xinjiang) of Central Asia. J. Asian Earth Sci. 2013, 66, 277–294. [Google Scholar]
- Zhang, T.; He, J.; Li, J.; Cao, Y.; Gong, L. Major Ionic Features and Possible Controls in the Groundwater in the Hamatong River Basin. Environ. Sci. 2018, 39, 4981–4990. [Google Scholar]
- He, J.; Ma, J.; Zhang, P.; Tian, L.; Zhu, G.; Edmunds, W.; Zhang, Q. Groundwater Recharge Environments and Hydrogeochemical Evolution in the Jiuquan Basin, Northwest China. Appl. Geochem. 2012, 27, 866–878. [Google Scholar]
- Qu, S.; Liang, X.; Liao, F.; Mao, H.; Xiao, B.; Duan, L.; Shi, Z.; Wang, G.; Yu, R. Geochemical Fingerprint and Spatial Pattern of Mine Water Quality in the Shaanxi-Inner Mongolia Coal Mine Base, Northwest China. Sci. Total Environ. 2023, 854, 158812. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).