Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Marineo WWTP
2.2. Biochar and Zeolite Characteristics
2.3. Experimental Setup
2.4. Experimental Campaign and Analytical Methods
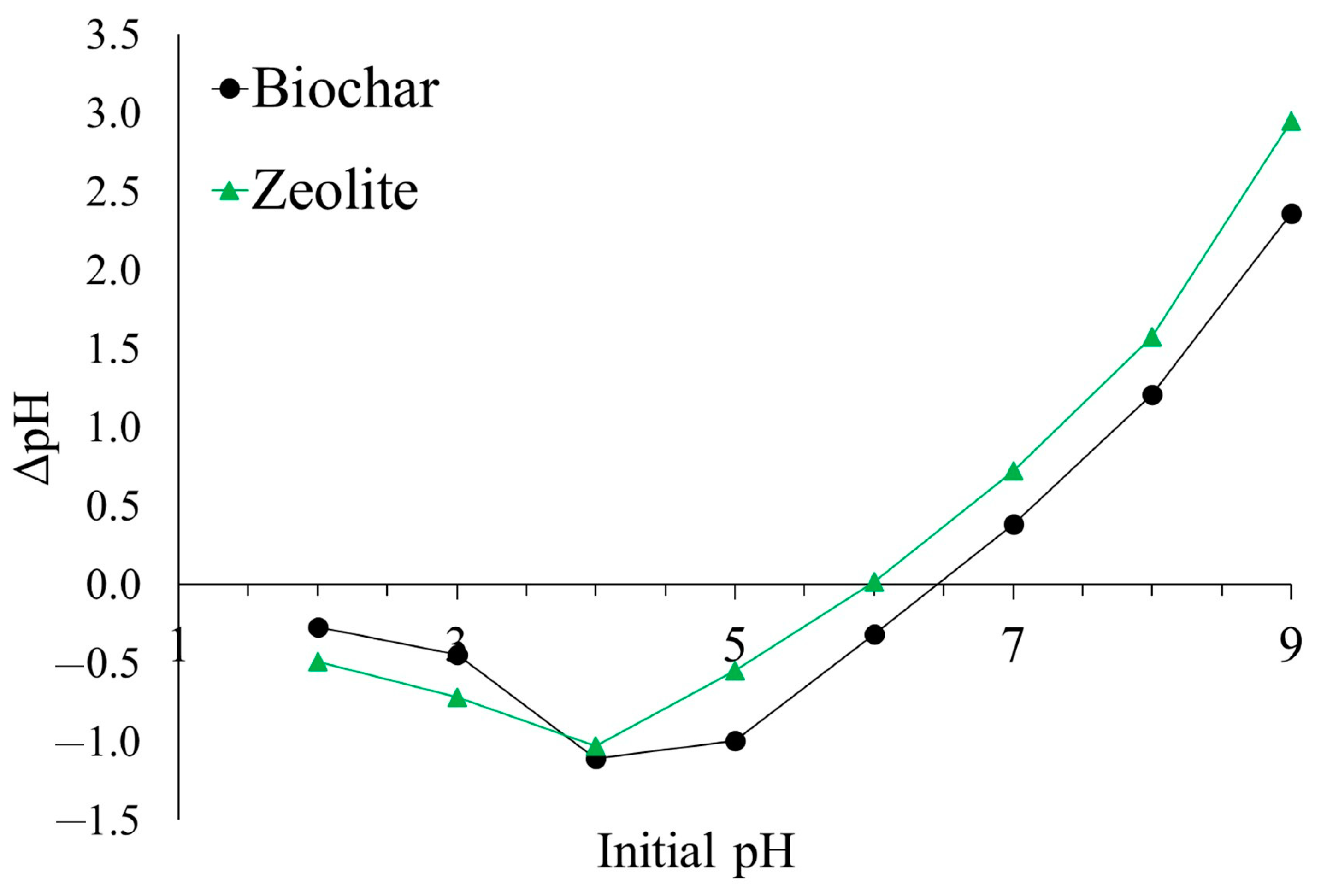
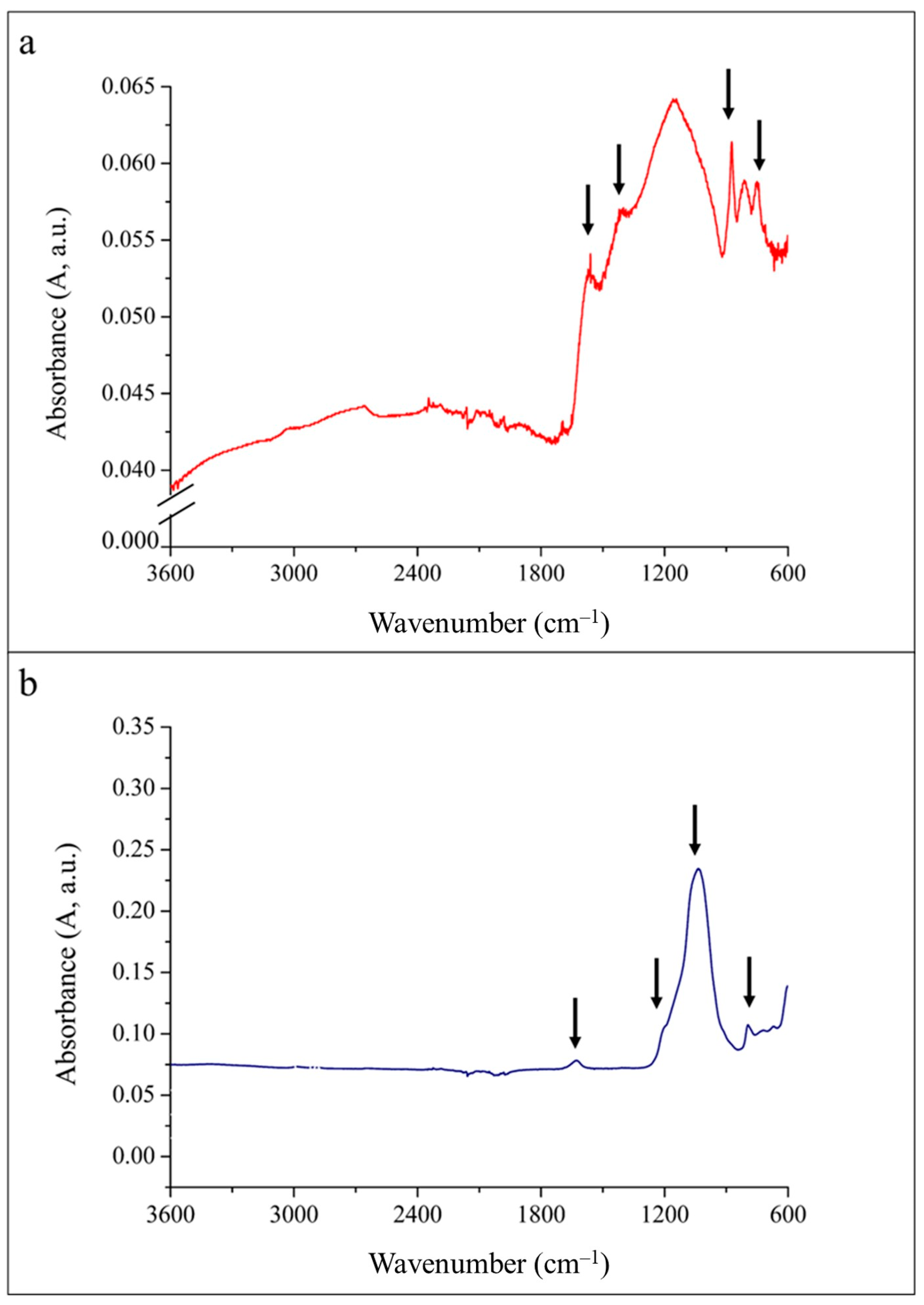
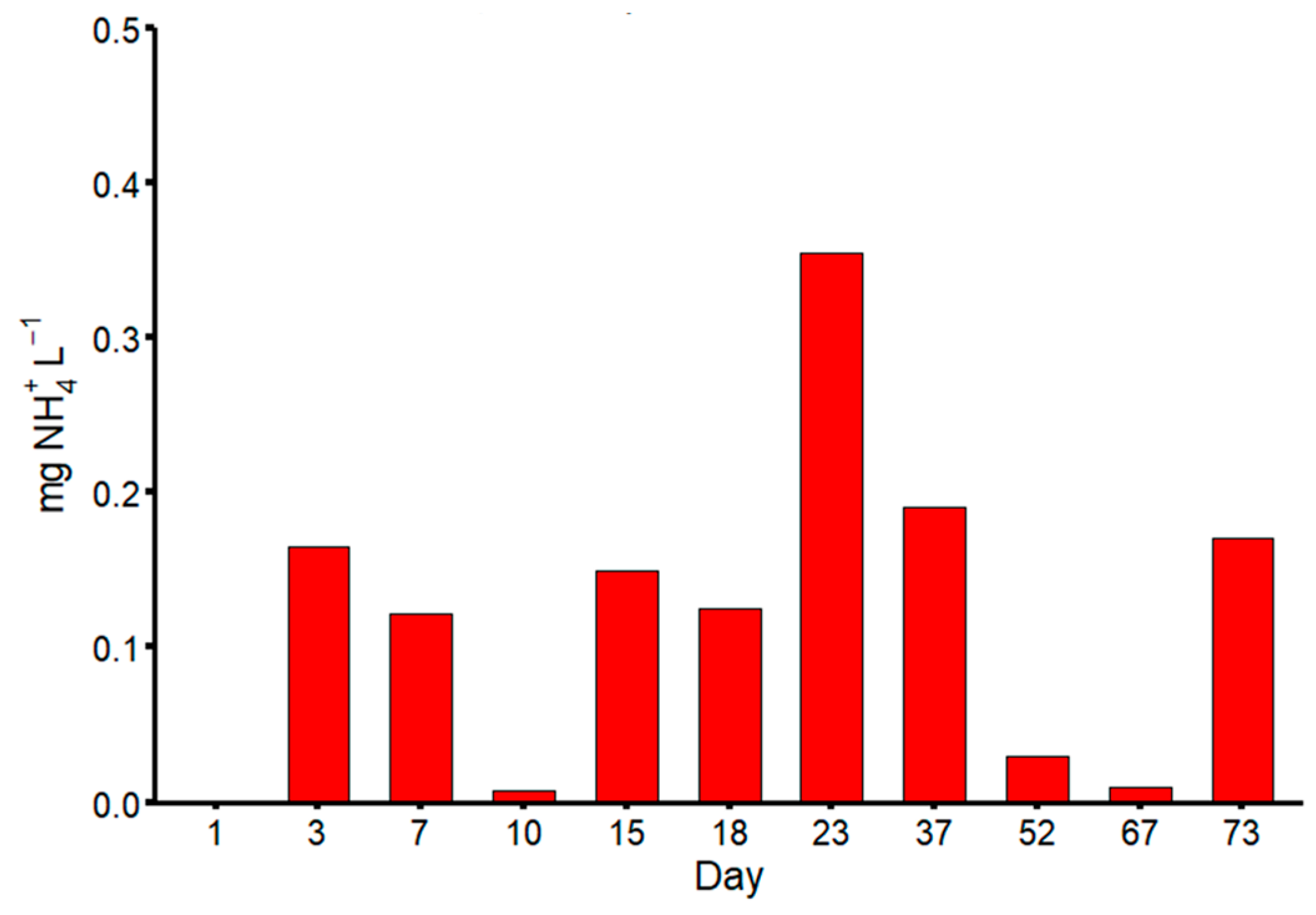
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patra, A.K.; Coumar, M.V. Sustainable Soil Resource Management for Food and Nutritional Security under Changing Climate Scenario. Indian J. Agron. 2023, 68, S78–S97. [Google Scholar]
- Gatto, A.; Chepeliev, M. Global Food Loss and Waste Estimates Show Increasing Nutritional and Environmental Pressures. Nat. Food 2024, 5, 136–147. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, J.; Mahmood, H.; Khalid, S. Natural Resource Depletion and Carbon Inequality: An Empirical Insight from Developed and Developing Countries. Energy Env. 2024. [Google Scholar] [CrossRef]
- Abebe, T.G.; Tamtam, M.R.; Abebe, A.A.; Abtemariam, K.A.; Shigut, T.G.; Dejen, Y.A.; Haile, E.G. Growing Use and Impacts of Chemical Fertilizers and Assessing Alternative Organic Fertilizer Sources in Ethiopia. Appl. Environ. Soil Sci. 2022, 2022, 4738416. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological Functions of Mineral Macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of Macronutrients in Plant Growth and Acclimation: Recent Advances and Future Prospective. In Improvement of Crops in the Era of Climatic Changes: Volume 2; Springer: New York, NY, USA, 2014; pp. 197–216. [Google Scholar]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. ISBN 978-981-10-9044-8. [Google Scholar]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Meshalkin, V.; Malyavin, A.; Kostyleva, V.; Ivanova, V.; Burvikova, J. Life Cycle Assessment and Sustainable Development of Mineral Fertilizers Production. E3S Web Conf. 2024, 510, 02007. [Google Scholar] [CrossRef]
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Ye, Q. Soil Pollution Status, Sources, and Control Methods in China. Acad. J. Sci. Technol. 2024, 9, 155–161. [Google Scholar] [CrossRef]
- Du, J.; Waite, T.D.; Feng, J.; Lei, Y.; Tang, W. Coupled Electrochemical Methods for Nitrogen and Phosphorus Recovery from Wastewater: A Review. Environ. Chem. Lett. 2023, 21, 885–909. [Google Scholar] [CrossRef]
- Theregowda, R.B.; González-Mejía, A.M.; Ma, X.; Garland, J. Nutrient Recovery from Municipal Wastewater for Sustainable Food Production Systems: An Alternative to Traditional Fertilizers. Environ. Eng. Sci. 2019, 36, 833–842. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Sutton, M.A.; Reay, D.S.; Heal, K.V.; Hermann, L.; Kabbe, C.; Spears, B.M. Global Actions for a Sustainable Phosphorus Future. Nat. Food 2021, 2, 71–74. [Google Scholar] [CrossRef]
- El Wali, M.; Golroudbary, S.R.; Kraslawski, A. Circular Economy for Phosphorus Supply Chain and Its Impact on Social Sustainable Development Goals. Sci. Total Environ. 2021, 777, 146060. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key Sustainability Challenges for the Global Phosphorus Resource, Their Implications for Global Food Security, and Options for Mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Jakobsson, A.K. The Phosphorus Challenge in International Politics: Geopolitics, Human Security, and the Grey Zone. FindResearcher 2021. [Google Scholar]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and Solutions. In Eutrophication: Causes, Consequences and Control: Volume 2; Ansari, A.A., Gill, S.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–15. ISBN 978-94-007-7814-6. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.; Ridoutt, B.G.; Wang, X.; Ren, P. Nitrogen and Phosphorus Losses and Eutrophication Potential Associated with Fertilizer Application to Cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- El-Nahhal, I.Y.; Al-Najar, H.; El-Nahhal, Y. Cations and Anions in Sewage Sludge from Gaza Waste Water Treatment Plant. Am. J. Anal. Chem. 2014, 5, 655. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Kalantari, N.; Sharifidoost, M.; Kazemi, M. Quality Assessment of Treated Wastewater to Be Reused in Agriculture. Glob. J. Environ. Sci. Manag. 2018, 4, 217–230. [Google Scholar] [CrossRef]
- Gholipour, M.; Mehrabanjoubani, P.; Abdolzadeh, A.; Raghimi, M.; Seyedkhademi, S.; Karimi, E.; Sadeghipour, H.R. Facilitated Decrease of Anions and Cations in Influent and Effluent of Sewage Treatment Plant by Vetiver Grass (Chrysopogon zizanioides): The Uptake of Nitrate, Nitrite, Ammonium, and Phosphate. Environ. Sci. Pollut. Res. 2020, 27, 21506–21516. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, J.; Liang, S. Nutrient Recovery from Wastewater: From Technology to Economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for Recovering Nutrients from Wastewater: A Critical Review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New Frontiers from Removal to Recycling of Nitrogen and Phosphorus from Wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Gorazda, K.; Szopa, D.; Trzaska, K.; Moustakas, K.; Chojnacka, K. Phosphorus Recovery from Wastewater and Bio-Based Waste: An Overview. Bioengineered 2022, 13, 13474–13506. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of Pyrolysis Temperature on Characteristics and Phosphate Adsorption Capability of Biochar Derived from Waste-Marine Macroalgae (Undaria Pinnatifida Roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium Adsorption, Desorption and Recovery by Acid and Alkaline Treated Zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef]
- Günal, A.; Erdogan, B. Ammonia Removal by Natural and Modified Clinoptilolite: Scientific Paper. J. Serbian Chem. Soc. 2022, 87, 1395–1407. [Google Scholar] [CrossRef]
- Banik, C.; Bakshi, S.; Andersen, D.S.; Laird, D.A.; Smith, R.G.; Brown, R.C. The Role of Biochar and Zeolite in Enhancing Nitrogen and Phosphorus Recovery: A Sustainable Manure Management Technology. Chem. Eng. J. 2023, 456, 141003. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Espinoza-Santos, N. Cation Exchange of Natural Zeolites: Worldwide Research. Sustainability 2021, 13, 7751. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Laudicina, V.A.; Mannina, G. Chapter 5—Zeolites for the Nutrient Recovery from Wastewater. In Current Developments in Biotechnology and Bioengineering; Mannina, G., Pandey, A., Sirohi, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 95–114. ISBN 978-0-323-99920-5. [Google Scholar]
- Muscarella, S.M.; Laudicina, V.A.; Badalucco, L.; Conte, P.; Mannina, G. Ammonium Recovery from Synthetic Wastewaters by Using Zeolitic Mixtures: A Desorption Batch-Study. Water 2023, 15, 3479. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.N.; Khorasani, Z.; Gorjiara, M. Ammonia Removal from Aqueous Solutions by Iranian Natural Zeolite. Sep. Sci. Technol. 2008, 43, 960–978. [Google Scholar] [CrossRef]
- Karapınar, N. Application of Natural Zeolite for Phosphorus and Ammonium Removal from Aqueous Solutions. J. Hazard. Mater. 2009, 170, 1186–1191. [Google Scholar] [CrossRef]
- Steiner, C. Considerations in Biochar Characterization. In SSSA Special Publications; Guo, M., He, Z., Uchimiya, S.M., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2015; pp. 87–100. ISBN 978-0-89118-967-1. [Google Scholar]
- Wang, Y.; Qiu, L.P.; Hu, M.F. Magnesium Ammonium Phosphate Crystallization: A Possible Way for Recovery of Phosphorus from Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 032032. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.P.; Purakayastha, T.J. Characterization of Biochar and Their Influence on Microbial Activities and Potassium Availability in an Acid Soil. Arch. Agron. Soil Sci. 2019, 65, 1302–1315. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar Elemental Composition and Factors Influencing Nutrient Retention. In Biochar for Environmental Management; Routledge: London, UK, 2015; ISBN 978-0-203-76226-4. [Google Scholar]
- Muscarella, S.M.; Badalucco, L.; Laudicina, V.A.; Conte, P.; Mannina, G. Phosphorus Recovery from P-Enriched Solution by Biochar Activated with Chloride Salts. In Resource Recovery from Wastewater Treatment; Mannina, G., Cosenza, A., Mineo, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 36–42. [Google Scholar]
- Trazzi, P.A.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and Desorption of Phosphate on Biochars. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Yuan, M.; Cai, R.; Macazo, F.C.; Minteer, S.D. Modified Biochar for Phosphate Adsorption in Environmentally Relevant Conditions. Chem. Eng. J. 2020, 380, 122375. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Zhang, Y.; Ni, F. Comparative Study on Characteristics and Mechanism of Phosphate Adsorption on Mg/Al Modified Biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of Phosphorus by Different Biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of Agro-Waste Rice Husk Ash for the Removal of Phosphate from the Wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Bulacio Fischer, P.T.; Di Trapani, D.; Laudicina, V.A.; Mineo, A.; Muscarella, S.M.; Mannina, G. Adsorption and Desorption of Ammonium from Treated Wastewater by Zeolite Filled Columns: An Experimental Study at the Water Resource Recovery Facility of Palermo University—Italy. J. Environ. Manag. 2025, 375, 124241. [Google Scholar] [CrossRef] [PubMed]
- Januševičius, T.; Mažeikienė, A.; Stepova, K.; Danila, V.; Paliulis, D. The Removal of Phosphorus from Wastewater Using a Sewage Sludge Biochar: A Column Study. Water 2024, 16, 1104. [Google Scholar] [CrossRef]
- Mannina, G.; Badalucco, L.; Barbara, L.; Cosenza, A.; Di Trapani, D.; Gallo, G.; Laudicina, V.; Marino, G.; Muscarella, S.; Presti, D.; et al. Enhancing a Transition to a Circular Economy in the Water Sector: The EU Project Wider Uptake. Water 2021, 13, 946. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Laudicina, V.A.; Cano, B.; Badalucco, L.; Conte, P.; Mannina, G. Recovering Ammonium by Treated and Untreated Zeolitic Mixtures: A Comprehensive Experimental and Modelling Study. Microporous Mesoporous Mater. 2023, 349, 112434. [Google Scholar] [CrossRef]
- Nasiruddin Khan, M.; Sarwar, A. Determination of Points of Zero Charge of Natural and Treated Adsorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Mikelionienė, A.; Baltušnikas, A.; Kantautas, A.; Radzevičius, A. Removal of Ammonium Ion from Aqueous Solutions by Using Unmodified and H2O2-Modified Zeolitic Waste. Sci. Rep. 2020, 10, 352. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Geoffrey Chan, W.; Hajaligol, M.R. Characterization of Chars from Pyrolysis of Lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Fischer, P.T.B.; Di Trapani, D.; Laudicina, V.A.; Muscarella, S.M.; Mannina, G. Nutrient Recovery from Columns Filled with Zeolite and Biochar: The Case Study of Marineo (ITALY) Wastewater Treatment Plant. In Resource Recovery from Wastewater Treatment; Mannina, G., Cosenza, A., Mineo, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 20–25. [Google Scholar]
- Kocaturk, N.P. Recovery of Nutrients from Biogas Digestate with Biochar and Clinoptilolite. Ph.D. Thesis, Sustainable Soil Use, Soil Biology, PE&RC, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Canellas-Garriga, J. Tertiary Ammonium Removal with Zeolites; Cranfield University: Bedford, UK, 2018. [Google Scholar]
- Michalski, R. Ion Chromatography Applications in Wastewater Analysis. Separations 2018, 5, 16. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 1123–1184. ISBN 978-0-89118-866-7. [Google Scholar]
- Jung, K.-W.; Jeong, T.-U.; Choi, J.-W.; Ahn, K.-H.; Lee, S.-H. Adsorption of Phosphate from Aqueous Solution Using Electrochemically Modified Biochar Calcium-Alginate Beads: Batch and Fixed-Bed Column Performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.G.; Izquierdo, C.G.; Fernández, M.T.H. Changes in Humic Fraction Characteristics and Humus-Enzyme Complexes Formation in Semiarid Degraded Soils Restored with Fresh and Composted Urban Wastes. A 5-Year Field Experiment. J. Soils Sediments 2018, 18, 1376–1388. [Google Scholar] [CrossRef]
- Tomin, O.; Yazdani, M.R. Production and Characterization of Porous Magnetic Biochar: Before and after Phosphate Adsorption Insights. J. Porous Mater. 2022, 29, 849–859. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Belviso, C.; Cavalcante, F.; Lettino, A.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Ammonium from Wastewater by Zeolite Synthetized from Volcanic Ash: Batch and Column Tests. J. Environ. Chem. Eng. 2022, 10, 107539. [Google Scholar] [CrossRef]
- Cyrus, J.S.; Reddy, G.B. Sorption and Desorption of Ammonium by Zeolite: Batch and Column Studies. J. Environ. Sci. Health Part A 2011, 46, 408–414. [Google Scholar] [CrossRef]
- Aydın Temel, F.; Cağcağ Yolcu, Ö.; Kuleyin, A. A Multilayer Perceptron-Based Prediction of Ammonium Adsorption on Zeolite from Landfill Leachate: Batch and Column Studies. J. Hazard. Mater. 2021, 410, 124670. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Tanner, C.C. Ammonium Removal from Wastewaters Using Natural New Zealand Zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Moreno Sayavedra, S.; Dockx, L.; Sigurnjak, I.; Akyol, Ç.; Meers, E. Post-Treatment of High-Rate Activated Sludge Effluent via Zeolite Adsorption and Recovery of Ammonium-Nitrogen as Alternative Fertilising Products. Bioresour. Technol. 2024, 403, 130837. [Google Scholar] [CrossRef]
- Chen, D.; Yin, Y.; Xu, Y.; Liu, C. Adsorptive Recycle of Phosphate by MgO-Biochar from Wastewater: Adsorbent Fabrication, Adsorption Site Energy Analysis and Long-Term Column Experiments. J. Water Process Eng. 2023, 51, 103445. [Google Scholar] [CrossRef]
- Tran, T.C.P.; Nguyen, T.P.; Nguyen, X.C.; Nguyen, X.H.; Nguyen, T.A.H.; Nguyen, T.T.N.; Vo, T.Y.B.; Nguyen, T.H.G.; Nguyen, T.T.H.; Vo, T.D.H.; et al. Adsorptive Removal of Phosphate from Aqueous Solutions Using Low-Cost Modified Biochar-Packed Column: Effect of Operational Parameters and Kinetic Study. Chemosphere 2022, 309, 136628. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, B.; Amano, Y.; Machida, M. Removal and Recovery of Phosphate from Water by Mg-Laden Biochar: Batch and Column Studies. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 429–437. [Google Scholar] [CrossRef]
- Saltalı, K.; Sarı, A.; Aydın, M. Removal of Ammonium Ion from Aqueous Solution by Natural Turkish (Yıldızeli) Zeolite for Environmental Quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.-J.; Tay, J.H. Adsorption Mechanisms of High-Levels of Ammonium onto Natural and NaCl-Modified Zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Taddeo, R.; Prajapati, S.; Lepistö, R. Optimizing Ammonium Adsorption on Natural Zeolite for Wastewaters with High Loads of Ammonium and Solids. J. Porous Mater. 2017, 24, 1545–1554. [Google Scholar] [CrossRef]
- Lucero, J.M.; Crawford, J.M.; Wolden, C.A.; Carreon, M.A. Tunability of Ammonia Adsorption over NaP Zeolite. Microporous Mesoporous Mater. 2021, 324, 111288. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of Ammonium from Greywater Using Natural Zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Chen, H.-F.; Lin, Y.-J.; Chen, B.-H.; Yoshiyuki, I.; Liou, S.Y.-H.; Huang, R.-T. A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals 2018, 8, 499. [Google Scholar] [CrossRef]
- Uygur, V.; Çelik, C.Ş.; Sukusu, E. The Effect of Particle Sizes on Ammonium Adsorption Kinetics and Desorption by Natural Zeolites. Int. J. Agric. Life Sci. 2019, 3, 371–377. [Google Scholar]
- He, W.; Gong, H.; Fang, K.; Peng, F.; Wang, K. Revealing the Effect of Preparation Parameters on Zeolite Adsorption Performance for Low and Medium Concentrations of Ammonium. J. Environ. Sci. 2019, 85, 177–188. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Laudicina, V.A.; Di Trapani, D.; Mannina, G. Recovering Ammonium from Real Treated Wastewater by Zeolite Packed Columns: The Effect of Flow Rate and Particle Diameter. Sustain. Chem. Pharm. 2024, 41, 101659. [Google Scholar] [CrossRef]
- Zabochnicka, M.; Mali, K. Removal of Ammonia by Clinoptilolite. Glob. NEST J. 2010, 12, 256–261. [Google Scholar]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Henriksen, S.W.; Andersen, M.N. Mechanism of Orthophosphate (PO4-P) Adsorption onto Different Biochars. Environ. Technol. Innov. 2020, 17, 100572. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of Biochar Formation from Various Agricultural By-Products Using FTIR Spectroscopy. Mod. Appl. Sci. 2015, 9, p246. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Trapani, D.D.; Laudicina, V.A.; Mannina, G. Phosphorus Recovery from Ultrafiltered Membrane Wastewater by Biochar Adsorption Columns: The Effect of Loading Rates. Heliyon 2024, 10, e34659. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.S.; Stenström, Y.; Jebrane, M.; Hylander, L.D.; Daniel, G.; Heinmaa, I. Efficiency of Iron- and Calcium-Impregnated Biochar in Adsorbing Phosphate From Wastewater in Onsite Wastewater Treatment Systems. Front. Environ. Sci. 2020, 8, 538539. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and Mechanisms of Phosphate Adsorption on Iron-Modified Biochars Derived from Waste Activated Sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Chernicharo, A.D.L.; Von Sperling, M. Biological Wastewater Treatment in Warm Climate Regions; IWA Publishing: London, UK, 2005; ISBN 978-1-78040-273-4. [Google Scholar]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal; IWA Publishing: London, UK, 2007; ISBN 978-1-84339-161-6. [Google Scholar]
- Grady, C.L., Jr.; Daigger, G.T.; Love, N.G.; Filipe, C.D.M. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-0963-7. [Google Scholar] [CrossRef]
- Jenkins, D.; Wanner, J. Activated Sludge: 100 Years and Counting; Jenkins, D., Wanner, J., Eds.; IWA Publishing: London, UK, 2014; 288p. [Google Scholar]






| WWTP Marineo | |||
|---|---|---|---|
| Parameter | Units | Influent | Effluent |
| TSS | [mg L−1] | 283 | 33 |
| pH | - | 7.8 | |
| BOD5 | [mg L−1] | 278 | 20 |
| COD | [mg L−1] | 566 | 43 |
| TP | [mg L−1] | 13 | 3 |
| NH4+ | [mg L−1] | 24 | 0.16 |
| NO2− | [mg L−1] | n.a. | 1.03 |
| NO3− | [mg L−1] | n.a. | 18 |
| Parameters | Unit | Value |
|---|---|---|
| Bulk density | g L−1 | 180 |
| Surface area | m2 g−1 | 194 |
| Total pore volume | cm3 g−1 | 38 |
| pH | 9.1 | |
| Electrical conductivity | dS m−1 | 1.3 |
| Total carbon | % | 62 |
| Total limestone | % | 5 |
| Total nitrogen | % | 0.8 |
| Total sulfur | % | 0.1 |
| Fe | mg g−1 | 22 |
| Zn | mg g−1 | 0.0017 |
| Molar ratio H:C | 0.7 |
| Parameters | Unit | Value |
|---|---|---|
| Bulk density | g cm−3 | 0.98 |
| Surface area | m2 g−1 | 40 |
| Si/Al ratio | 4.8–5.5 | |
| pH | 7.6 | |
| Clinoptilolite | % | 85 |
| Cristobalite | % | 8 |
| Illite | % | 4 |
| Plagioclase | % | 3 |
| Material | Nutrient Concentration | Type of Solution | Type of Experiment | Reference |
|---|---|---|---|---|
| Zeolite from volcanic ash | 10–40 mg NH4+ L−1 | Deionized water vs. secondary effluent wastewater | Batch and column | [65] |
| Natural and modified zeolites | 500 mg NH4+ L−1 | Swine wastewater | Batch and column | [66] |
| Natural zeolite | 263.2–1363.6 mg NH4+ L−1 | Wastewater | Batch and column | [67] |
| Natural zeolite | 0.2–300 g NH4-N m−3 | Wastewater | Column | [68] |
| Natural zeolite | 60–800 mg NH4+ L−1 | Wastewater | Column | [69] |
| Natural zeolite | 22 mg NH4+ L−1 | Wastewater | Column | [49] |
| Magnesium modified biochar | 10 mg PO43− L−1 | Wastewater | Column | [70] |
| Aluminum modified biochar | 25–100 mg PO43− L−1 | Aqueous solution | Column | [71] |
| Natural biochar | 25 mg PO43− L−1 | wastewater | Column | [50] |
| Calcium modified biochar | 2000 mg PO43− L−1 | Aqueous solution | Batch and Column | [62] |
| Magnesium modified biochar | 20–500 mg PO43− L−1 | Water | Batch and Column | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulacio Fischer, P.T.; Di Trapani, D.; Laudicina, V.A.; Muscarella, S.M.; Mannina, G. Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant. Water 2025, 17, 848. https://doi.org/10.3390/w17060848
Bulacio Fischer PT, Di Trapani D, Laudicina VA, Muscarella SM, Mannina G. Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant. Water. 2025; 17(6):848. https://doi.org/10.3390/w17060848
Chicago/Turabian StyleBulacio Fischer, Pedro Tomas, Daniele Di Trapani, Vito Armando Laudicina, Sofia Maria Muscarella, and Giorgio Mannina. 2025. "Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant" Water 17, no. 6: 848. https://doi.org/10.3390/w17060848
APA StyleBulacio Fischer, P. T., Di Trapani, D., Laudicina, V. A., Muscarella, S. M., & Mannina, G. (2025). Nutrient Recovery from Zeolite and Biochar Columns: The Case Study of Marineo (Italy) Wastewater Treatment Plant. Water, 17(6), 848. https://doi.org/10.3390/w17060848










