Evaluating Pretreatment Strategies with Modeling for Reducing Scaling Potential of Reverse Osmosis Concentrate: Insights from Ion Exchange and Activated Alumina
Abstract
1. Introduction
- Developing high recovery and ultra high-pressure primary desalination processes to minimize ROC volume and potentially achieve near-to-zero liquid discharge (ZLD). This approach increases water recovery for brackish water desalination, minimizes brine volume, and reduces brine disposal costs and environmental impacts. Examples of high recovery desalination systems include low salt rejection RO [24,25], closed circuit reverse osmosis (CCRO) [26,27], and high-pressure spiral wound RO elements that can operate up to 120 bar and concentrate brine up to ~120 g/L TDS [28,29]. Recently, the techno-economic assessment of new ion-exchange membrane technologies [30]—electrodialysis brine concentrator [31] and electrodialysis metathesis [32,33]—showed their potential to overcome the limitations of concentrate management through enhanced performance and economic viability. Despite these advances, the high scaling potential and remaining brine generated from brackish water desalination still remain as major concerns.
- Directly treating ROC. For existing facilities, it may be more practical to directly treat the ROC (such as using the high recovery processes in strategy 1 as secondary or tertiary desalination processes) rather than retrofitting the facility with high-recovery processes, although the goal of minimizing the concentrate volume is the same as in strategy 1. The selected ROC treatment technologies should consider technically feasible, cost-effective, and energy-efficient processes in existing and planned desalination facilities.
- Valorization of ROC. Instead of concentrating the ROC and disposing of the solids, valorization aims to recover valuable resources for a circular economy by finding beneficial uses for the extracted compounds, for example, the development of an integrated treatment train using selective electrodialysis (SED), electrodialysis brine concentrator (EDBC), and bipolar membrane electrodialysis (BPED) to convert ROC to low-salinity water for irrigation and acids and caustic streams for industrial uses [34,35].
| Treatment | Water Source | Concentration | Experimental Procedure | Hardness Results | Silica Results | Regeneration Results | Ref. |
|---|---|---|---|---|---|---|---|
| IX resin/SBA | Tap water | TDS = N/A Silica = 20 mg/L | Bench scale column experiments: 100 mL of IX resin in a small column (18 cm × 5 cm) with Q = 55 mL/min | N/A | 94% average silica removal before breakthrough (at 50 BV) | N/A | [46] |
| BW ROC | TDS = 12,445 mg/L Silica = 160 mg/L | Bench scale isotherm experiments: IX resin concentrations of 2.5–100 g/L in 200 mL of ROC for 24 h | N/A | Langmuir constant: the maximum capacity (qmax) is 19.65 mg/g | N/A | This study | |
| Bench scale column experiments: 40 mL of IX resin in a 50 mL burette, Q = 8 mL/min (12 BV/h) | N/A | Saturation at 5 BV (0.2 L), with an operational capacity of 5.23 mg SiO2/g of resin | Regeneration in column: regeneration achieved at 5 BV (5 L/L of resin) using 2% NaOH (0.5 M) | ||||
| IX resin/three types of WAC | Synthetic water | TDS = 8093 mg/L Hardness = 57.1 mg/L as CaCO3 | Bench isotherm experiments A | N/A | N/A | N/A | [47] |
| Bench scale column experiments: 22 mL of IX resin was packed in a small column (25 cm high) with Q = 7.5 mL/min (20 BV/h) | Saturation at 1922, 2577, and 3232 BV (70 L), total operational capacity of 2–4 meq/g of resin (100–200 mg CaCO3/g of resin B) | N/A | Regeneration in column: regeneration requirement of 2.3–3 meq HCl/meq adsorbed (3.88–4.97 L/L of resin B) using 5% HCl w/v (1.37 N) | ||||
| IX resin/SAC | Seawater ROC | TDS = 39.13 g/L Hardness = 4200 mg/L as CaCO3 | Bench scale batch experiments: IX resin concentration of 10–60 mg/L in 1 L of seawater ROC for 60 min (contact time) | 8.23–41.24% hardness removal | N/A | Some regeneration results were reported (75 and 54% of Ca and Mg elution, respectively) using 15% HCl w/v (4.11 N) A | [48] |
| Synthetic water | TDS = 5900, 28,800, and 52,600 mg/L Hardness = 1818, 8266, and 11,172 mg/L as CaCO3 B | Bench scale column experiments: glass columns (15 cm × 2.5 cm) with a Q = 10 mL/min and 5 min as contact time | Saturation at 65, 12, and 6 BV | N/A | Regeneration was conducted using 10% NaCl (1.71 M) at Q = 2.5 and 10 mL/min. No results were reported. | [49] | |
| BW ROC | TDS = 12,445 mg/L Hardness = 3000 mg/L as CaCO3 | Bench scale isotherm experiments: IX resin doses of 2.5–100 g/L in 200 mL of ROC for 24 h | Langmuir constant: maximum capacity (qmax): 133.33 mg/g | N/A | N/A | This study | |
| Bench scale column experiments: 40 mL of IX resin was packed in a 50 mL burette, Q = 8 mL/min (12 BV/h) | Saturation at 17 and 33 BV (0.68 and 1.32 L), total operational capacity of 65.63 and 98.25 g CaCO3/L of resin | N/A | Regeneration in column: regeneration was achieved at 15 and 20 BV (15 and 20 L/L of resin) using 8% NaCl (1.37 M) | ||||
| AA | BW ROC | TDS = 5800 mg/L Silica = 160 mg/L | Bench scale batch experiments: AA dose 10 g/L, for 60 min (contact time) at 20 °C | N/A | 71.90% silica removal | Regeneration batches: 10 g AA (adsorption capacity of 50 mg/g) in 100 mL of 2% NaOH (0.5 M) achieved 80% of silica desorbed after 9 batches | [45] |
| Synthetic water | TDS = N/A Silica = 50 mg/L | Bench scale isotherm experiments: AA dose 25 g/L in 100 mL of synthetic water at pH = 8–8.5 and 20 °C | N/A | Langmuir constant: maximum capacity (qmax): 7.943 mg/g Freundlich constant: adsorption capacity (K) 0.379 mg/g | N/A | [44] | |
| Bench scale batch experiments: AA dose 5–25 g/L, for 30 min | N/A | ~42–90% silica removal | N/A | ||||
| Cooling tower water | TDS = N/A Silica = 100 mg/L | Bench scale batch experiments: AA dose of 2 g/L in 50 mL of cooling tower water for 2 h at pH = 8.8 and 25 °C | N/A | 29% silica removal | N/A | [50] | |
| BW ROC | TDS = 12,445 mg/L Silica = 160 mg/L | Bench scale isotherm experiments: AA concentrations of 2.5–100 g/L in 200 mL of ROC for 24 h | N/A | Langmuir constant: maximum capacity (qmax) 625 mg/g | 26.6% regeneration efficiency after three 1 h batches using 0.1 N HCl | This study | |
| Bench scale column experiments: 40 mL of AA was packed in a 50 mL burette, Q = 8 mL/min (12 BV/h) | N/A | Column operation until 870 BV (34.8 L), with an adsorption capacity of 217.5 mg SiO2/g of AA | Regeneration in column: 16% of regeneration achieved after 80 BV (80 L/L of resin) using 1 N HCl |
2. Materials and Methods
2.1. Water Source
2.2. Ion Exchange Resins
IX Experimental Conditions
2.3. Activated Alumina
AA Experimental Conditions
2.4. Modeling Methodology
3. Results
3.1. Silica Removal by Ion Exchange Resins
3.1.1. Isotherm Experiments: Silica Removal Using IX
3.1.2. Continuous Column Testing Experiments: Silica Removal Using IX
3.2. Silica Removal by Activated Alumina
3.2.1. Isotherm Experiments: Silica Removal Using AA
3.2.2. Continuous Column Testing Experiments: Silica Removal Using AA
3.3. Hardness Removal by IX Resins
3.3.1. Isotherm Experiments: Hardness Removal Using IX
3.3.2. Continuous Column Testing Experiments: Hardness Removal Using IX
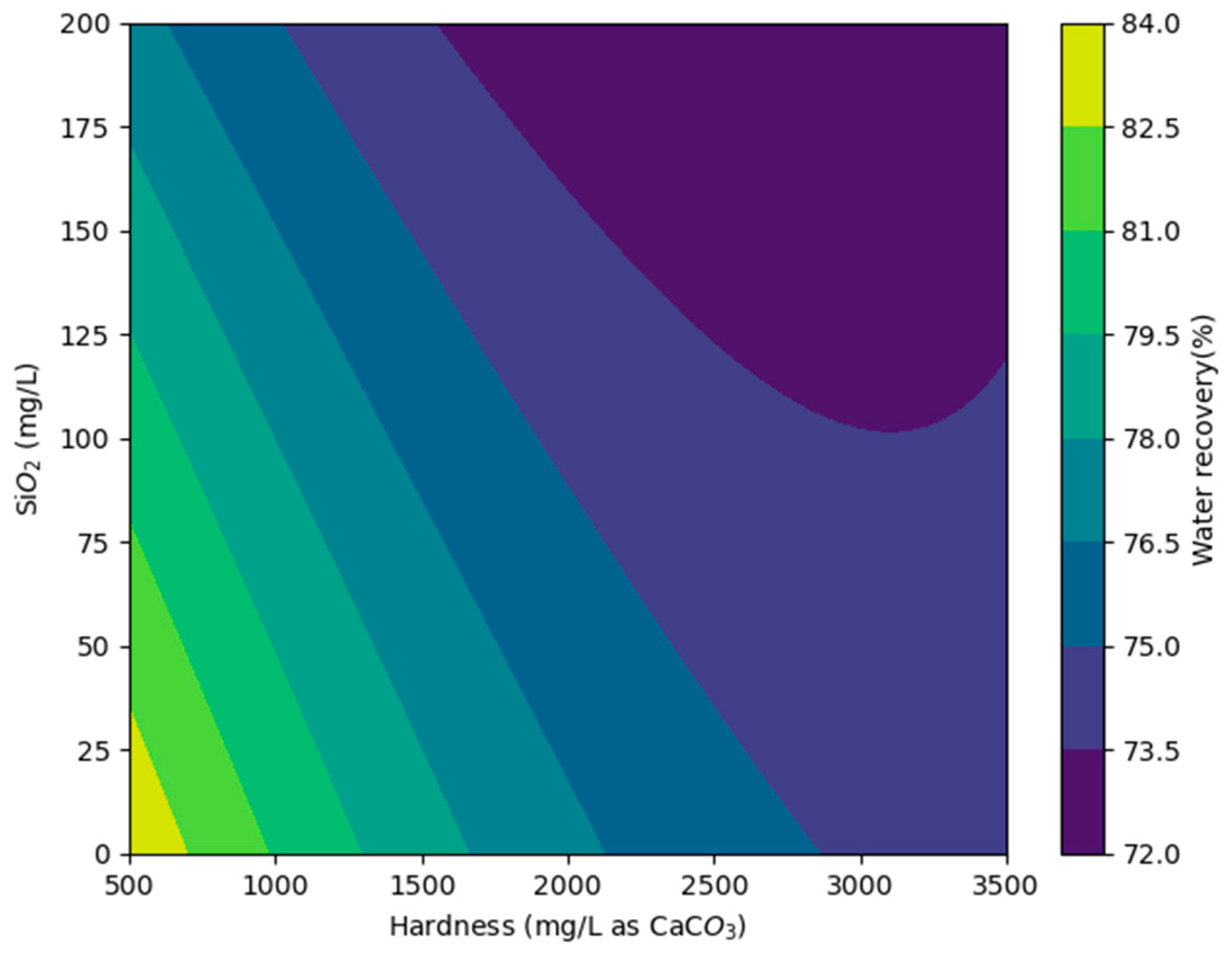
3.4. Modeling Results and Preliminary Cost Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahdab, Y.D.; Lienhard, J.H. Chapter 41—Desalination of Brackish Groundwater to Improve Water Quality and Water Supply. In Global Groundwater; Mukherjee, A., Scanlon, B.R., Aureli, A., Langan, S., Guo, H., McKenzie, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 559–575. [Google Scholar]
- Xu, X.; Ness, J.E.; Miara, A.; Sitterley, K.A.; Talmadge, M.; O’Neill, B.; Coughlin, K.; Akar, S.; Edirisooriya, E.T.; Kurup, P.; et al. Analysis of Brackish Water Desalination for Municipal Uses: Case Studies on Challenges and Opportunities. ACS EST Eng. 2022, 2, 306–322. [Google Scholar] [CrossRef]
- Patel, S.K.; Biesheuvel, P.M.; Elimelech, M. Energy Consumption of Brackish Water Desalination: Identifying the Sweet Spots for Electrodialysis and Reverse Osmosis. ACS EST Eng. 2021, 1, 851–864. [Google Scholar] [CrossRef]
- Felix, V.; Hardikar, M.; Hickenbottom, K.L. Concentrate circularity: A comparative techno-economic analysis of membrane distillation and conventional inland concentrate management technologies. Desalination 2024, 574, 117213. [Google Scholar] [CrossRef]
- Kum, S.; Tang, X.; Liu, H. Recovery of fresh water and minerals from inland brackish desalination brine via persulfate-based photochemical treatment and demineralization. Sep. Purif. Technol. 2024, 342, 126994. [Google Scholar] [CrossRef]
- Bond, R.; Veerapaneni, S. Zero liquid discharge for inland desalination. World Water Environ. Eng. 2007, 30, 28–29. [Google Scholar]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Panagopoulos, A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process. Process Intensif. 2022, 176, 108944. [Google Scholar] [CrossRef]
- Mickley, M. US Municipal Desalination Plants. Available online: https://www.multi-statesalinitycoalition.com/wp-content/uploads/2020-Mickley.pdf (accessed on 30 December 2024).
- Congressional Research Service (CRS). Desalination and Membrane Technologies: Federal Research and Adoption Issues. 2015. Available online: https://crsreports.congress.gov/product/pdf/R/R40477 (accessed on 28 September 2024).
- Oron, G.; Appelbaum, S.; Guy, O. Reuse of brine from inland desalination plants with duckweed, fish and halophytes toward increased food production and improved environmental control. Desalination 2023, 549, 116317. [Google Scholar] [CrossRef]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.Y.; Robertson, A.P.; Reinhard, M.; Leckie, J.O.; Drewes, J.E. Critical Review of Desalination Concentrate Management, Treatment and Beneficial Use. Environ. Eng. Sci. 2013, 30, 502–514. [Google Scholar] [CrossRef]
- Tang, F.; Li, J.; Yan, M.; Jiang, N.; Hu, Y.; Xu, X.; Ye, W.; Bao, Y.; Bao, L.; Huang, M. Enhancing the antifouling performance of surfactant-contaminated brackish water in the reverse osmosis process through a super-hydrophilic layer. J. Water Process Eng. 2024, 63, 105538. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Lugo, A.; Mejía-Saucedo, C.; Senanayake, P.S.; Stoll, Z.; Sitterley, K.; Wang, H.; Kota, K.; Kuravi, S.; Fthenakis, V.; Kurup, P.; et al. Technical, Economic, Energetic, and Environmental Evaluation of Pretreatment Strategies for Scaling Control in Brackish Water Desalination Brine Treatment. Water 2025, 17, 708. [Google Scholar] [CrossRef]
- Sanciolo, P.; Ostarcevic, E.; Atherton, P.; Leslie, G.; Fane, T.; Cohen, Y.; Payne, M.; Gray, S. Enhancement of reverse osmosis water recovery using interstage calcium precipitation. Desalination 2012, 295, 43–52. [Google Scholar] [CrossRef]
- Park, Y.-M.; Yeon, K.-M.; Park, C.-H. Silica treatment technologies in reverse osmosis for industrial desalination: A review. Environ. Eng. Res. 2020, 25, 819–829. [Google Scholar] [CrossRef]
- Comstock, S.E.H.; Boyer, T.H.; Graf, K.C. Treatment of nanofiltration and reverse osmosis concentrates: Comparison of precipitative softening, coagulation, and anion exchange. Water Res. 2011, 45, 4855–4865. [Google Scholar] [CrossRef]
- Ordóñez, R.; Moral, A.; Hermosilla, D.; Blanco, Á. Combining coagulation, softening and flocculation to dispose reverse osmosis retentates. J. Ind. Eng. Chem. 2012, 18, 926–933. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Subramani, A.; Cryer, E.; Liu, L.; Lehman, S.; Ning, R.Y.; Jacangelo, J.G. Impact of intermediate concentrate softening on feed water recovery of reverse osmosis process during treatment of mining contaminated groundwater. Sep. Purif. Technol. 2012, 88, 138–145. [Google Scholar] [CrossRef]
- O’Connell, M.G.; Rajendran, N.; Elimelech, M.; Gilron, J.; Dunn, J.B. Water, Energy, and Cost: A Nexus Approach to Zero/Minimal Liquid Discharge Desalination Technologies. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, D.; Chen, Y.; He, D.; Elimelech, M. Comparison of Energy Consumption of Osmotically Assisted Reverse Osmosis and Low-Salt-Rejection Reverse Osmosis for Brine Management. Environ. Sci. Technol. 2021, 55, 10714–10723. [Google Scholar] [CrossRef] [PubMed]
- Warsinger, D.M.; Tow, E.W.; Nayar, K.G.; Maswadeh, L.A.; Lienhard, J.H.V. Energy efficiency of batch and semi-batch (CCRO) reverse osmosis desalination. Water Res. 2016, 106, 272–282. [Google Scholar] [CrossRef]
- Nayar, K.G.; Lienhard, J.H.V. Brackish water desalination for greenhouse agriculture: Comparing the costs of RO, CCRO, EDR, and monovalent-selective EDR. Desalination 2020, 475, 114188. [Google Scholar] [CrossRef]
- DuPont. Product Data Sheet. DuPont™ XUS180808 Reverse Osmosis Element Ultra-High Pressure, High-Rejection, Reverse Osmosis Element for Industrial Water Purification. Available online: https://dws.octochemstore.com/wp-content/uploads/2020/01/FilmTec-XUS180808-Ultra-High-Pressure-RO.pdf (accessed on 7 December 2024).
- Saltworks. Applying Ultra-Hjigh Pressure Reverse Osmosis in Brine Management. Available online: https://www.saltworkstech.com/articles/applying-ultra-high-pressure-reverse-osmosis-in-brine-management/ (accessed on 26 January 2024).
- Reimonn, G.; Kamcev, J. Techno-economic perspective on the limitations and prospects of ion-exchange membrane technologies. Curr. Opin. Chem. Eng. 2025, 47, 101077. [Google Scholar] [CrossRef]
- Senanayake, P.S.; Ahmed, M.F.; Lugo, A.; Stoll, Z.; Moe, N.E.; Ehsani, M.; Barber, J.; Xu, P.; Wang, H. Enhancing the efficiency of electrodialysis brine concentrator to reduce the levelized cost of salt production through dynamic model-based simulations. Desalination 2025, 598, 118416. [Google Scholar] [CrossRef]
- Cappelle, M.A. High Recovery Inland Desalination: A Technical and Economic Performance Evaluation of Zero Discharge Desalination and Other Technologies. Ph.D. Thesis, University of Texas at El Paso, El Paso, TX, USA, 2018. [Google Scholar]
- Oddonetto, T.L.; Deemer, E.; Lugo, A.; Cappelle, M.; Xu, P.; Santiago, I.; Walker, W.S. Assessment of salt-free electrodialysis metathesis: A novel process for brine management in brackish water desalination using monovalent selective ion exchange membranes. Desalination 2024, 592, 118160. [Google Scholar] [CrossRef]
- Xu, P. Concentrate Treatment and Chemical Production Using Innovative Electrodialysis Processes for Near Zero-Waste Discharge. 2024. Available online: https://www.nawihub.org/wp-content/uploads/sites/16/2024/03/3.15-Pei-Xu-Concentrate-treatment-and-chemical-production-using-innovative-electrodialysis-processes-for-near-zero-waste-discharge.pdf (accessed on 24 December 2024).
- Senanayake, P.S.; Lugo, A.; Ahmed, M.F.; Stoll, Z.; Moe, N.E.; Barber, J.; Walker, W.S.; Xu, P.; Wang, H. Electrodialysis modeling for desalination and resource recovery. Curr. Opin. Chem. Eng. 2025, 47, 101081. [Google Scholar] [CrossRef]
- Comstock, S.E.H.; Boyer, T.H. Combined magnetic ion exchange and cation exchange for removal of DOC and hardness. Chem. Eng. J. 2014, 241, 366–375. [Google Scholar] [CrossRef]
- Yaqub, M.; Nguyen, M.N.; Lee, W. Treating reverse osmosis concentrate to address scaling and fouling problems in zero-liquid discharge systems: A scientometric review of global trends. Sci. Total Environ. 2022, 844, 157081. [Google Scholar] [CrossRef]
- Crittenden, J.C. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Miranda, R.; Latour, I.; Blanco, A. Silica Removal from a Paper Mill Effluent by Adsorption on Pseudoboehmite and γ-Al2O3. Water 2021, 13, 2031. [Google Scholar] [CrossRef]
- Chuuman, T.; Eguchi, K.; Akinaga, M.; Kawamoto, D.; Horii, S.; Yonezu, K.; Watanabe, K.; Yokoyama, T. Inhibition of Silicic Acid Elution during the Regeneration of Strong Base Anion Exchange Resin Column. Bull. Chem. Soc. Jpn. 2019, 92, 869–874. [Google Scholar] [CrossRef]
- Milne, N.A.; O’Reilly, T.; Sanciolo, P.; Ostarcevic, E.; Beighton, M.; Taylor, K.; Mullett, M.; Tarquin, A.J.; Gray, S.R. Chemistry of silica scale mitigation for RO desalination with particular reference to remote operations. Water Res. 2014, 65, 107–133. [Google Scholar] [CrossRef]
- Iler, R. Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry of Silica; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Mbedzi, N. An Investigation into the Removal of Aluminosilicates Scaling Species by Activated Alumina. Master Thesis, University of Cape Town, Cape Town, South Africa, 2010. Available online: http://hdl.handle.net/11427/5443 (accessed on 28 November 2024).
- Bouguerra, W.; Ali, M.B.S.; Hamrouni, B.; Dhahbi, M. Equilibrium and kinetic studies of adsorption of silica onto activated alumina. Desalination 2007, 206, 141–146. [Google Scholar] [CrossRef]
- Sanciolo, P.; Milne, N.A.; Taylor, K.; Mullet, M.; Gray, S.R. Silica scale mitigation for high recovery reverse osmosis of groundwater for a mining process. Desalination 2014, 340, 49–58. [Google Scholar] [CrossRef]
- Cob, S.S.; Hofs, B.; Maffezzoni, C.; Adamus, J.; Siegers, W.G.; Cornelissen, E.R.; Güner, F.G.; Witkamp, G.J. Silica removal to prevent silica scaling in reverse osmosis membranes. Desalination 2014, 344, 137–143. [Google Scholar] [CrossRef]
- Janson, A.; Minier-Matar, J.; Al-Shamari, E.; Hussain, A.; Sharma, R.; Rowley, D.; Adham, S. Evaluation of new ion exchange resins for hardness removal from boiler feedwater. Emergent Mater. 2018, 1, 77–87. [Google Scholar] [CrossRef]
- Dahmani, K.; Kherroub, D.; Boucherdoud, A.; Benaouda, B. Removal of Ca(II) and Mg(II) hardness by ion exchange resins and soda ash for seawater pretreatment to reduce scale formation in evaporators multi-stage flash desalination. Desalination Water Treat. 2021, 221, 23–30. [Google Scholar] [CrossRef]
- Thomson, B.M.; Tandukar, S.; Shahi, A.; Lee, C.O.; Howe, K.J. Mineral Recovery Enhanced Desalination (MRED) process: An innovative technology for desalinating hard brackish water. Desalination 2020, 496, 114761. [Google Scholar] [CrossRef]
- Sasan, K.; Brady, P.V.; Krumhansl, J.L.; Nenoff, T.M. Exceptional selectivity for dissolved silicas in industrial waters using mixed oxides. J. Water Process Eng. 2017, 20, 187–192. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Hayani, A.; Mountadar, S.; Tahiri, S.; Mountadar, M.M. Softening of hard water by ion-exchange with strongly acidic cationic resin. Application to the brackish groundwater of the coastal area of El Jadida province (Morocco). J. Mater. Environ. Sci. 2016, 7, 3875–3884. [Google Scholar]
- Zaman, M. Silica Characterization in Coal Seam Gas Water and Its Removal by Activated Alumina. Master’s Thesis, The University of Queensland, Brisbane, QLD, Australia, 2016. [Google Scholar]
- Lencka, M.M.; Springer, R.D.; Wang, P.; Anderko, A. Modeling Mineral Scaling in Oil and Gas Environments Up to Ultra High Pressures and Temperatures. In Proceedings of the CORROSION 2018, Phoenix, AZ, USA, 15–19 April 2018; p. NACE–2018-10828. [Google Scholar]
- Advisor Ci Online. Available online: https://avistamembranesolutions.com/resources/avista-advisorci-online-registration/ (accessed on 23 January 2025).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- WaterTAP. WaterTAP: An Open-Source Water Treatment Model Library. Available online: https://github.com/watertap-org/watertap (accessed on 16 March 2024).
- Ali, M.B.S.; Hamrouni, B.; Bouguecha, S.; Dhahbi, M. Silica removal using ion-exchange resins. Desalination 2004, 167, 273–279. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep. Purif. Technol. 2005, 42, 265–271. [Google Scholar] [CrossRef]
- Shemer, H.; Melki-Dabush, N.; Semiat, R. Removal of silica from brackish water by integrated adsorption/ultrafiltration process. Environ. Sci. Pollut. Res. 2019, 26, 31623–31631. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.; Sorg, T.J.; Ghurye, G.L. Ion exchange and adsorption of inorganic contaminants. In Water Quality & Treatment: A Handbook on Drinking Water, 6th ed.; Edzwald, J., Ed.; McGraw-Hill Education: New York, NY, USA, 1999. [Google Scholar]
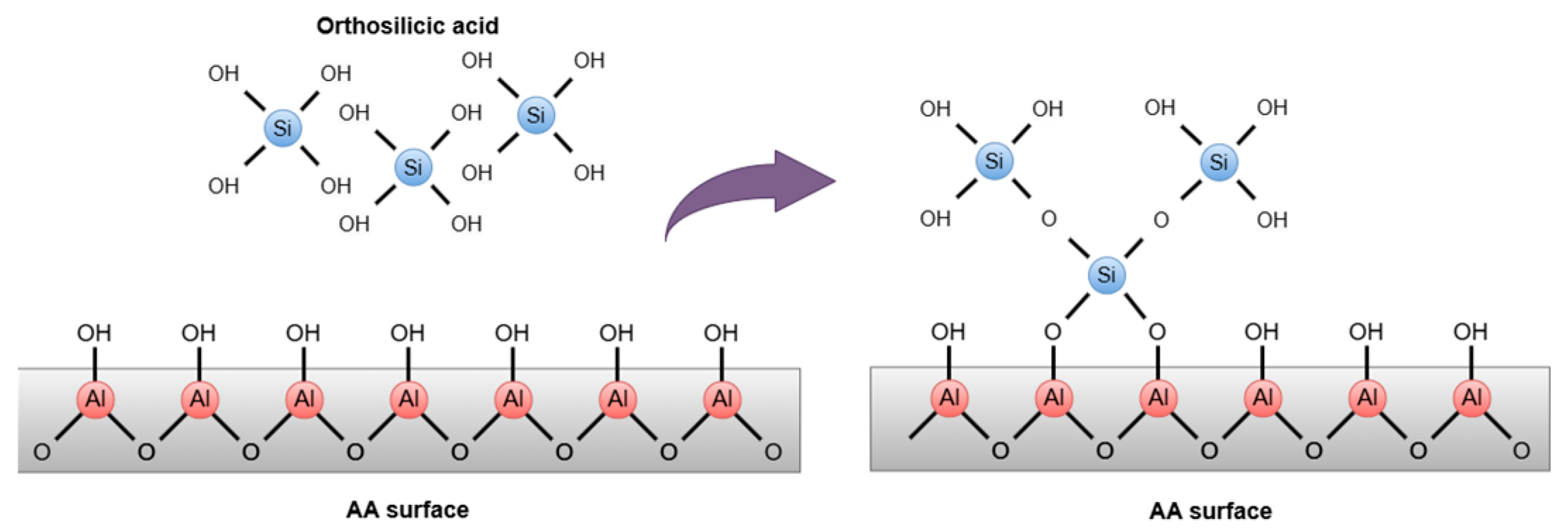


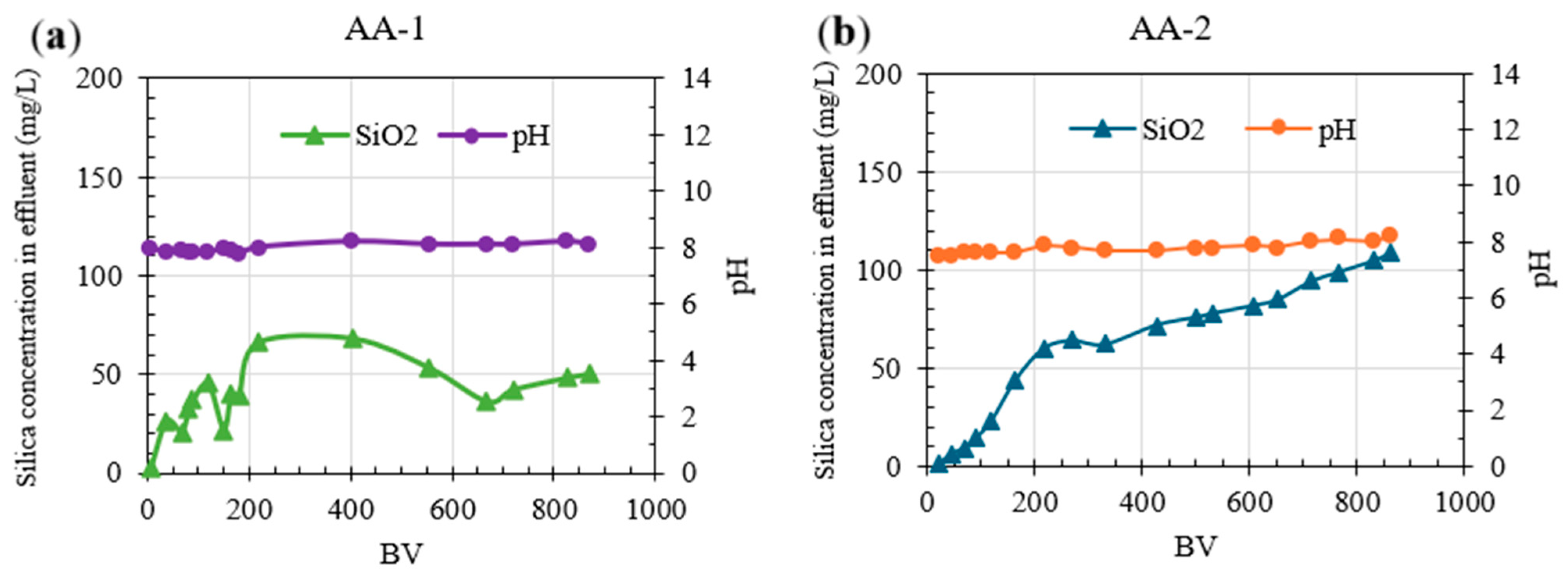





| Parameters | Unit | ROC |
|---|---|---|
| pH | - | 8.5 ± 0.1 |
| Electrical conductivity (EC) | mS/cm | 18.8 ± 0.2 |
| Dissolved organic carbon (DOC) | mg/L | 9.1 ± 0.21 |
| Hardness (as CaCO3) | mg/L | 3000 ± 50 |
| Alkalinity (as CaCO3) | mg/L | 350 ± 26.5 |
| Silica (as SiO2) | mg/L | 160 ± 2 |
| Sodium | mg/L | 3382 ± 160.7 |
| Ammonium | mg/L | ND |
| Potassium | mg/L | 90.2 ± 2.7 |
| Magnesium | mg/L | 211.67 ± 9.4 |
| Calcium | mg/L | 846.7 ± 29.7 |
| Fluoride | mg/L | 17.1 ± 1.4 |
| Chloride | mg/L | 5634 ± 163.1 |
| Nitrite | mg/L | 25.3 ± 2.2 |
| Bromide | mg/L | 21.3 ± 2.6 |
| Nitrate | mg/L | 15 ± 0.7 |
| Sulfate | mg/L | 1393 ± 30.4 |
| Phosphate | mg/L | ND |
| Total dissolved solids (TDS) | mg/L | 12,445 ± 188.6 |
| Predicted scalants | Formula | SI |
| Anhydrite | CaSO4 | −0.58 |
| Aragonite | CaCO3 | 1.78 |
| Calcite | CaCO3 | 1.93 |
| Chalcedony | SiO2 | 0.65 |
| Chrysotile | Mg3Si2O5(OH)4 | 5.09 |
| Dolomite | CaMg(CO3)2 | 3.6 |
| Fluorite | CaF2 | 1.79 |
| Gypsum | CaSO4٠2H2O | −0.28 |
| Quartz | SiO2 | 1.07 |
| Sepiolite | Mg2Si3O7٠5OH:3H2O | 4.25 |
| Sepiolite (disordered) | Mg2Si3O7٠5OH:3H2O | 1.35 |
| SiO2 (amorphous) | SiO2 | −0.19 |
| Physicochemical Properties | Silica Removal | Hardness Removal | |||
|---|---|---|---|---|---|
| SRIX-1 | SRIX-2 | HRIX-1 | HRIX-2 | HRIX-3 | |
| Copolymer | Styrene/divinylbenzene | Polystyrene crosslinked with divinylbenzene | Crosslinked acrylic | Polystyrene crosslinked with divinylbenzene | Acrylic gel |
| Matrix | Gel | Macroporous | Macroporous | Gel | Gel |
| Type | SBA | SBA | WAC | SAC | WAC |
| Functional group | Trimethyl ammonium | Quaternary ammonium | Carboxylic acid | Sulfonic acid | Carboxylic acid |
| Ionic form as shipped | Cl− | Cl− | H+ | Na+ | H+ |
| Total exchange capacity | 1.2 eq/L (Cl− form) | 1.2 eq/L (Cl− form) | ≥4.7 eq/L (H+ form) | ≥4.56 eq/L (Na+ form) | ≥4.2 eq/L (H+ form) |
| Water retention capacity | 49–59% (Cl− form) | 50–60% (Cl− form) | 40–50% (H+ form) | 37–47% (Na+ form) | 43–60% (H+ form) |
| Particle diameter | 600–750 µm | 300–1200 µm | 500–700 µm | 650 ± 50 µm | 297–1190 µm |
| Temperature range | 5–100 °C (41–212 °F) | <100 °C (212 °F) | 5–120 °C (41–248 °F) | <60 °C (140 °F) | <100 °C (212 °F) |
| pH range | 1–14 | 1–14 | 6–14 | 1–14 | >7 |
| Service Cycle | Resin Bed Volume (BV) | Service Flow Rate (SFR) | Flow Rate (Q) | Empty Bed Contact Time (EBCT) | Hydraulic Loading Rate (HLR) |
|---|---|---|---|---|---|
| mL | BV/h | mL/min | min | mL/cm2/min | |
| Column operation | 40 | 12 | 8 | 5 | 7.84 |
| Backwash | 40 | 75 | 50 | 0.8 | 49.02 |
| Regeneration | 40 | 12 | 8 | 5 | 7.84 |
| DI water rinse | 40 | 12 | 8 | 5 | 7.84 |
| Typical Chemical Composition, % | AA-1 (CPN) | AA-2 (DD-6) | AA-3 (DD-2) |
|---|---|---|---|
| Al2O3 | 92 | 92 | - |
| SiO2 | 0.02 | 0.03 | <0.02 |
| Fe2O3 | 0.03 | 0 | <0.01 |
| Na2O | 0.3 | 0.35 | <0.4 |
| Typical physical properties | |||
| Particle size, μm | 1180 × 600 | 600 × 300 | 1180 × 600 |
| Surface area, m2/g | 315 | 380 | 275 |
| Packed bulk density, lb/ft3 (kg/m3) | 47 (752) | 40 (641) | - |
| IX Resin Using ROC | AA Using ROC | AA-2 Using High-Salinity Synthetic Water | ||||||
|---|---|---|---|---|---|---|---|---|
| SRIX-1 | SRIX-2 | AA-1 | AA-2 | AA-3 | 5% NaCl | 10% NaCl | 20% NaCl | |
| qmax (mg/g) | 12.44 | 19.65 | 625.00 | 322.58 | 238.10 | 312.5 | 357.14 | 294.12 |
| KL | 0.0231 | 0.0101 | 0.0012 | 0.0025 | 0.0016 | 0.0004 | 0.0004 | 0.003 |
| AA | Silica Adsorbed | Regenerant | Regenerant Concentration | # of Batches | Temperature | Contact Time | Regeneration/ Elution |
|---|---|---|---|---|---|---|---|
| AA-1 | 23.5 mg/g | NaOH | 2, 8 and 10% | 3 | 25 °C | 1 h | 0 |
| H2SO4 | 0.1 N | 3 | 25 °C | 1 h | 10.1% | ||
| NaOH/H2SO4 | NaOH 2% H2SO4 0.1 N | 1 | 25 °C | 1 h each | 4.5% | ||
| AA-2 | 42 mg/g | NaOH | 2% | 1 | 25 °C | 1, 12, and 24 h | 0 |
| 50 °C | 1 h | 0 | |||||
| 6% | 1 | 25 °C | 1, 12, and 24 h | 0 | |||
| 50 °C | 1 h | 0 | |||||
| 8% | 1 | 25 °C | 1, 12, and 24 h | 0 | |||
| 50 °C | 1 h | 0 | |||||
| H2SO4 | 0.1 N | 3 | 25 °C | 1 h | 10.4% | ||
| NaOH/H2SO4 | NaOH 2% H2SO4 0.1 N | 1 | 25 °C | 1 h each | 5.3% | ||
| 59 mg/g | HCl | 0.05 N | 3 | 25 °C | 1 h | 2.9% | |
| 0.1 N | 3 | 25 °C | 1 h | 10.7% | |||
| 1 N | 3 | 25 °C | 1 h | 19.8% | |||
| 33.3 mg/g | HCl | 0.1 N | 3 | 25 °C | 2 h | 9.6% | |
| 3 h | 12.8% | ||||||
| 12 h | 22.8% | ||||||
| 40 °C | 1 h | 18.6% | |||||
| 50 °C | 1 h | 22.9% | |||||
| 60 °C | 1 h | 26.6% |
| IX Resin | |||
|---|---|---|---|
| HRIX-1 | HRIX-2 | HRIX-3 | |
| qmax (mg/g) | 133.33 | 129.87 | 101.01 |
| qmax (eq/L) | 3.23 | 3.12 | 1.56 |
| KL | 0.0019 | 0.0012 | 0.0013 |
| Maximum Water Recovery (%) with Vitec 7400 | ||||||
|---|---|---|---|---|---|---|
| Silica Concentration (mg/L) | ||||||
| 10 | 37.5 | 75 | 112.5 | 160 | ||
| Hardness (mg/L as CaCO3) | 980 | 80 (AA + IX effluent) | − | − | − | − |
| 1417 | 79 | 78 | − | − | − | |
| 1882 | 77 | 76 | 75 | − | − | |
| 2336 | 76 | 74 | 72 | 68 | − | |
| 2800 | 75 (AA effluent) | 74 | 72 | 70 | 64 (ROC) | |
| Maximum Water Recovery (%) with Vitec 1070 | ||||||
| Silica Concentration (mg/L) | ||||||
| 10 | 37.5 | 75 | 112.5 | 160 | ||
| Hardness (mg/L as CaCO3) | 980 | 80 (AA + IX effluent) | − | − | − | − |
| 1417 | 78 | 72 | − | − | − | |
| 1882 | 77 | 68 | 52 | − | − | |
| 2336 | 76 | 65 | 53 | 28 | − | |
| 2800 | 75 (AA effluent) | 64 | 53 | 30 | 4 (ROC) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Saucedo, C.; Stoll, Z.; Senanayake, P.S.; Xu, P.; Wang, H. Evaluating Pretreatment Strategies with Modeling for Reducing Scaling Potential of Reverse Osmosis Concentrate: Insights from Ion Exchange and Activated Alumina. Water 2025, 17, 828. https://doi.org/10.3390/w17060828
Mejía-Saucedo C, Stoll Z, Senanayake PS, Xu P, Wang H. Evaluating Pretreatment Strategies with Modeling for Reducing Scaling Potential of Reverse Osmosis Concentrate: Insights from Ion Exchange and Activated Alumina. Water. 2025; 17(6):828. https://doi.org/10.3390/w17060828
Chicago/Turabian StyleMejía-Saucedo, Carolina, Zachary Stoll, Punhasa S. Senanayake, Pei Xu, and Huiyao Wang. 2025. "Evaluating Pretreatment Strategies with Modeling for Reducing Scaling Potential of Reverse Osmosis Concentrate: Insights from Ion Exchange and Activated Alumina" Water 17, no. 6: 828. https://doi.org/10.3390/w17060828
APA StyleMejía-Saucedo, C., Stoll, Z., Senanayake, P. S., Xu, P., & Wang, H. (2025). Evaluating Pretreatment Strategies with Modeling for Reducing Scaling Potential of Reverse Osmosis Concentrate: Insights from Ion Exchange and Activated Alumina. Water, 17(6), 828. https://doi.org/10.3390/w17060828









