Distribution Characteristics and Adsorption Performance of Microplastics in Domestic Sewage: A Case Study of Guilin, China
Abstract
1. Introduction
2. Materials and Methods
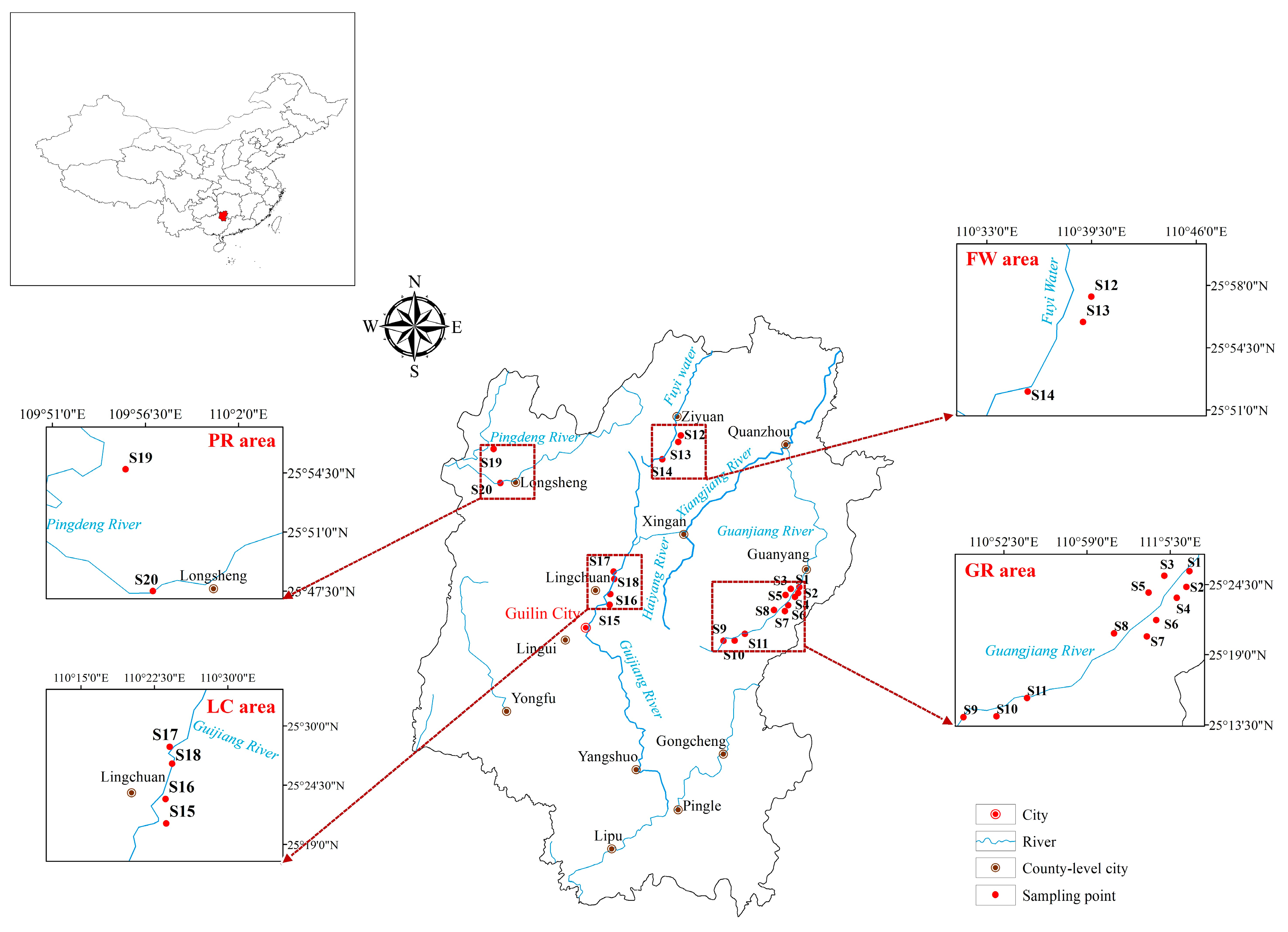
2.1. Sampling and Experiments
2.2. Hydrochemical Analysis
2.3. MP Identification
2.4. Adsorption Experiments and Models
2.5. Quality Control and Data Analysis
3. Results and Discussion
3.1. Hydrochemical Characteristics
3.2. MP Distribution Characteristics
3.2.1. Abundance of MPs
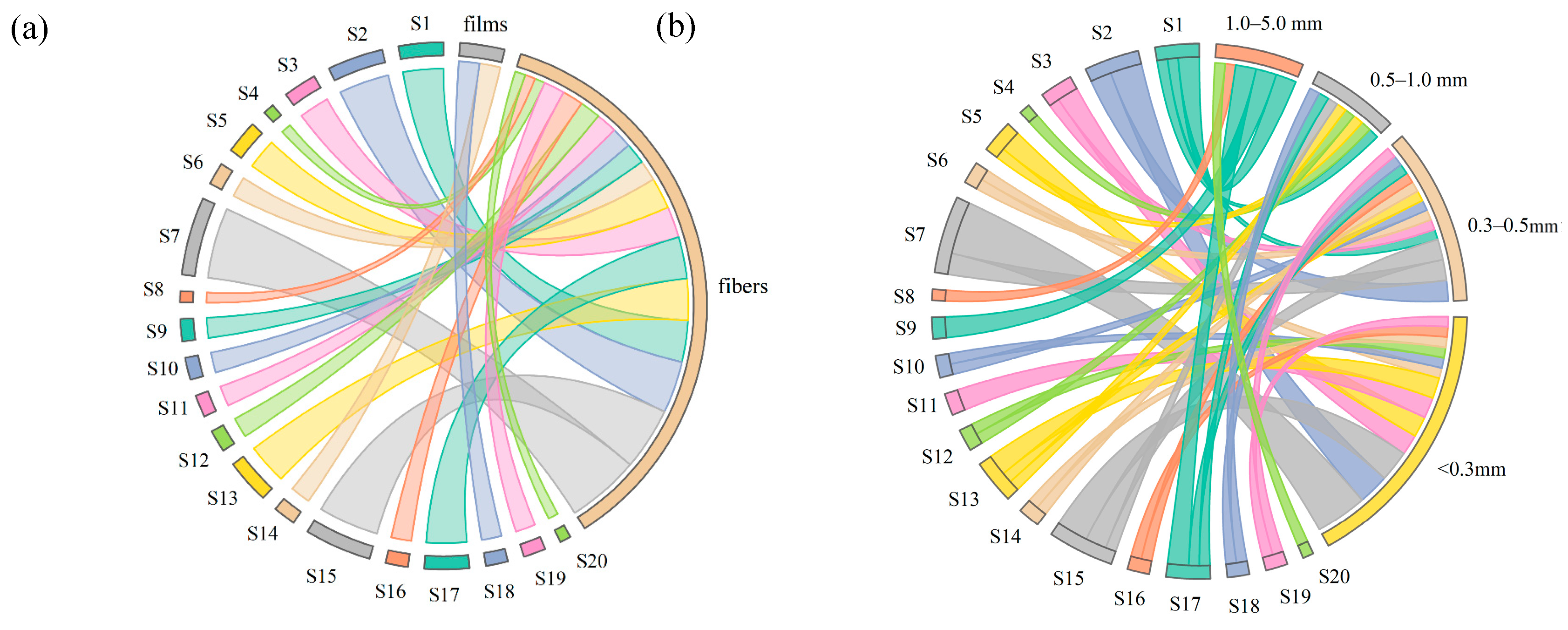
3.2.2. Morphology of MPs
3.2.3. Sizes of MPs
3.3. Relationship Analysis
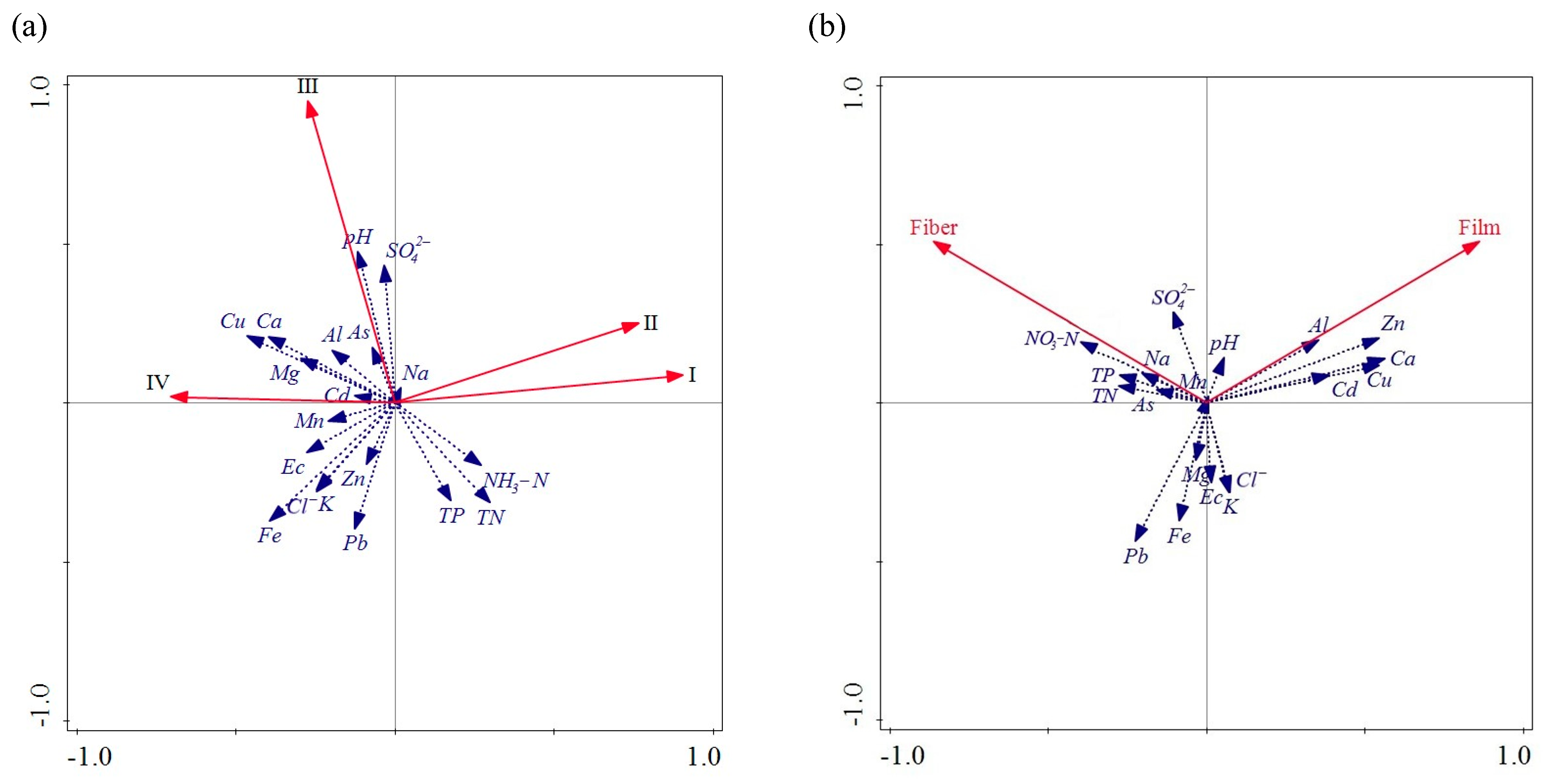
3.3.1. Relationship of MP Abundance with Hydrochemical Parameters
3.3.2. Relationship of MP Size and Morphology with Hydrochemical Parameters
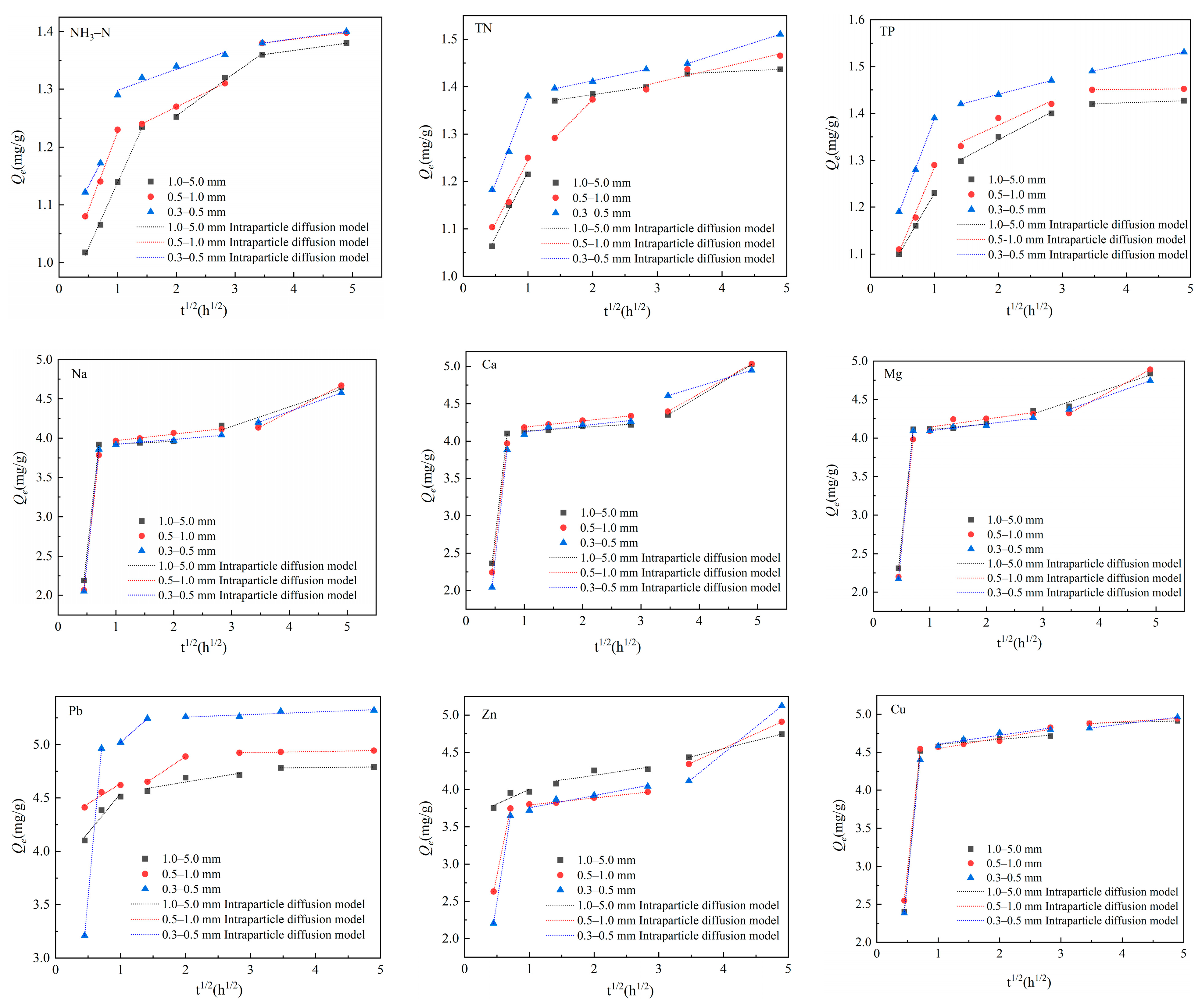
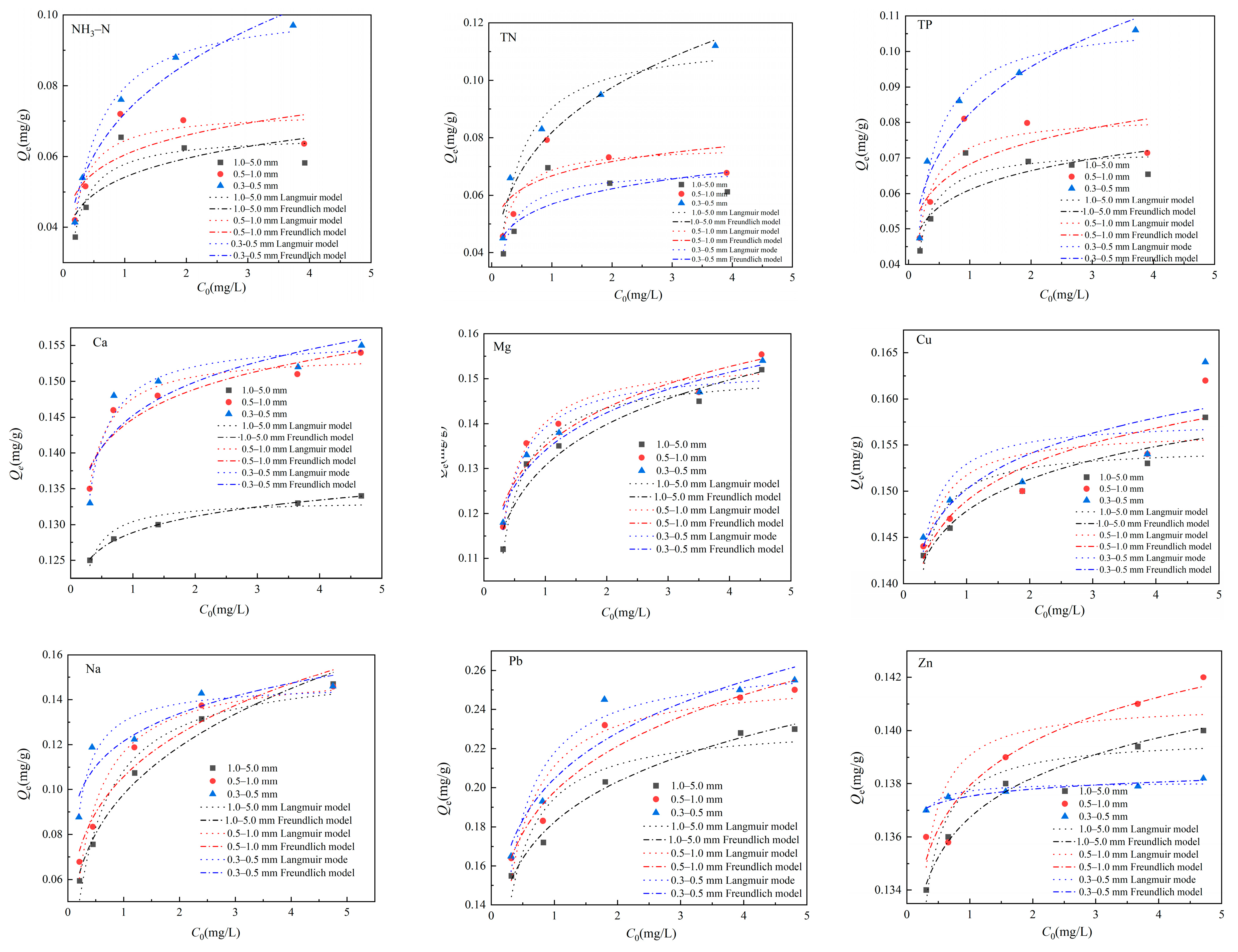
3.4. Adsorption Characteristics and Models
3.4.1. Adsorption Kinetics
3.4.2. Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Zhong, S.; Liu, J.; Qin, Y.; Gao, P. Microplastics in freshwater and wild fishes from Lijiang River in Guangxi, Southwest China. Sci. Total Environ. 2021, 755, 142428. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhu, Z.-R.; Fu, W.; Dai, X.; Ni, B.-J. The atmospheric microplastics deposition contributes to microplastic pollution in urban waters. Water Res. 2022, 225, 119116. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Aleixo, M.; De Pablo, H.; Lopes, C.; Raimundo, J. Microplastics in wastewater: Microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. 2020, 27, 26643–26649. [Google Scholar] [CrossRef] [PubMed]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Distribution of microplastic in relation to water quality parameters in the Brantas River, East Java, Indonesia. Environ. Technol. Innov. 2021, 24, 101915. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Feng, S.; Lu, H.; Yao, T.; Liu, Y.; Tian, P.; Lu, J. Microplastic footprints in the Qinghai-Tibet Plateau and their implications to the Yangtze River Basin. J. Hazard. Mater. 2021, 407, 124776. [Google Scholar] [CrossRef]
- Xu, H.; Nakano, H.; Tokai, T.; Miyazaki, T.; Hamada, H.; Arakawa, H. Contamination of sea surface water offshore the Tokai region and Tokyo Bay in Japan by small microplastics. Mar. Pollut. Bull. 2022, 185, 114245. [Google Scholar] [CrossRef]
- Aransiola, S.A.; Victor-Ekwebelem, M.O.; Daza, B.X.; Oladoye, P.O.; Alli, Y.A.; Bamisaye, A.; Aransiola, A.B.; Oni, A.O.; Maddela, N.R. Micro- and nano-plastics pollution in the marine environment: Progresses, drawbacks and future guidelines. Chemosphere 2025, 374, 144211. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Chen, B.; Booth, A.M.; Liu, S.; Wang, R.; Zhu, L.; Zhao, X.; Qu, K.; Xia, B. Factors influencing the occurrence and distribution of microplastics in coastal sediments: From source to sink. J. Hazard. Mater. 2021, 410, 124982. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, e1398. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Xu, P.; Zeng, G.; Huang, D.; Yin, L.; Yin, Q.; Hu, L.; Wan, J.; Zhang, J.; et al. Microplastic pollution in surface sediments of urban water areas in Changsha, China: Abundance, composition, surface textures. Mar. Pollut. Bull. 2018, 136, 414–423. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Aryani, D.; Khalifa, M.A.; Herjayanto, M.; Pratama, G.; Rahmawati, A.; Putra, R.D.; Munandar, E. Correlation of Water Quality with Microplastic Exposure Prevalence in Tilapia (Oreochromis niloticus). In Proceedings of the E3S Web of Conferences Maritime Continent Fulcrum International Conference (MaCiFIC 2021); EDP Sciences: Les Ulis, France, 2021. [Google Scholar]
- Liu, Q.; Wu, H.; Chen, J.; Guo, B.; Zhao, X.; Lin, H.; Li, H.; Li, W.; Zhao, X.; Lv, S.; et al. Adsorption mechanism of trace heavy metals on microplastics and simulating their effect on microalgae in river. Environ. Res. 2022, 214, 113777. [Google Scholar] [CrossRef]
- Li, S.; Yang, M.; Wang, H.; Jiang, Y. Adsorption of microplastics on aquifer media: Effects of the action time, initial concentration, ionic strength, ionic types and dissolved organic matter. Environ. Pollut. 2022, 308, 119482. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef]
- Xue, X.-D.; Fang, C.-R.; Zhuang, H.-F. Adsorption behaviors of the pristine and aged thermoplastic polyurethane microplastics in Cu(II)-OTC coexisting system. J. Hazard. Mater. 2021, 407, 124835. [Google Scholar] [CrossRef] [PubMed]
- Hasenmueller, E.A.; Baraza, T.; Hernandez, N.F.; Finegan, C.R. Cave sediment sequesters anthropogenic microparticles (including microplastics and modified cellulose) in subsurface environments. Sci. Total Environ. 2023, 893, 164690. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro- and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Gong, X.; Tian, L.; Wang, P.; Wang, Z.; Zeng, L.; Hu, J. Microplastic pollution in the groundwater under a bedrock island in the South China sea. Environ. Res. 2023, 239, 117277. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Shu, X.; Xu, L.; Yang, M.; Qin, Z.; Zhang, Q.; Zhang, L. Spatial distribution characteristics and migration of microplastics in surface water, groundwater and sediment in karst areas: The case of Yulong River in Guilin, Southwest China. Sci. Total Environ. 2023, 868, 161578. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment; NOAA Technical Memorandum NOS-OR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, S.; Wang, K.; Li, D.; Liu, H.; Li, F. Distribution characteristics and pollution risk assessment of microplastics in urban rivers: A case study in Yitong River, China. J. Water Process Eng. 2024, 61, 105277. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef]
- Lagergreen, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Namasivayam, C.; Senthilkumar, S. Removal of Arsenic(V) from Aqueous Solution Using Industrial Solid Waste: Adsorption Rates and Equilibrium Studies. Ind. Eng. Chem. Res. 1998, 37, 4816–4822. [Google Scholar] [CrossRef]
- Adomat, Y.; Grischek, T. Sampling and processing methods of microplastics in river sediments—A review. Sci. Total Environ. 2021, 758, 143691. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Huang, Y.; Jiang, Y.; Li, J.; Michal, J.J.; Jiang, Z.; Xu, Y.; Lan, W. Long-term trends of microplastics in seawater and farmed oysters in the Maowei Sea, China. Environ. Pollut. 2021, 273, 116450. [Google Scholar] [CrossRef]
- Xie, Y.H.; Liu, Z.; Gou, X.; Qin, Y.H.; Li, X.Y.; Chen, S.Y.; Shi, G.Z. Analysis on quality of domestic sewage and investigation of villages ’willingness from the rural areas of southwest China. Environ. Eng. 2018, 36, 165–169+188. [Google Scholar]
- Sefie, A.; Aris, A.Z.; Ramli, M.F.; Narany, T.S.; Shamsuddin, M.K.N.; Saadudin, S.B.; Zali, M.A. Hydrogeochemistry and groundwater quality assessment of the multilayered aquifer in Lower Kelantan Basin, Kelantan, Malaysia. Environ. Earth Sci. 2018, 77, 397. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Peterse, I.F.; Hendriks, L.; Weideveld, S.T.; Smolders, A.J.; Lamers, L.P.; Lücker, S.; Veraart, A.J. Wastewater-effluent discharge and incomplete denitrification drive riverine CO2, CH4 and N2O emissions. Sci. Total Environ. 2024, 951, 175797. [Google Scholar] [CrossRef] [PubMed]
- Dailey, K.R.; Welch, K.A.; Lyons, W.B. Evaluating the influence of road salt on water quality of Ohio rivers over time. Appl. Geochem. 2014, 47, 25–35. [Google Scholar] [CrossRef]
- Kukkola, A.; Runkel, R.L.; Schneidewind, U.; Murphy, S.F.; Kelleher, L.; Smith, G.H.S.; Nel, H.A.; Lynch, I.; Krause, S. Prevailing impacts of river management on microplastic transport in contrasting US streams: Rethinking global microplastic flux estimations. Water Res. 2023, 240, 120112. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Shi, H.; Yu, D.; Yin, L.; Sui, Y.; Liu, Y.; Qiao, S.; Wang, W.; Zheng, W.; Ding, D. Source-sink process of microplastics in watershed-estuary-offshore system. J. Clean. Prod. 2022, 338, 130612. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, M.; Chen, Y.; Jin, X.; Shangguan, J.; Cui, J.; Chang, S.; Guo, M.; Wang, Y. The neglected potential source of microplastics from daily necessities: A study on protective mobile phone cases. J. Hazard. Mater. 2023, 441, 129911. [Google Scholar] [CrossRef]
- Guo, X.; Yin, Y.; Yang, C.; Dang, Z. Maize straw decorated with sulfide for tylosin removal from the water. Ecotoxicol. Environ. Saf. 2018, 152, 16–23. [Google Scholar] [CrossRef]
- Ding, R.; Ouyang, F.; Peng, D.; You, J.; Ding, L.; Ouyang, Z.; Liu, P.; Guo, X. A case study of distribution and characteristics of microplastics in surface water and sediments of the seas around Shenzhen, southern coastal area of China. Sci. Total Environ. 2022, 838, 156063. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Corbaz, M.; Baecher, H. Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch. Sci. 2012, 65, 157–164. [Google Scholar]
- Mintenig, S.; Int-Veen, I.; Löder, M.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Imbulana, S.; Tanaka, S.; Oluwoye, I. Quantifying annual microplastic emissions of an urban catchment: Surface runoff vs wastewater sources. J. Environ. Manag. 2024, 360, 121123. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential microplastic release from beached fishing gear in Great Britain’s region of highest fishing litter density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Okoffo, E.D. COVID-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Yang, X.; Huang, Q.; Zhu, K.; Sun, Y.; Van Hulle, S.; Jia, H. Generation of environmental persistent free radicals (EPFRs) enhances ecotoxicological effects of the disposable face mask waste with the COVID-19 pandemic. Environ. Pollut. 2022, 301, 119019. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Xu, Y.; Xiao, C.; Liu, J.; Wu, R.; Guo, X. Different functional areas and human activities significantly affect the occurrence and characteristics of microplastics in soils of the Xi’an metropolitan area. Sci. Total Environ. 2022, 852, 158581. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake—Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef]
- Cai, M.; Liu, M.; Qi, H.; Cui, Y.; Zhang, M.; Huang, P.; Wang, L.; Xie, M.; Li, Y.; Wang, W.; et al. Transport of microplastics in the South China Sea: A review. Gondwana Res. 2022, 108, 49–59. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, W.; Chen, Y.; Wang, J. Microplastics in surface waters of Dongting Lake and Hong Lake, China. Sci. Total Environ. 2018, 633, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I.; Efimova, I.; Bagaeva, M.; Bagaev, A.; Isachenko, I. On mechanical fragmentation of single-use plastics in the sea swash zone with different types of bottom sediments: Insights from laboratory experiments. Mar. Pollut. Bull. 2020, 150, 110726. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Tang, N.; Liu, X.; Xing, W. Microplastics in wastewater treatment plants of Wuhan, Central China: Abundance, removal, and potential source in household wastewater. Sci. Total Environ. 2020, 745, 141026. [Google Scholar] [CrossRef]
- Sedgwick, P. Pearson’s correlation coefficient. BMJ 2012, 345, e4483. [Google Scholar] [CrossRef]
- Kothari, V.; Vij, S.; Sharma, S.; Gupta, N. Correlation of various water quality parameters and water quality index of districts of Uttarakhand. Environ. Sustain. Indic. 2021, 9, 100093. [Google Scholar] [CrossRef]
- Asuero, A.G.; Sayago, A.; González, A.G. The Correlation Coefficient: An Overview. Crit. Rev. Anal. Chem. 2006, 36, 41–59. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Teng, Y. Source apportionment and source-oriented risk assessment of heavy metals in the sediments of an urban river-lake system. Sci. Total Environ. 2020, 737, 140310. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Jeong, E.; Lee, J.-Y.; Chia, R.W.; Raza, M. Microplastic contamination in groundwater on a volcanic Jeju Island of Korea. Environ. Res. 2023, 226, 115682. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Sahoo, B.K.; Das, A.; Nag, S.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021, 192, 116853. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, X.; Song, Y.; Chang, S.X. Coexistence of polyethylene microplastics and biochar increases ammonium sorption in an aqueous solution. J. Hazard. Mater. 2021, 405, 124260. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’connell, B.; Healy, M.G.; O’connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Radford, F.; Horton, A.A.; Felgate, S.; Lichtschlag, A.; Hunt, J.; Andrade, V.; Sanders, R.; Evans, C. Factors influencing microplastic abundances in the sediments of a seagrass-dominated tropical atoll. Environ. Pollut. 2024, 357, 124483. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, C.; Li, J.; Lu, S.; Xu, G. Effect of Polypropylene Microplastics Concentration on Wastewater Denitrification. Sci. J. Chem. 2022, 10, 53. [Google Scholar] [CrossRef]
- Parrish, K.L.; Fahrenfeld, N. Microplastic biofilm in fresh- and wastewater as a function of microparticle type and size class. Environ. Sci. Water Res. Technol. 2019, 5, 495–505. [Google Scholar] [CrossRef]
- Nguyen, T.L.H.; Duong, T.L.; Nguyen, T.L.H.; Dang, T.Q.; Nguyen, T.H.; Dao, N.N.; Nguyen, K.T.; Duong, C.D.; Pham, N.N.; Nguyen, B.Q. Microplastics and trace metals in river sediment: Prevalence and correlation with multiple factors. Sci. Total Environ. 2023, 895, 165145. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Radhakrishnan, N. Establishment the relationship between water quality parameter and micro plastic concentration for Adyar and Cooum estuary. Environ. Qual. Manag. 2023, 33, 121–133. [Google Scholar] [CrossRef]
- Conowall, P.; Schreiner, K.M.; Minor, E.C.; Hrabik, T.; Schoenebeck, C.W. Variability of microplastic loading and retention in four inland lakes in Minnesota, USA. Environ. Pollut. 2023, 328, 121573. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, R.; Keerthivasan, R.; Janani, R.; Ramasundaram, S.; Venkatesan, R.; Murthy, M.R.; Sudhakar, T. Seasonal distribution of microplastics in surface waters of the Northern Indian Ocean. Mar. Pollut. Bull. 2023, 190, 114838. [Google Scholar] [CrossRef]
- Jin, X.; Fu, X.; Lu, W.; Wang, H. The effects of riverside cities on microplastics in river water: A case study on the Southern Jiangsu Canal, China. Sci. Total Environ. 2023, 858, 159783. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Y.; Bao, X.; Zhang, X.; Ling, X.; Lu, G.; Liu, J.; Nie, Y. Microplastic pollution in an urbanized river affected by water diversion: Combining with active biomonitoring. J. Hazard. Mater. 2021, 417, 126058. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Tao, W.; Zhang, W.; Wang, L.; Shi, X.; Lu, X.; Li, X. Pollution characteristics and health risk assessment of phthalate esters in urban soil in the typical semi-arid city of Xi’an, Northwest China. Chemosphere 2018, 191, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Oksanen, J.; ter Braak, C.J.F. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Xu, N.; Jiang, L.; Zhang, Y.; Shen, Y.; Wang, Y.; Wang, S.; Yuan, Q. Microplastic pollution in the offshore sea, rivers and wastewater treatment plants in Jiangsu coastal area in China. Mar. Environ. Res. 2023, 188, 105992. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Hazardous metal additives in plastics and their environmental impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Almeida CM, R.; Manjate, E.; Ramos, S. Adsorption of Cd and Cu to different types of microplastics in estuarine salt marsh medium. Mar. Pollut. Bull. 2020, 151, 110797. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Shi, L.; He, W.; Zhang, W.; Li, E.; Zhang, C.; Pang, H.; Tan, X. Interaction of Pb(II) with microplastic-sediment complexes: Critical effect of surfactant. Environ. Pollut. 2024, 361, 124815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, L.; Arulmani, S.R.B.; Yan, J.; Wu, L.; Wu, T.; Zhang, H.; Xiao, T. Adsorption of Different Pollutants by Using Microplastic with Different Influencing Factors and Mechanisms in Wastewater: A Review. Nanomaterials 2022, 12, 2256. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Teng, F.; Zhou, C. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem. Eng. J. 2021, 405, 126989. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Chen, Y.; Wei, L.; Wang, Y. Activation of Firmiana Simplex leaf and the enhanced Pb(II) adsorption performance: Equilibrium and kinetic studies. J. Hazard. Mater. 2009, 169, 386–394. [Google Scholar] [CrossRef]
- Qi, K.; Lu, N.; Zhang, S.; Wang, W.; Wang, Z.; Guan, J. Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: Adsorption and combined toxicity in comparison to natural solid substrates. J. Hazard. Mater. 2021, 411, 125115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, J.; Yang, Q.; Dang, Z.; Zhang, L. Effect of carbon chain structure on the phthalic acid esters (PAEs) adsorption mechanism by mesoporous cellulose biochar. Chem. Eng. J. 2019, 362, 383–391. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, Y.; Yuan, Y.; Hu, B.; Wang, B. Comparative analysis of kinetics and mechanisms for Pb(II) sorption onto three kinds of microplastics. Ecotoxicol. Environ. Saf. 2021, 208, 111451. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Chapter 7—Adsorption Processes for the Removal of Contaminants from Wastewater: The Perspective Role of Nanomaterials and Nanotechnology; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar]
- Li, Y.; Li, M.; Li, Z.; Yang, L.; Liu, X. Effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic. Chemosphere 2019, 231, 308–314. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Yu, A.; Sun, X. Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu(II), Ni(II), Zn(II)) in aqueous solutions. J. Hazard. Mater. 2021, 403, 123548. [Google Scholar] [CrossRef]




| Regions | Parameters | Max | Min | Mean | Mean ± SE | CV |
|---|---|---|---|---|---|---|
| GR area | NH3–N (mg/L) | 0.495 | 0.026 | 0.128 | 0.128 ± 0.040 | 104.347 |
| TN (mg/L) | 1.357 | 0.739 | 0.986 | 0.986 ± 0.052 | 17.501 | |
| TP (mg/L) | 0.380 | 0.010 | 0.135 | 0.135 ± 0.041 | 106.832 | |
| NO3-N (mg/L) | 4.239 | 0.816 | 2.183 | 2.183 ± 0.345 | 52.434 | |
| Cl− (mg/L) | 8.994 | 1.715 | 3.867 | 3.867 ± 0.697 | 59.774 | |
| SO42− (mg/L) | 9.332 | 1.466 | 3.977 | 3.977 ± 0.676 | 56.438 | |
| pH | 7.84 | 6.59 | 7.314 | 7.314 ± 0.119 | 5.401 | |
| Ec (μS/cm) | 219.6 | 66.8 | 136.991 | 136.991 ± 15.308 | 37.077 | |
| Ca (mg/L) | 57.960 | 2.980 | 32.681 | 32.681 ± 6.050 | 61.401 | |
| K (mg/L) | 6.660 | 1.750 | 3.327 | 3.327 ± 0.539 | 53.711 | |
| Na (mg/L) | 16.580 | 4.640 | 9.027 | 9.027 ± 0.982 | 36.088 | |
| Mg (mg/L) | 6.670 | 1.420 | 3.934 | 3.934 ± 0.566 | 47.760 | |
| As (μg/L) | 8.216 | 4.520 | 5.891 | 5.891 ± 0.357 | 20.134 | |
| Cu (mg/L) | 0.010 | 0.001 | 0.006 | 0.006 ± 0.001 | 50.000 | |
| Fe (mg/L) | 0.070 | 0.000 | 0.019 | 0.019 ± 0.006 | 110.526 | |
| Al (mg/L) | 0.150 | 0.030 | 0.093 | 0.093 ± 0.012 | 44.086 | |
| Mn (mg/L) | 0.110 | 0.030 | 0.071 | 0.071 ± 0.010 | 45.070 | |
| Pb (mg/L) | 0.130 | 0.060 | 0.095 | 0.095 ± 0.006 | 22.105 | |
| Zn (mg/L) | 1.070 | 0.100 | 0.536 | 0.536 ± 0.117 | 72.574 | |
| Cd (mg/L) | 0.020 | 0.000 | 0.009 | 0.009 ± 0.002 | 66.667 | |
| LC area | NH3–N (mg/L) | 0.057 | 0.025 | 0.038 | 0.038 ± 0.007 | 35.771 |
| TN (mg/L) | 0.596 | 0.49 | 0.543 | 0.543 ± 0.027 | 9.672 | |
| TP (mg/L) | 0.027 | 0.008 | 0.017 | 0.017 ± 0.004 | 47.303 | |
| NO3-N (mg/L) | 2.46 | 0.868 | 1.294 | 1.294 ± 0.389 | 60.126 | |
| Cl− (mg/L) | 2.001 | 0.885 | 1.477 | 1.477 ± 0.273 | 36.965 | |
| SO42− (mg/L) | 6.594 | 2.465 | 4.034 | 4.034 ± 0.987 | 48.922 | |
| pH | 7.790 | 7.450 | 7.630 | 7.630 ± 0.087 | 2.295 | |
| Ec (μS/cm) | 349 | 64 | 158.500 | 158.50 ± 66.713 | 84.180 | |
| Ca (mg/L) | 66.850 | 11.460 | 48.253 | 48.253 ± 17.868 | 64.136 | |
| K (mg/L) | 3.780 | 1.620 | 2.580 | 2.580 ± 0.658 | 44.147 | |
| Na (mg/L) | 12.480 | 8.730 | 10.033 | 10.033 ± 1.163 | 20.085 | |
| Mg (mg/L) | 3.600 | 1.100 | 2.257 | 2.257 ± 0.769 | 59.017 | |
| As (μg/L) | 8.096 | 6.272 | 6.881 | 6.881 ± 0.586 | 14.752 | |
| Cu (mg/L) | 0.011 | 0.003 | 0.008 | 0.008 ± 0.002 | 50.000 | |
| Fe (mg/L) | 0.000 | 0.000 | 0 | 0.000 ± 0.000 | 0.000 | |
| Al (mg/L) | 0.150 | 0.030 | 0.103 | 0.103 ± 0.037 | 62.136 | |
| Mn (mg/L) | 0.060 | 0.020 | 0.037 | 0.037 ± 0.012 | 56.757 | |
| Pb (mg/L) | 0.100 | 0.090 | 0.093 | 0.093 ± 0.003 | 6.452 | |
| Zn (mg/L) | 0.870 | 0.010 | 0.317 | 0.317 ± 0.266 | 145.110 | |
| Cd (mg/L) | 0.020 | 0.000 | 0.010 | 0.010 ± 0.006 | 100.000 | |
| FW area | NH3–N (mg/L) | 0.023 | 0.013 | 0.017 | 0.017 ± 0.003 | 29.605 |
| TN (mg/L) | 0.850 | 0.394 | 0.648 | 0.648 ± 0.134 | 35.864 | |
| TP (mg/L) | 0.023 | 0.006 | 0.014 | 0.014 ± 0.005 | 63.085 | |
| NO3-N (mg/L) | 2.028 | 0.816 | 1.333 | 1.333 ± 0.361 | 46.887 | |
| Cl− (mg/L) | 3.803 | 1.481 | 2.543 | 2.543 ± 0.678 | 46.141 | |
| SO42− (mg/L) | 7.026 | 2.266 | 3.879 | 3.879 ± 1.574 | 70.267 | |
| pH | 7.920 | 7.210 | 7.480 | 7.480 ± 0.222 | 5.138 | |
| Ec (μS/cm) | 355 | 48.40 | 159.20 | 159.20 ± 98.244 | 106.820 | |
| Ca (mg/L) | 50.320 | 15.690 | 25.040 | 25.040 ± 8.056 | 64.351 | |
| K (mg/L) | 1.610 | 0.810 | 1.152 | 1.152 ± 0.168 | 29.167 | |
| Na (mg/L) | 8.170 | 7.140 | 7.657 | 7.657 ± 0.198 | 5.161 | |
| Mg (mg/L) | 3.120 | 1.290 | 2.077 | 2.077 ± 0.411 | 39.576 | |
| As (μg/L) | 5.396 | 4.628 | 4.960 | 4.960 ± 0.162 | 6.532 | |
| Cu (mg/L) | 0.015 | 0.004 | 0.009 | 0.009 ± 0.002 | 55.556 | |
| Fe (mg/L) | 0.010 | 0.000 | 0.005 | 0.005 ± 0.003 | 120.000 | |
| Al (mg/L) | 0.190 | 0.040 | 0.098 | 0.098 ± 0.035 | 70.408 | |
| Mn (mg/L) | 0.050 | 0.020 | 0.030 | 0.030 ± 0.007 | 46.667 | |
| Pb (mg/L) | 0.090 | 0.020 | 0.067 | 0.067 ± 0.017 | 50.746 | |
| Zn (mg/L) | 0.320 | 0.120 | 0.192 | 0.192 ± 0.045 | 46.354 | |
| Cd (mg/L) | 0.010 | 0.010 | 0.010 | 0.010 ± 0.000 | 0.000 | |
| PR area | NH3–N (mg/L) | 0.039 | 0.028 | 0.033 | 0.033 ± 0.006 | 23.218 |
| TN (mg/L) | 0.775 | 0.672 | 0.724 | 0.724 ± 0.052 | 10.067 | |
| TP (mg/L) | 0.033 | 0.023 | 0.028 | 0.028 ± 0.005 | 25.254 | |
| NO3-N (mg/L) | 2.735 | 0.405 | 1.570 | 1.570 ± 1.165 | 104.940 | |
| Cl− (mg/L) | 408.369 | 4.378 | 206.374 | 206.374 ± 202.025 | 138.421 | |
| SO42− (mg/L) | 8.233 | 2.135 | 5.184 | 5.184 ± 1.025 | 83.178 | |
| pH | 7.790 | 7.290 | 7.540 | 7.540 ± 0.250 | 4.6989 | |
| Ec (μS/cm) | 1873 | 166.8 | 1019.9 | 1019.9 ± 853.179 | 118.293 | |
| Ca (mg/L) | 70.780 | 41.990 | 56.385 | 56.385 ± 14.395 | 36.167 | |
| K (mg/L) | 719.900 | 3.180 | 361.540 | 361.540 ± 358.36 | 91.590 | |
| Na (mg/L) | 11.170 | 6.970 | 9.070 | 9.070 ± 2.100 | 32.745 | |
| Mg (mg/L) | 3.450 | 2.120 | 2.785 | 2.785 ± 0.665 | 33.752 | |
| As (μg/L) | 6.292 | 4.820 | 5.556 | 5.556 ± 0.736 | 18.755 | |
| Cu (mg/L) | 0.011 | 0.009 | 0.010 | 0.010 ± 0.001 | 10.000 | |
| Fe (mg/L) | 0.030 | 0.000 | 0.015 | 0.015 ± 0.015 | 94.000 | |
| Al (mg/L) | 0.190 | 0.100 | 0.145 | 0.145 ± 0.045 | 44.138 | |
| Mn (mg/L) | 0.040 | 0.020 | 0.030 | 0.030 ± 0.010 | 46.667 | |
| Pb (mg/L) | 0.090 | 0.080 | 0.085 | 0.085 ± 0.005 | 8.235 | |
| Zn (mg/L) | 0.280 | 0.220 | 0.250 | 0.250 ± 0.030 | 16.800 | |
| Cd (mg/L) | 0.010 | 0.000 | 0.005 | 0.005 ± 0.005 | 91.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Shan, H.; Zhan, H.; Shi, Y.; Lan, X.; Liu, Y. Distribution Characteristics and Adsorption Performance of Microplastics in Domestic Sewage: A Case Study of Guilin, China. Water 2025, 17, 868. https://doi.org/10.3390/w17060868
Lu M, Shan H, Zhan H, Shi Y, Lan X, Liu Y. Distribution Characteristics and Adsorption Performance of Microplastics in Domestic Sewage: A Case Study of Guilin, China. Water. 2025; 17(6):868. https://doi.org/10.3390/w17060868
Chicago/Turabian StyleLu, Meiyuan, Huimei Shan, Hongbin Zhan, Yuxin Shi, Xujun Lan, and Yunquan Liu. 2025. "Distribution Characteristics and Adsorption Performance of Microplastics in Domestic Sewage: A Case Study of Guilin, China" Water 17, no. 6: 868. https://doi.org/10.3390/w17060868
APA StyleLu, M., Shan, H., Zhan, H., Shi, Y., Lan, X., & Liu, Y. (2025). Distribution Characteristics and Adsorption Performance of Microplastics in Domestic Sewage: A Case Study of Guilin, China. Water, 17(6), 868. https://doi.org/10.3390/w17060868







